Abstract
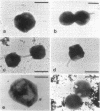
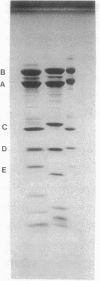
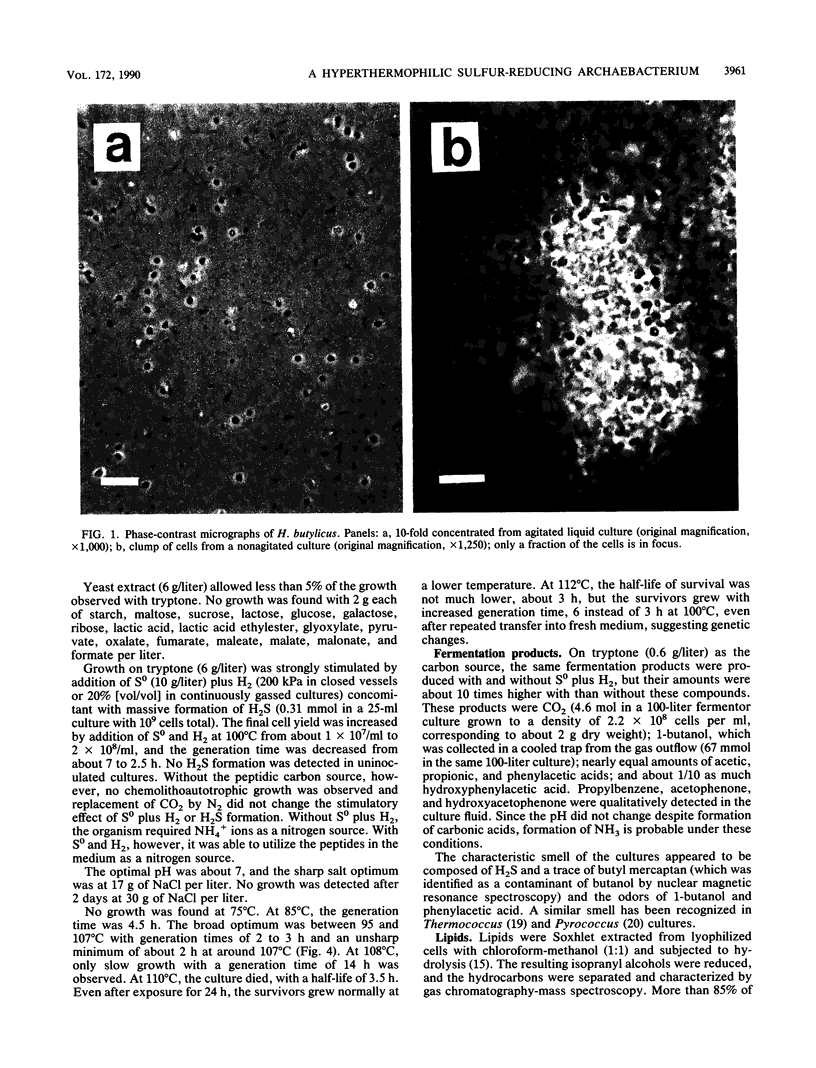
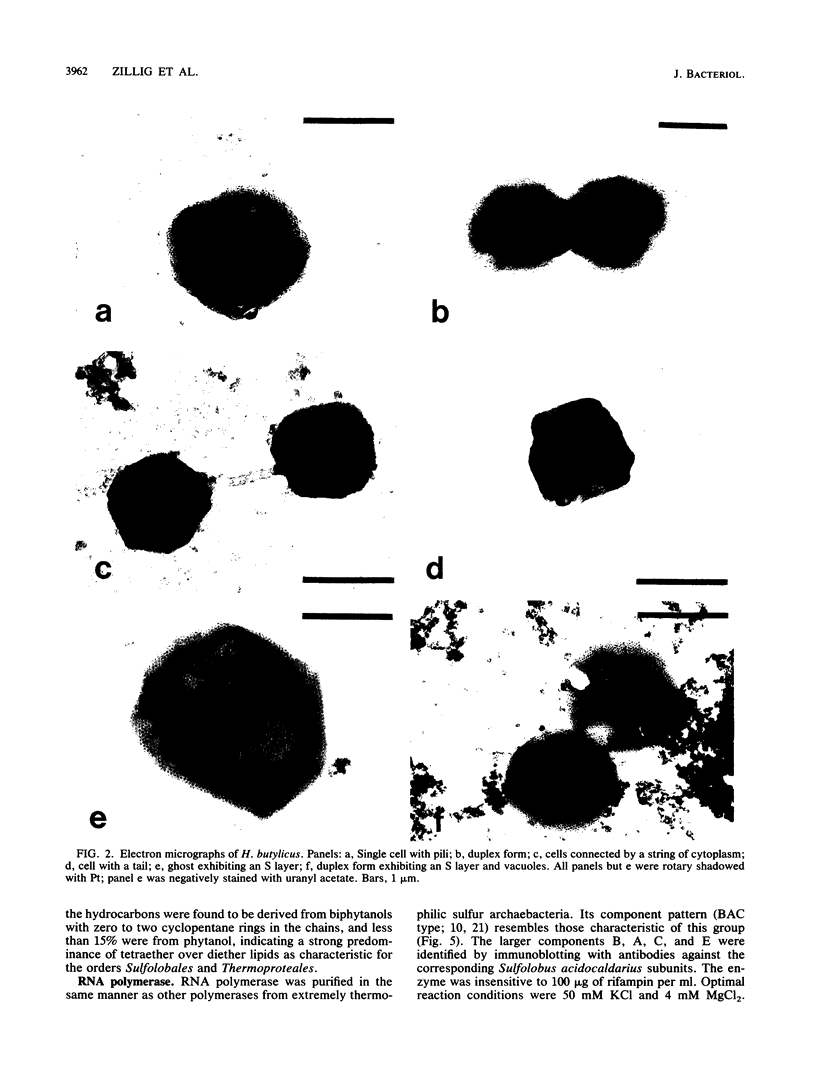
The hyperthermophilic peptide-fermenting sulfur archaebacterium Hyperthermus butylicus was isolated from the sea floor of a solfataric habitat with temperatures of up to 112 degrees C on the coast of the island of São Miguel, Azores. The organism grows at up to 108 degrees C, grows optimally between 95 and 106 degrees C at 17 g of NaCl per liter and pH 7.0, utilizes peptide mixtures as carbon and energy sources, and forms H2S from elemental sulfur and molecular hydrogen as a growth-stimulating accessory energy source but not by sulfur respiration. The same fermentation products, CO2, 1-butanol, acetic acid, phenylacetic acid, and a trace of hydroxyphenylacetic acid, are formed both with and without of S0 and H2. Its ether lipids, the absence of a mureine sacculus, the nature of the DNA-dependent RNA polymerase, and phylogenetic classification by DNA-rRNA cross-hybridization characterize H. butylicus as part of a novel genus of the major branch of archaebacteria comprising the orders Thermoproteales and Sulfolobales, representing a particularly long lineage bifurcating with the order Sulfolobales above the branching off of the genus Thermoproteus and distinct from the genera Desulfurococcus and Pyrodictium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Scherrer K. Isolation of preribosomes from HeLa cells and their characterization by electrophoresis on uniform and exponential-gradient-polyacrylamide gels. Eur J Biochem. 1971 Nov 11;23(2):372–386. doi: 10.1111/j.1432-1033.1971.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Prangishvilli D., Zillig W., Gierl A., Biesert L., Holz I. DNA-dependent RNA polymerase of thermoacidophilic archaebacteria. Eur J Biochem. 1982 Mar 1;122(3):471–477. doi: 10.1111/j.1432-1033.1982.tb06461.x. [DOI] [PubMed] [Google Scholar]
