Abstract
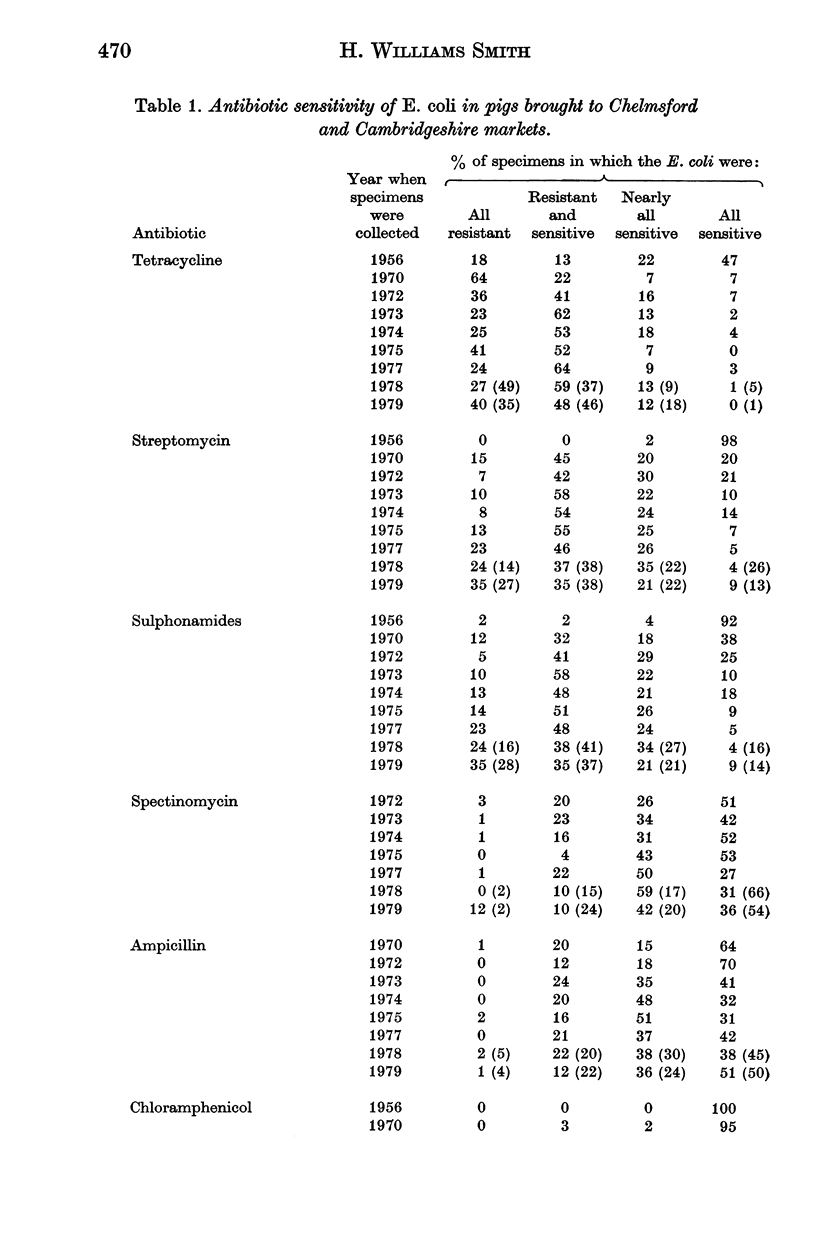
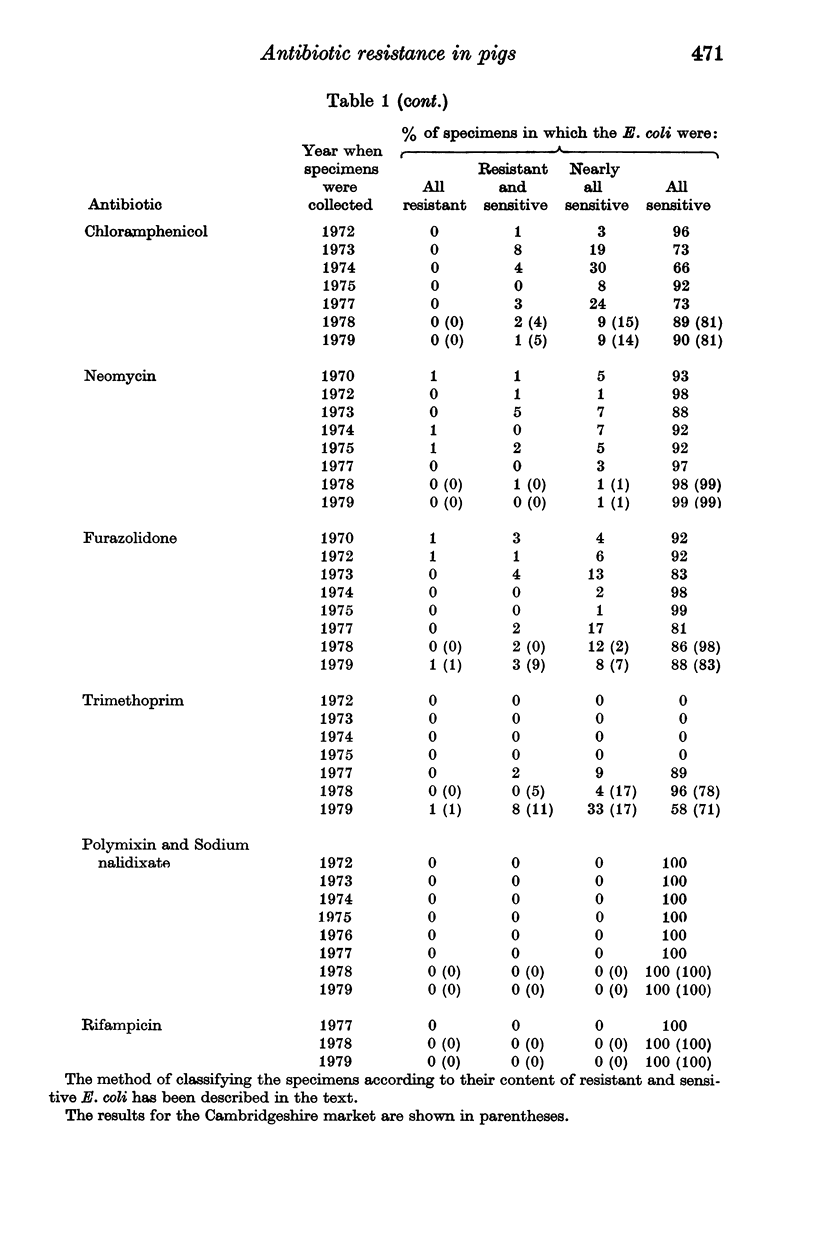
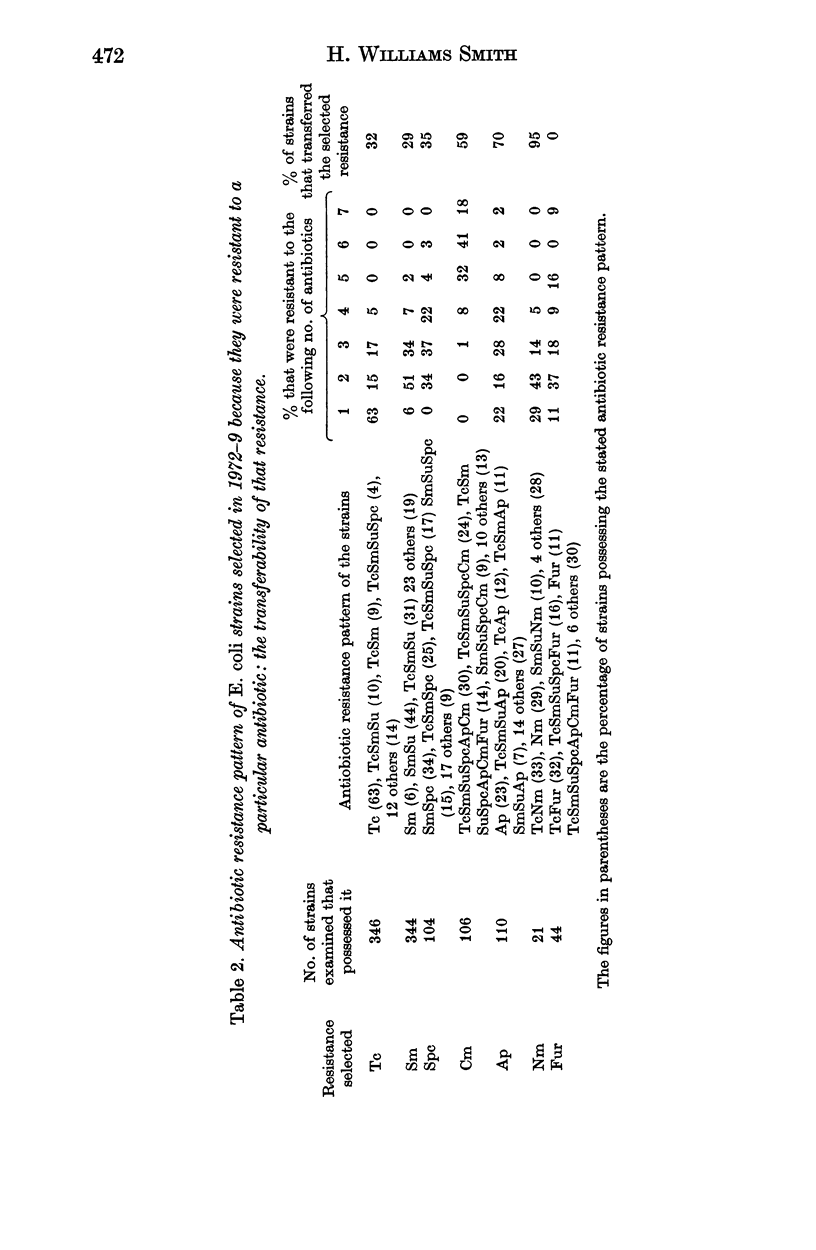
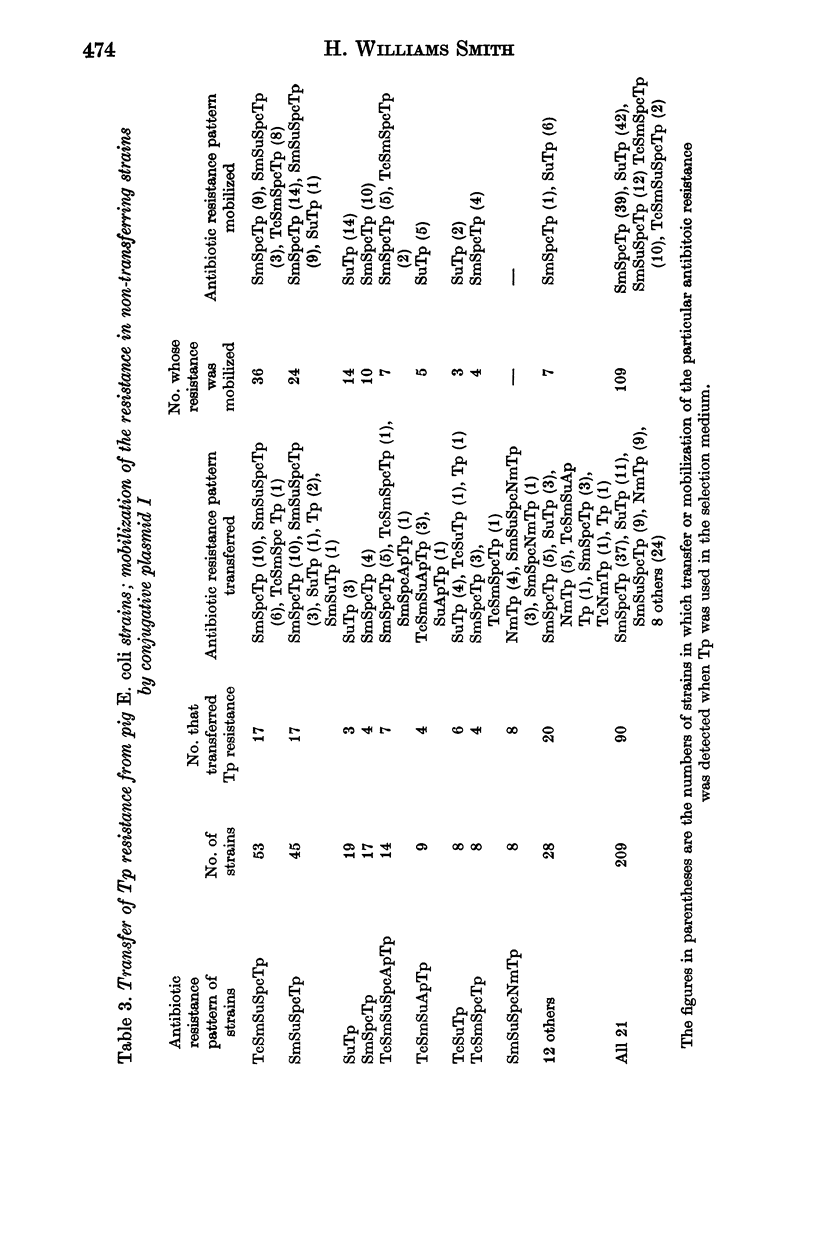
Surveys conducted since 1956 on the antibiotic resistance of the Escherichia coli in faecal specimens from pigs entering Chelmsford Market have revealed that despite the implementation of the Swann Report in 1971 pigs are still an enormous reservoir of tetracycline-resistant E. coli with conjugative ability. Increasingly large amounts of E. coli resistant to streptomycin and sulphonamides were found in specimens examined in recent years until in 1979 the amounts present approached those of tetracycline-resistant organisms. E. coli resistant to chloramphenicol, ampicillin, neomycin, furazolidone or spectinomycin were present, usually in low concentration, in a considerable proportion of the specimens