Abstract
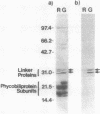
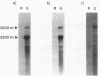
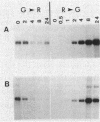
Many biological processes in photosynthetic organisms can be regulated by light quantity or light quality or both. A unique example of the effect of specific wavelengths of light on the composition of the photosynthetic apparatus occurs in cyanobacteria that undergo complementary chromatic adaptation. These organisms alter the composition of their light-harvesting organelle, the phycobilisome, and exhibit distinct morphological features as a function of the wavelength of incident light. Fremyella diplosiphon, a filamentous cyanobacterium, responds to green light by activating transcription of the cpeBA operon, which encodes the pigmented light-harvesting component phycoerythrin. We have isolated and determined the complete nucleotide sequence of another operon, cpeCD, that encodes the linker proteins associated with phycoerythrin hexamers in the phycobilisome. The cpeCD operon is activated in green light and expressed as two major transcripts with the same 5' start site but differing 3' ends. Analysis of the kinetics of transcript accumulation in cultures of F. diplosiphon shifted from red light to green light and vice versa shows that the cpeBA and cpeCD operons are regulated coordinately. A common 17-base-pair sequence is found upstream of the transcription start sites of both operons. A comparison of the predicted amino acid sequences of the phycoerythrin-associated linker proteins CpeC and CpeD with sequences of other previously characterized rod linker proteins shows 49 invariant residues, most of which are in the amino-terminal half of the proteins.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. K., Grossman A. R. Structure and light-regulated expression of phycoerythrin genes in wild-type and phycobilisome assembly mutants of Synechocystis sp. strain PCC 6701. J Bacteriol. 1990 Mar;172(3):1297–1305. doi: 10.1128/jb.172.3.1297-1305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. K., Rayner M. C., Sweet R. M., Eiserling F. A. Regulation of Nostoc sp. phycobilisome structure by light and temperature. J Bacteriol. 1983 Sep;155(3):1407–1416. doi: 10.1128/jb.155.3.1407-1416.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Belknap W. R., Haselkorn R. Cloning and light regulation of expression of the phycocyanin operon of the cyanobacterium Anabaena. EMBO J. 1987 Apr;6(4):871–884. doi: 10.1002/j.1460-2075.1987.tb04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973 Aug;58(2):419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns B. U., Briggs W. R., Grossman A. R. Molecular characterization of phycobilisome regulatory mutants of Fremyella diplosiphon. J Bacteriol. 1989 Feb;171(2):901–908. doi: 10.1128/jb.171.2.901-908.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. A. The photoregulated expression of multiple phycocyanin species. A general mechanism for the control of phycocyanin synthesis in chromatically adapting cyanobacteria. Eur J Biochem. 1981 Oct;119(2):425–429. doi: 10.1111/j.1432-1033.1981.tb05625.x. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Beatty J. T., Cohen S. N., Belasco J. G. An intercistronic stem-loop structure functions as an mRNA decay terminator necessary but insufficient for puf mRNA stability. Cell. 1988 Feb 26;52(4):609–619. doi: 10.1016/0092-8674(88)90473-4. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. Molecular characterization and evolution of sequences encoding light-harvesting components in the chromatically adapting cyanobacterium Fremyella diplosiphon. J Mol Biol. 1988 Feb 5;199(3):447–465. doi: 10.1016/0022-2836(88)90617-1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Füglistaller P., Suter F., Zuber H. Linker polypeptides of the phycobilisome from the cyanobacterium Mastigocladus laminosus: amino-acid sequences and relationships. Biol Chem Hoppe Seyler. 1985 Oct;366(10):993–1001. doi: 10.1515/bchm3.1985.366.2.993. [DOI] [PubMed] [Google Scholar]
- Gendel S., Ohad I., Bogorad L. Control of Phycoerythrin Synthesis during Chromatic Adaptation. Plant Physiol. 1979 Nov;64(5):786–790. doi: 10.1104/pp.64.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich J. C., Blaha L. K., Glazer A. N. Rod substructure in cyanobacterial phycobilisomes: analysis of Synechocystis 6701 mutants low in phycoerythrin. J Cell Biol. 1982 Feb;92(2):261–268. doi: 10.1083/jcb.92.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich J. C., Williams R. C., Glazer A. N. Rod substructure in cyanobacterial phycobilisomes: phycoerythrin assembly in synechocystis 6701 phycobilisomes. J Cell Biol. 1982 Oct;95(1):170–178. doi: 10.1083/jcb.95.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Light harvesting by phycobilisomes. Annu Rev Biophys Biophys Chem. 1985;14:47–77. doi: 10.1146/annurev.bb.14.060185.000403. