Abstract
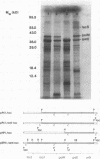
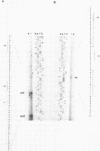
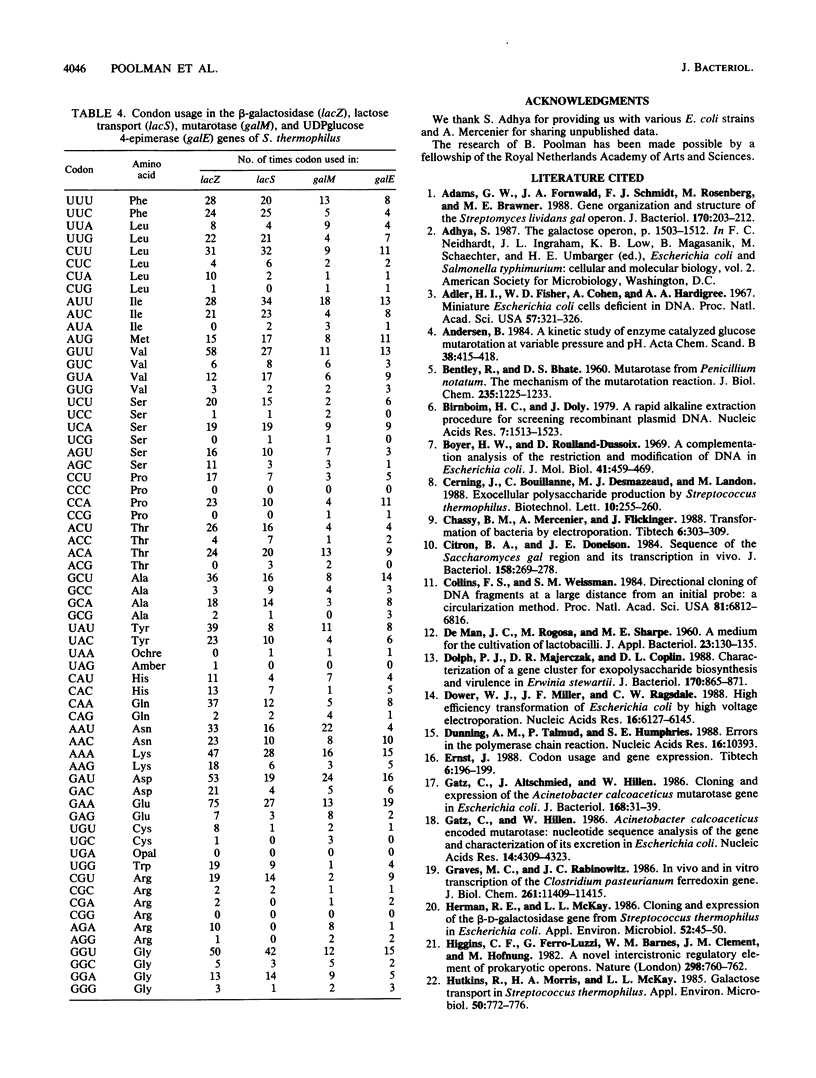
The complete nucleotide sequences of the genes encoding aldose 1-epimerase (mutarotase) (galM) and UDPglucose 4-epimerase (galE) and flanking regions of Streptococcus thermophilus have been determined. Both genes are located immediately upstream of the S. thermophilus lac operon. To facilitate the isolation of galE, a special polymerase chain reaction-based technique was used to amplify the region upstream of galM prior to cloning. The galM protein was homologous to the mutarotase of Acinetobacter calcoaceticus, whereas the galE protein was homologous to UDPglucose 4-epimerase of Escherichia coli and Streptomyces lividans. The amino acid sequences of galM and galE proteins also showed significant similarity with the carboxy-terminal and amino-terminal domains, respectively, of UDPglucose 4-epimerase from Kluyveromyces lactis and Saccharomyces cerevisiae, suggesting that the yeast enzymes contain an additional, yet unidentified (mutarotase) activity. In accordance with the open reading frames of the structural genes, galM and galE were expressed as polypeptides with apparent molecular masses of 39 and 37 kilodaltons, respectively. Significant activities of mutarotase and UDPglucose 4-epimerase were detected in lysates of E. coli cells containing plasmids encoding galM and galE. Expression of galE in E. coli was increased 300-fold when the gene was placed downstream of the tac promoter. The gene order for the gal-lac gene cluster of S. thermophilus is galE-galM-lacS-lacZ. The flanking regions of these genes were searched for consensus promoter sequences and further characterized by primer extension analysis. Analysis of mRNA levels for the gal and lac genes in S. thermophilus showed a strong reduction upon growth in medium containing glucose instead of lactose. The activities of the lac (lactose transport and beta-galactosidase) and gal (UDPglucose 4-epimerase) proteins of lactose- and glucose-grown S. thermophilus cells matched the mRNA levels.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Fornwald J. A., Schmidt F. J., Rosenberg M., Brawner M. E. Gene organization and structure of the Streptomyces lividans gal operon. J Bacteriol. 1988 Jan;170(1):203–212. doi: 10.1128/jb.170.1.203-212.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENTLEY R., BHATE D. S. Mutarotase from Penicillium notatum. II. The mechanism of the mutarotation reaction. J Biol Chem. 1960 May;235:1225–1233. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Citron B. A., Donelson J. E. Sequence of the Saccharomyces GAL region and its transcription in vivo. J Bacteriol. 1984 Apr;158(1):269–278. doi: 10.1128/jb.158.1.269-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. Directional cloning of DNA fragments at a large distance from an initial probe: a circularization method. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6812–6816. doi: 10.1073/pnas.81.21.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph P. J., Majerczak D. R., Coplin D. L. Characterization of a gene cluster for exopolysaccharide biosynthesis and virulence in Erwinia stewartii. J Bacteriol. 1988 Feb;170(2):865–871. doi: 10.1128/jb.170.2.865-871.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning A. M., Talmud P., Humphries S. E. Errors in the polymerase chain reaction. Nucleic Acids Res. 1988 Nov 11;16(21):10393–10393. doi: 10.1093/nar/16.21.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz C., Altschmied J., Hillen W. Cloning and expression of the Acinetobacter calcoaceticus mutarotase gene in Escherichia coli. J Bacteriol. 1986 Oct;168(1):31–39. doi: 10.1128/jb.168.1.31-39.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz C., Hillen W. Acinetobacter calcoaceticus encoded mutarotase: nucleotide sequence analysis of the gene and characterization of its secretion in Escherichia coli. Nucleic Acids Res. 1986 May 27;14(10):4309–4323. doi: 10.1093/nar/14.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves M. C., Rabinowitz J. C. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. Evidence for "extended" promoter elements in gram-positive organisms. J Biol Chem. 1986 Aug 25;261(24):11409–11415. [PubMed] [Google Scholar]
