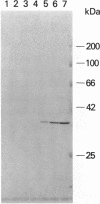
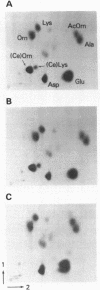
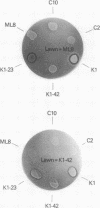
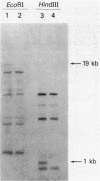
Abstract
A spontaneous derivative of Lactococcus lactis subsp. lactis K1 (formerly Streptococcus lactis K1) lacking N5-(carboxyethyl)ornithine synthase (EC 1.5.1.24) was isolated. This mutant had also lost the abilities to ferment sucrose and to produce the antibiotic nisin. Hybridization studies indicate that these linked traits are encoded on the chromosome of L. lactis K1 and that they may be located on a conjugative transposon.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Plasmids, loss of lactose metabolism, and appearance of partial and full lactose-fermenting revertants in Streptococcus cremoris B1. J Bacteriol. 1977 Jan;129(1):367–377. doi: 10.1128/jb.129.1.367-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Davey G. P., Pearce L. E., Thomas T. D. Plasmid linkage of the D-tagatose 6-phosphate pathway in Streptococcus lactis: effect on lactose and galactose metabolism. J Bacteriol. 1983 Jan;153(1):76–83. doi: 10.1128/jb.153.1.76-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd H. M., Horn N., Gasson M. J. Analysis of the genetic determinant for production of the peptide antibiotic nisin. J Gen Microbiol. 1990 Mar;136(3):555–566. doi: 10.1099/00221287-136-3-555. [DOI] [PubMed] [Google Scholar]
- Fouet A., Arnaud M., Klier A., Rapoport G. Bacillus subtilis sucrose-specific enzyme II of the phosphotransferase system: expression in Escherichia coli and homology to enzymes II from enteric bacteria. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8773–8777. doi: 10.1073/pnas.84.24.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Transfer of Sucrose-Fermenting Ability and Nisin Production Phenotype among Lactic Streptococci. Appl Environ Microbiol. 1985 Mar;49(3):627–633. doi: 10.1128/aem.49.3.627-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack E., Kemp J. D. Comparison of octopine, histopine, lysopine, and octopinic acid synthesizing activities in sunflower crown gall tissues. Biochem Biophys Res Commun. 1977 Sep 23;78(2):785–791. doi: 10.1016/0006-291x(77)90248-0. [DOI] [PubMed] [Google Scholar]
- Kaletta C., Entian K. D. Nisin, a peptide antibiotic: cloning and sequencing of the nisA gene and posttranslational processing of its peptide product. J Bacteriol. 1989 Mar;171(3):1597–1601. doi: 10.1128/jb.171.3.1597-1601.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar W., Rajchert-Trzpil M., Dobrzański W. T. The effect of proflavin, ethidium bromide and an elevated temperature on the appearance of nisin-negative clones in nisin-producing strains of Streptococcus lactis. J Gen Microbiol. 1974 Aug;83(2):295–302. doi: 10.1099/00221287-83-2-295. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Crow V. L., Lee L. N., Garon C. F. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J Bacteriol. 1979 Feb;137(2):878–884. doi: 10.1128/jb.137.2.878-884.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L. Functional properties of plasmids in lactic streptococci. Antonie Van Leeuwenhoek. 1983 Sep;49(3):259–274. doi: 10.1007/BF00399502. [DOI] [PubMed] [Google Scholar]
- Miller S. P., Thompson J. Biosynthesis and stereochemical configuration of N5-(1-carboxyethyl)ornithine. An unusual amino acid produced by Streptococcus lactis. J Biol Chem. 1987 Nov 25;262(33):16109–16115. [PubMed] [Google Scholar]
- Murphey-Corb M., Nolan-Willard M., Daum R. S. Integration of plasmid DNA coding for beta-lactamase production in the Haemophilus influenzae chromosome. J Bacteriol. 1984 Nov;160(2):815–817. doi: 10.1128/jb.160.2.815-817.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Poy F., Jacobson G. R., Kuramitsu H. K. Characterization and sequence analysis of the scrA gene encoding enzyme IIScr of the Streptococcus mutans phosphoenolpyruvate-dependent sucrose phosphotransferase system. J Bacteriol. 1989 Jan;171(1):263–271. doi: 10.1128/jb.171.1.263-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Ebner R., Altenbuchner J., Schmitt R., Lengeler J. W. Plasmid-mediated sucrose metabolism in Escherichia coli K12: mapping of the scr genes of pUR400. Mol Microbiol. 1988 Jan;2(1):1–8. doi: 10.1111/j.1365-2958.1988.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steele J. L., McKay L. L. Partial characterization of the genetic basis for sucrose metabolism and nisin production in Streptococcus lactis. Appl Environ Microbiol. 1986 Jan;51(1):57–64. doi: 10.1128/aem.51.1.57-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J. L., Polzin K. M., McKay L. L. Characterization of the genetic element coding for lactose metabolism in Lactococcus lactis subsp. lactis KP3. Plasmid. 1989 Jul;22(1):44–51. doi: 10.1016/0147-619x(89)90034-6. [DOI] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Uptake and metabolism of sucrose by Streptococcus lactis. J Bacteriol. 1981 Aug;147(2):543–551. doi: 10.1128/jb.147.2.543-551.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Curtis M. A., Miller S. P. N5-(1-carboxyethyl)-ornithine, a new amino acid from the intracellular pool of Streptococcus lactis. J Bacteriol. 1986 Aug;167(2):522–529. doi: 10.1128/jb.167.2.522-529.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. N5-(L-1-carboxyethyl)-L-ornithine:NADP+ oxidoreductase from Streptococcus lactis. Purification and partial characterization. J Biol Chem. 1989 Jun 5;264(16):9592–9601. [PubMed] [Google Scholar]
- Tsai H. J., Sandine W. E. Conjugal transfer of nisin plasmid genes from Streptococcus lactis 7962 to Leuconostoc dextranicum 181. Appl Environ Microbiol. 1987 Feb;53(2):352–357. doi: 10.1128/aem.53.2.352-357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]