Abstract
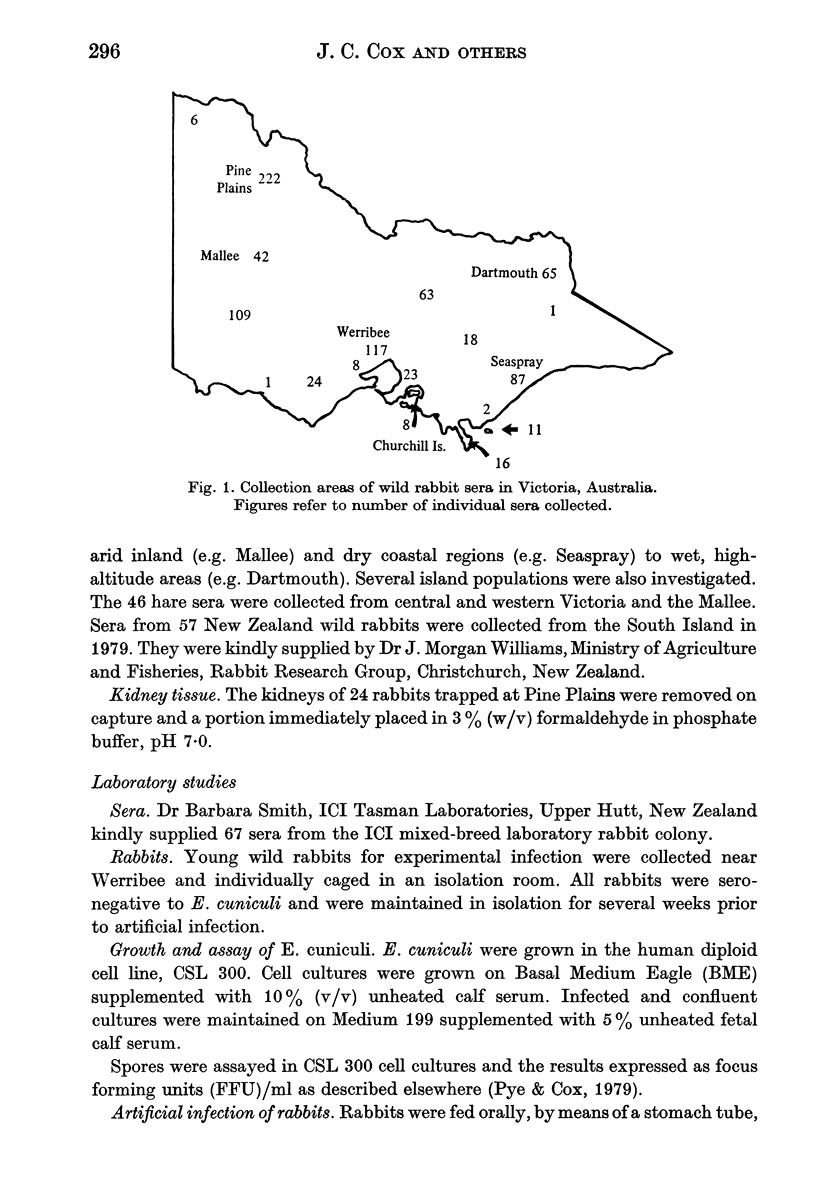
Sera from 823 wild rabbits (Oryctolagus cuniculus) collected from a number of geographic regions of Victoria, Australia over the past eight years were examined for antibodies to Encephalitozoon cuniculi, along with sera from 46 hares (Lepus europaeus) (Pallas) and 57 New Zealand wild rabbits. No sera were positive, implying that this common laboratory rabbit parasite is absent from wild rabbits in these areas. However, wild rabbits were found to be readily infected by the oral route with small numbers of tissue-culture-grown spores of E. cuniculi. A possible explanation for the absence of encephalitozoonosis in wild rabbits is that E. cuniculi infection places them at a biological disadvantage for survival. The natural hygiene habit of wild rabbits may also significantly decrease post-natal infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox J. C. Altered immune responsiveness associated with Encephalitozoon cuniculi infection in rabbits. Infect Immun. 1977 Feb;15(2):392–395. doi: 10.1128/iai.15.2.392-395.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. C., Gallichio H. A., Pye D., Walden N. B. Application of immunofluorescence to the establishment of an Encephalitozoon cuniculi-free rabbit colony. Lab Anim Sci. 1977 Apr;27(2):204–209. [PubMed] [Google Scholar]
- Cox J. C., Hamilton R. C., Attwood H. D. An investigation of the route and progression of Encephalitozoon cuniculi infection in adult rabbits. J Protozool. 1979 May;26(2):260–265. doi: 10.1111/j.1550-7408.1979.tb02776.x. [DOI] [PubMed] [Google Scholar]
- Cox J. C., Pye D. Serodiagnosis of nosematosis by immunofluorescence using cell-culture-grown organisms. Lab Anim. 1975 Oct;9(4):297–304. doi: 10.1258/002367775780957124. [DOI] [PubMed] [Google Scholar]
- Cox J. C., Walden N. B. Presumptive diagnosis of Nosema cuniculi in rabbits by immunofluorescence. Res Vet Sci. 1972 Nov;13(6):595–597. [PubMed] [Google Scholar]
- Hunt R. D., King N. W., Foster H. L. Encephalitozoonosis: evidence for vertical transmission. J Infect Dis. 1972 Aug;126(2):212–214. doi: 10.1093/infdis/126.2.212. [DOI] [PubMed] [Google Scholar]
- Pye D., Cox J. C. Simple focus assay for Encephalitozoon cuniculi. Lab Anim. 1979 Jul;13(3):193–195. doi: 10.1258/002367779780937807. [DOI] [PubMed] [Google Scholar]
- Shepherd R. C., Edmonds J. W., Nolan I. F., Gocs A. Myxomatosis in the Mallee region of Victoria, Australia. J Hyg (Lond) 1978 Oct;81(2):239–243. doi: 10.1017/s0022172400025067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testoni F. J. Enzootic renal nosematosis in laboratory rabbits. Aust Vet J. 1974 Apr;50(4):159–163. doi: 10.1111/j.1751-0813.1974.tb06883.x. [DOI] [PubMed] [Google Scholar]
- Wilson J. M. Encephalitozoon cuniculi in wild European rabbits and a fox. Res Vet Sci. 1979 Jan;26(1):114–114. [PubMed] [Google Scholar]