- Herman R. E., McKay L. L. Cloning and expression of the beta-D-galactosidase gene from Streptococcus thermophilus in Escherichia coli. Appl Environ Microbiol. 1986 Jul;52(1):45–50. doi: 10.1128/aem.52.1.45-50.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F., Barnes W. M., Clement J. M., Hofnung M. A novel intercistronic regulatory element of prokaryotic operons. Nature. 1982 Aug 19;298(5876):760–762. doi: 10.1038/298760a0. [DOI] [PubMed] [Google Scholar]
- Hutkins R., Morris H. A., McKay L. L. Galactokinase activity in Streptococcus thermophilus. Appl Environ Microbiol. 1985 Oct;50(4):777–780. doi: 10.1128/aem.50.4.777-780.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutkins R., Morris H. A., McKay L. L. Galactose transport in Streptococcus thermophilus. Appl Environ Microbiol. 1985 Oct;50(4):772–776. doi: 10.1128/aem.50.4.772-776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lemaire H. G., Müller-Hill B. Nucleotide sequences of the gal E gene and the gal T gene of E. coli. Nucleic Acids Res. 1986 Oct 10;14(19):7705–7711. doi: 10.1093/nar/14.19.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa I. Rapid polarographic mutarotase assay with -D-glucose oxidase. Anal Biochem. 1972 Feb;45(2):441–447. doi: 10.1016/0003-2697(72)90205-9. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Ochman H., Gerber A. S., Hartl D. L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988 Nov;120(3):621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Royer T. J., Mainzer S. E., Schmidt B. F. Lactose transport system of Streptococcus thermophilus: a hybrid protein with homology to the melibiose carrier and enzyme III of phosphoenolpyruvate-dependent phosphotransferase systems. J Bacteriol. 1989 Jan;171(1):244–253. doi: 10.1128/jb.171.1.244-253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Konings W. N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987 Jun;169(6):2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B. F., Adams R. M., Requadt C., Power S., Mainzer S. E. Expression and nucleotide sequence of the Lactobacillus bulgaricus beta-galactosidase gene cloned in Escherichia coli. J Bacteriol. 1989 Feb;171(2):625–635. doi: 10.1128/jb.171.2.625-635.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Crow V. L. Selection of Galactose-Fermenting Streptococcus thermophilus in Lactose-Limited Chemostat Cultures. Appl Environ Microbiol. 1984 Jul;48(1):186–191. doi: 10.1128/aem.48.1.186-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varenne S., Buc J., Lloubes R., Lazdunski C. Translation is a non-uniform process. Effect of tRNA availability on the rate of elongation of nascent polypeptide chains. J Mol Biol. 1984 Dec 15;180(3):549–576. doi: 10.1016/0022-2836(84)90027-5. [DOI] [PubMed] [Google Scholar]
- WILSON D. B., HOGNESS D. S. THE ENZYMES OF THE GALACTOSE OPERON IN ESCHERICHIA COLI. I. PURIFICATION AND CHARACTERIZATION OF URIDINE DIPHOSPHOGALACTOSE 4-EPIMERASE. J Biol Chem. 1964 Aug;239:2469–2481. [PubMed] [Google Scholar]
- Webster T. D., Dickson R. C. Nucleotide sequence of the galactose gene cluster of Kluyveromyces lactis. Nucleic Acids Res. 1988 Aug 25;16(16):8192–8194. doi: 10.1093/nar/16.16.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster T. D., Dickson R. C. The organization and transcription of the galactose gene cluster of Kluyveromyces lactis. Nucleic Acids Res. 1988 Aug 25;16(16):8011–8028. doi: 10.1093/nar/16.16.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]