- Schnabel R., Thomm M., Gerardy-Schahn R., Zillig W., Stetter K. O., Huet J. Structural homology between different archaebacterial DNA-dependent RNA polymerases analyzed by immunological comparison of their components. EMBO J. 1983;2(5):751–755. doi: 10.1002/j.1460-2075.1983.tb01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss T., Melchers K., Scheirle G., Schäfer K. P. Structural comparison of recombinant pulmonary surfactant protein SP-A derived from two human coding sequences: implications for the chain composition of natural human SP-A. Am J Respir Cell Mol Biol. 1991 Jan;4(1):88–94. doi: 10.1165/ajrcmb/4.1.88. [DOI] [PubMed] [Google Scholar]
- Wildhaber I., Santarius U., Baumeister W. Three-dimensional structure of the surface protein of Desulfurococcus mobilis. J Bacteriol. 1987 Dec;169(12):5563–5568. doi: 10.1128/jb.169.12.5563-5568.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Klenk H. P., Palm P., Pühler G., Gropp F., Garrett R. A., Leffers H. The phylogenetic relations of DNA-dependent RNA polymerases of archaebacteria, eukaryotes, and eubacteria. Can J Microbiol. 1989 Jan;35(1):73–80. doi: 10.1139/m89-011. [DOI] [PubMed] [Google Scholar]
- Zillig W., Schnabel R., Stetter K. O. Archaebacteria and the origin of the eukaryotic cytoplasm. Curr Top Microbiol Immunol. 1985;114:1–18. doi: 10.1007/978-3-642-70227-3_1. [DOI] [PubMed] [Google Scholar]
- Zillig W., Stetter K. O., Janeković D. DNA-dependent RNA polymerase from the archaebacterium Sulfolobus acidocaldarius. Eur J Biochem. 1979 Jun 1;96(3):597–604. doi: 10.1111/j.1432-1033.1979.tb13074.x. [DOI] [PubMed] [Google Scholar]