Abstract
Three different types of bivalent influenza virus vaccine, a whole virus, an aqueous-surface-antigen vaccine and an adsorbed-surface-antigen vaccine were tested at three dosage levels in volunteers primed with respect to only one of the haemagglutinin antigens present in the vaccines. The local and systemic reactions to all three vaccine types were mild in nature and, following first immunization, the aqueous-surface-antigen vaccine was the least reactogenic. The serum haemagglutination-inhibiting antibody response to the A/Victoria/75 component of the vaccines to which the volunteer population was primed, was greatest following immunization with the aqueous-surface-antigen vaccine; the greatest antibody response to the A/New Jersey/76 component of the vaccines was observed following immunization with whole virus vaccine.
Full text
PDF



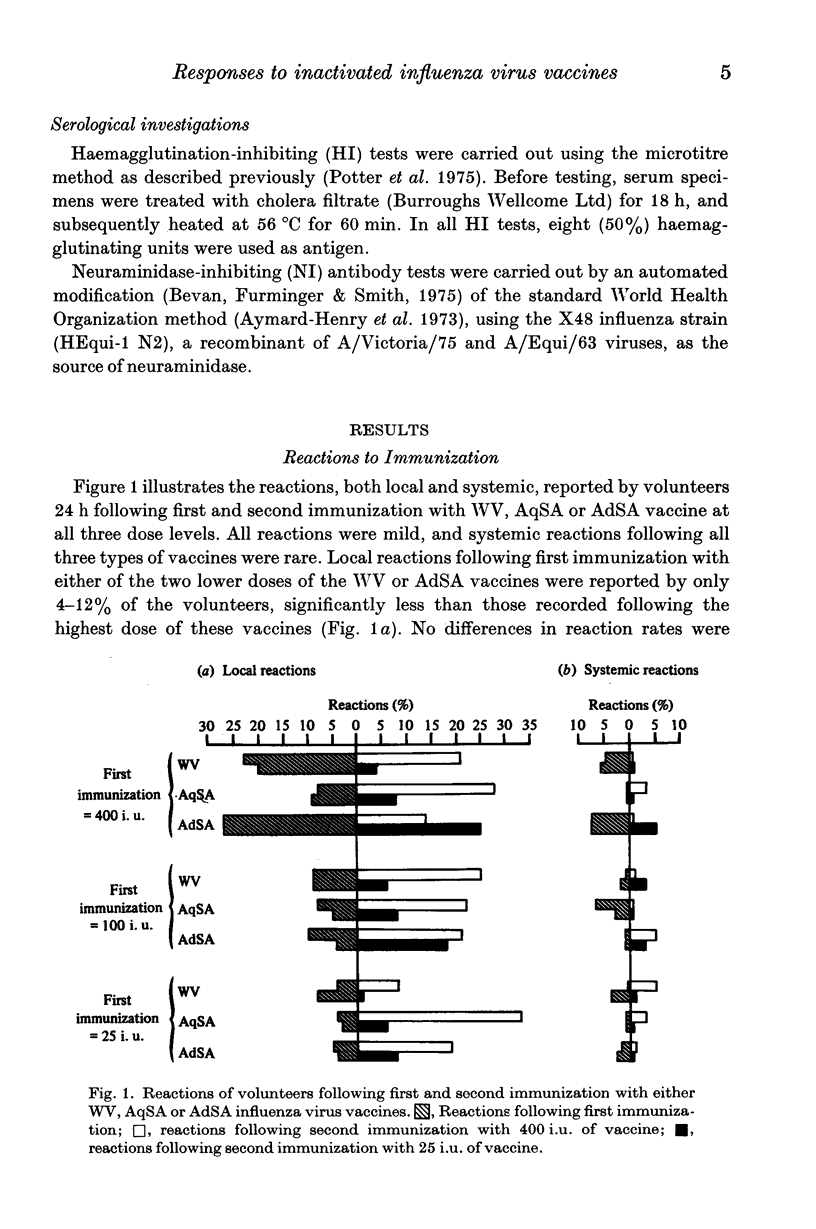

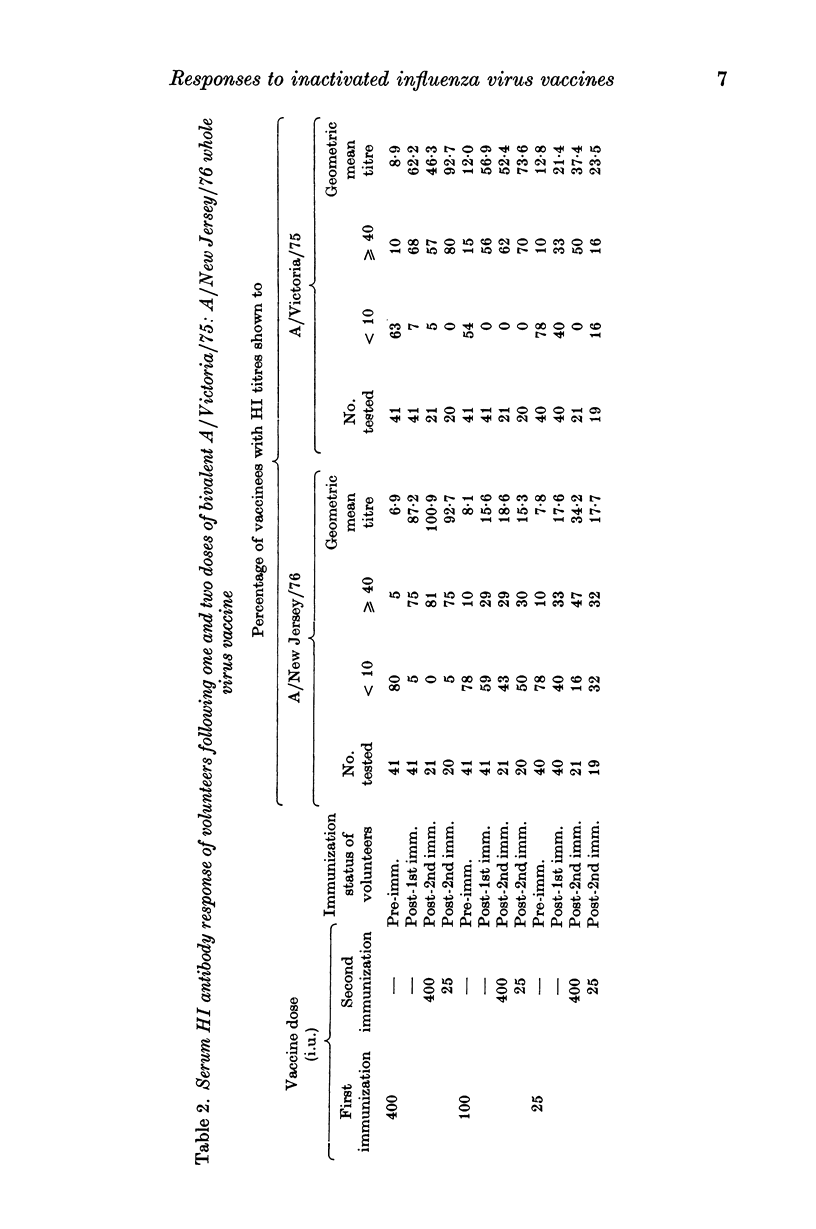
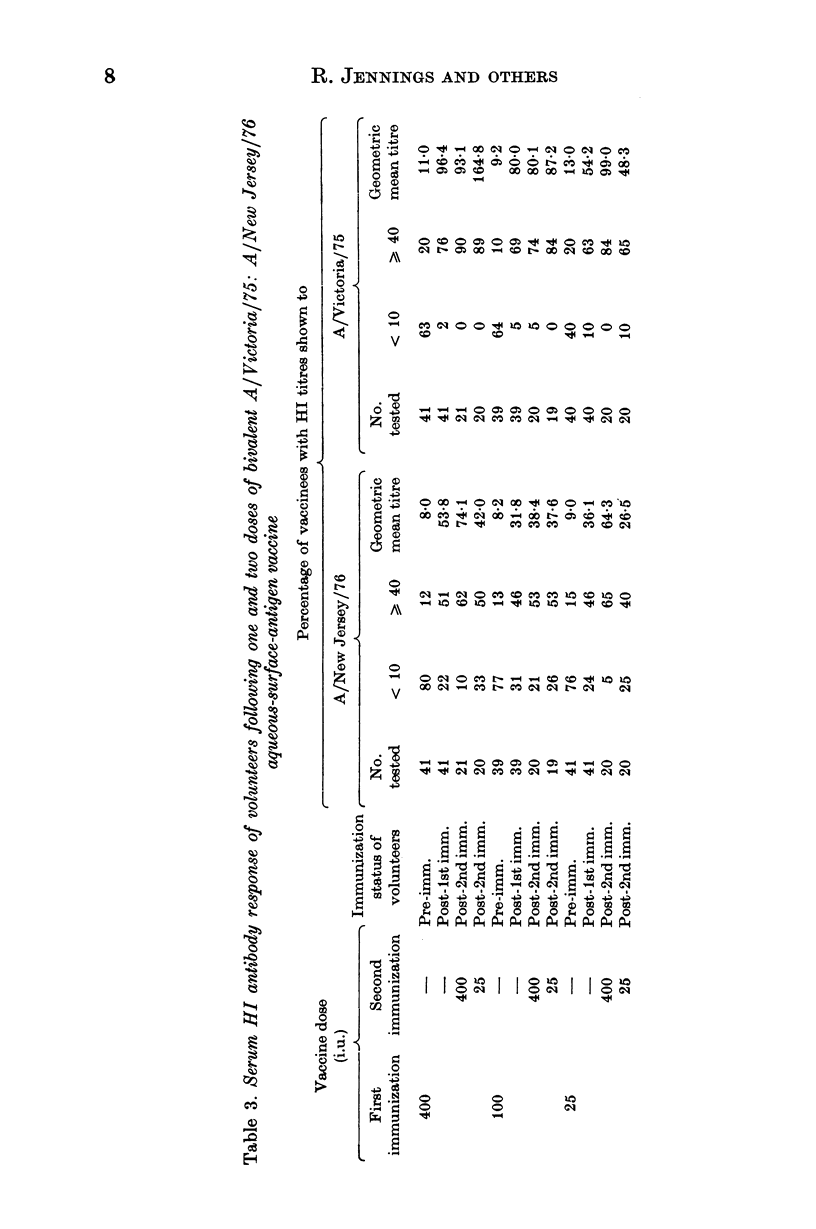

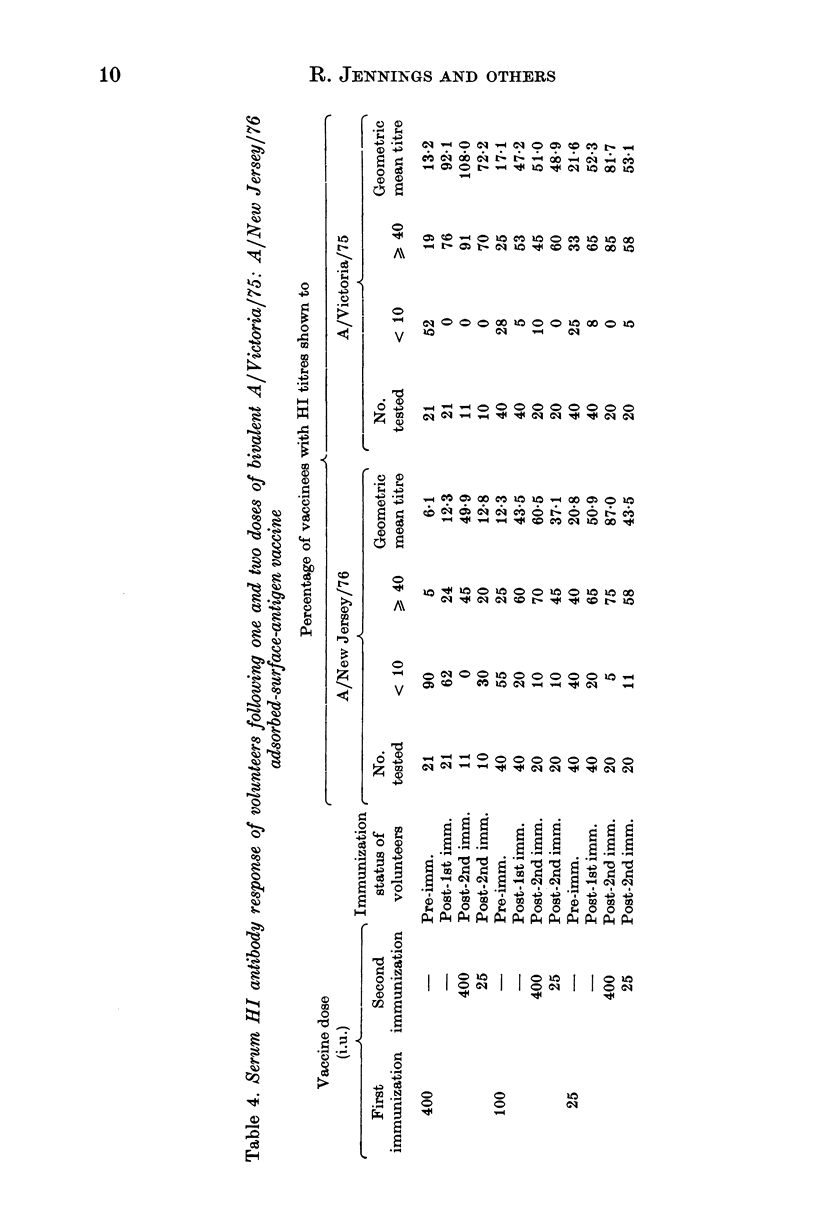





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Barry D. W., Mayner R. E., Hochstein H. D., Dunlap R. C., Rastogi S. C., Hannah J. E., Blackburn R. J., Sullivan J. L., Gerety R. J. Comparative trial of influenza vaccines. II. Adverse reactions in children and adults. Am J Epidemiol. 1976 Jul;104(1):47–59. doi: 10.1093/oxfordjournals.aje.a112273. [DOI] [PubMed] [Google Scholar]