at each yearly examination but the concentration and incidence of these organisms showed no obvious sign of increasing with time. Much of this resistance, except to furazolidone, was of the transferable type. Until 1979 the incidence of faecal specimens containing trimethoprim-resistant E. coli was very low. It increased significantly in that year, most of the resistance being plasmid-, or possibly transposon-determined. The results of surveys performed in a Cambridgeshire market in 1978 and 1979, which showed that a high proportion of faecal specimens contained low concentrations of trimethoprim-resistant E. coli, in general resembled those of the corresponding Chelmsford surveys, suggesting that all the Chelmsford surveys may have accurately reflected the position in the national pig herd.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Yamada T., Davies J. Enzymatic Adenylylation of Streptomycin and Spectinomycin by R-Factor-Resistant Escherichia coli. Infect Immun. 1970 Jan;1(1):109–119. doi: 10.1128/iai.1.1.109-119.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molton P., Williams J., Ponnamperuma C. Survival of common bacteria in liquid culture under carbon dioxide at high temperatures. Nature. 1973 May 25;243(5404):242–243. doi: 10.1038/243242a0. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Benveniste R., Tipper D., Davies J. Aminoglycoside antibiotics: inactivation by phosphorylation in Escherichia coli carrying R factors. J Bacteriol. 1969 Nov;100(2):1144–1146. doi: 10.1128/jb.100.2.1144-1146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards H., Datta N., Sojka W. J., Wray C. Trimethoprim-resistance plasmids and transposons in Salmonella. Lancet. 1978 Dec 2;2(8101):1194–1195. doi: 10.1016/s0140-6736(78)92171-2. [DOI] [PubMed] [Google Scholar]
- Smith H. W. Mobilization of non-conjugative tetracycline, streptomycin, spectinomycin and sulphonamide resistance determinants of Escherichia coli. J Gen Microbiol. 1977 May;100(1):189–196. doi: 10.1099/00221287-100-1-189. [DOI] [PubMed] [Google Scholar]
- Smith H. W. Mutants of Klebsiella pneumoniae resistant to several antibiotics. Nature. 1976 Jan 29;259(5541):307–308. doi: 10.1038/259307a0. [DOI] [PubMed] [Google Scholar]
- Smith H. W. Persistence of tetracycline resistance in pig E. coli. Nature. 1975 Dec 18;258(5536):628–630. doi: 10.1038/258628a0. [DOI] [PubMed] [Google Scholar]
- West B., White G. A survey of trimethoprim resistance in the enteric bacterial flora of farm animals. J Hyg (Lond) 1979 Jun;82(3):481–488. doi: 10.1017/s0022172400054000. [DOI] [PMC free article] [PubMed] [Google Scholar]