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Phycobilisomes: structure and dynamics. Annu Rev Microbiol. 1982;36:173–198. doi: 10.1146/annurev.mi.36.100182.001133. [DOI] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Green L. S., Laudenbach D. E., Grossman A. R. A region of a cyanobacterial genome required for sulfate transport. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1949–1953. doi: 10.1073/pnas.86.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haury J. F., Bogorad L. Action Spectra for Phycobiliprotein Synthesis in a Chromatically Adapting Cyanophyte, Fremyella diplosiphon. Plant Physiol. 1977 Dec;60(6):835–839. doi: 10.1104/pp.60.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Loeb L. A. An E. coli promoter that regulates transcription by DNA superhelix-induced cruciform extrusion. Science. 1988 Aug 5;241(4866):703–705. doi: 10.1126/science.2456617. [DOI] [PubMed] [Google Scholar]
- Houmard J., Capuano V., Coursin T., Tandeau de Marsac N. Genes encoding core components of the phycobilisome in the cyanobacterium Calothrix sp. strain PCC 7601: occurrence of a multigene family. J Bacteriol. 1988 Dec;170(12):5512–5521. doi: 10.1128/jb.170.12.5512-5521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., Mazel D., Moguet C., Bryant D. A., Tandeau de Marsac N. Organization and nucleotide sequence of genes encoding core components of the phycobilisomes from Synechococcus 6301. Mol Gen Genet. 1986 Dec;205(3):404–410. doi: 10.1007/BF00338074. [DOI] [PubMed] [Google Scholar]
- Houmard J., de Marsac N. T. Cyanobacterial genetic tools: current status. Methods Enzymol. 1988;167:808–847. doi: 10.1016/0076-6879(88)67092-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lomax T. L., Conley P. B., Schilling J., Grossman A. R. Isolation and characterization of light-regulated phycobilisome linker polypeptide genes and their transcription as a polycistronic mRNA. J Bacteriol. 1987 Jun;169(6):2675–2684. doi: 10.1128/jb.169.6.2675-2684.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel D., Guglielmi G., Houmard J., Sidler W., Bryant D. A., Tandeau de Marsac N. Green light induces transcription of the phycoerythrin operon in the cyanobacterium Calothrix 7601. Nucleic Acids Res. 1986 Nov 11;14(21):8279–8290. doi: 10.1093/nar/14.21.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel D., Marlière P. Adaptive eradication of methionine and cysteine from cyanobacterial light-harvesting proteins. Nature. 1989 Sep 21;341(6239):245–248. doi: 10.1038/341245a0. [DOI] [PubMed] [Google Scholar]
- Oelmüller R., Conley P. B., Federspiel N., Briggs W. R., Grossman A. R. Changes in Accumulation and Synthesis of Transcripts Encoding Phycobilisome Components during Acclimation of Fremyella diplosiphon to Different Light Qualities. Plant Physiol. 1988 Dec;88(4):1077–1083. doi: 10.1104/pp.88.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Riethman H. C., Mawhinney T. P., Sherman L. A. Characterization of phycobilisome glycoproteins in the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1988 Jun;170(6):2433–2440. doi: 10.1128/jb.170.6.2433-2440.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H. C., Mawhinney T. P., Sherman L. A. Phycobilisome-associated glycoproteins in the cyanobacterium Anacystis nidulans R 2. FEBS Lett. 1987 May 11;215(2):209–214. doi: 10.1016/0014-5793(87)80147-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R. Refined three-dimensional structures of two cyanobacterial C-phycocyanins at 2.1 and 2.5 A resolution. A common principle of phycobilin-protein interaction. J Mol Biol. 1987 Aug 5;196(3):677–695. doi: 10.1016/0022-2836(87)90040-4. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Huber R., Schneider M., Bode W., Miller M., Hackert M. L. Crystal structure analysis and refinement at 2.5 A of hexameric C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. The molecular model and its implications for light-harvesting. J Mol Biol. 1986 Apr 20;188(4):651–676. doi: 10.1016/s0022-2836(86)80013-4. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Berk A. J., Berget S. M. Transcription maps of adenovirus. Methods Enzymol. 1980;65(1):750–768. doi: 10.1016/s0076-6879(80)65071-x. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac N. Occurrence and nature of chromatic adaptation in cyanobacteria. J Bacteriol. 1977 Apr;130(1):82–91. doi: 10.1128/jb.130.1.82-91.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Yu M. H., Glazer A. N. Cyanobacterial phycobilisomes. Role of the linker polypeptides in the assembly of phycocyanin. J Biol Chem. 1982 Apr 10;257(7):3429–3433. [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marsac N. T., Cohen-bazire G. Molecular composition of cyanobacterial phycobilisomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1635–1639. doi: 10.1073/pnas.74.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]