- Barry D. W., Staton E., Mayner R. E. Inactivated influenza vaccine efficacy: diminished antigenicity of split-product vaccines in mice. Infect Immun. 1974 Dec;10(6):1329–1336. doi: 10.1128/iai.10.6.1329-1336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan A. M., Furminger I. G., Smith C. H. Neuraminidase assay of influenza vaccines. Dev Biol Stand. 1975;28:173–180. [PubMed] [Google Scholar]
- Brady M. I., Furminger I. G. A surface antigen influenza vaccine. 1. Purification of haemagglutinin and neuraminidase proteins. J Hyg (Lond) 1976 Oct;77(2):161–172. doi: 10.1017/s002217240002458x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady M. I., Furminger I. G., Stones P. B. An adsorbed surface-antigen influenza vaccine and its serological activity in volunteers. Postgrad Med J. 1976 Jun;52(608):368–372. doi: 10.1136/pgmj.52.608.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon F. B., Barrett C. D., Jr, Hook A. E., Lease G. O. Human febrile response to influenza virus or its ether isolated hemagglutinins. Proc Soc Exp Biol Med. 1967 Jul;125(3):683–686. doi: 10.3181/00379727-125-32180. [DOI] [PubMed] [Google Scholar]
- Eastwood L. M., Jennings R., Milner R. D., Potter C. W. Reactogenicity and immunogenicity of a surface-antigen-adsorbed influenza virus vaccine in children. J Clin Pathol. 1979 Jun;32(6):534–537. doi: 10.1136/jcp.32.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P. A., Ennis F. A. Influenza vaccine: split-product versus whole-virus types--How do they differ. N Engl J Med. 1977 Mar 10;296(10):567–568. doi: 10.1056/NEJM197703102961012. [DOI] [PubMed] [Google Scholar]
- Gross P. A. Reactogenicity and immunogenicity of bivalent influenza vaccine in one- and two-dose trials in children: a summary. J Infect Dis. 1977 Dec;136 (Suppl):S616–S625. doi: 10.1093/infdis/136.supplement_3.s616. [DOI] [PubMed] [Google Scholar]
- Hobson D., Curry R. L., Beare A. S., Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972 Dec;70(4):767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells C. H., Evans A. D., Vesselinova-Jenkins C. Effect of two doses of influenza vaccine in stimulating antibody in volunteers. Lancet. 1973 Jun 23;1(7817):1436–1438. doi: 10.1016/s0140-6736(73)91755-8. [DOI] [PubMed] [Google Scholar]
- Jennings R., Clark A., Oxford J. S., Hockley D. J., Potter C. W. Reactogenicity and immunogenicity of whole and ether-Tween-split influenza A virus vaccines in volunteers. J Infect Dis. 1978 Nov;138(5):577–586. doi: 10.1093/infdis/138.5.577. [DOI] [PubMed] [Google Scholar]
- Jennings R., Potter C. W., McLaren C., Brady M. A new, surface-antigen-adsorbed influenza virus vaccine. I. Studies on immunogenicity in hamsters. J Hyg (Lond) 1975 Dec;75(3):341–352. doi: 10.1017/s0022172400024402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marine W. M., Stuart-Harris C. Reactions and serologic responses in young children and infants after administration of inactivated monovalent influenza A vaccine. J Pediatr. 1976 Jan;88(1):26–30. doi: 10.1016/s0022-3476(76)80721-4. [DOI] [PubMed] [Google Scholar]
- McLaren C., Verbonitz M. W., Daniel S., Grubbs G. E., Ennis F. A. Effect of priming infection on serologic response to whole and subunit influenza virus vaccines in animals. J Infect Dis. 1977 Dec;136 (Suppl):S706–S711. doi: 10.1093/infdis/136.supplement_3.s706. [DOI] [PubMed] [Google Scholar]
- Monto A. S., Kendal A. P. Effect of neuraminidase antibody on Hong Kong influenza. Lancet. 1973 Mar 24;1(7804):623–625. doi: 10.1016/s0140-6736(73)92196-x. [DOI] [PubMed] [Google Scholar]
- Nicholson K. G., Tyrrell D. A., Harrison P., Potter C. W., Jennings R., Clark A., Schild G. C., Wood J. M., Yetts R., Seagroatt V. Clinical studies of monovalent inactivated whole virus and subunit A/USSR/77 (H1N1) vaccine: serological responses and clinical reactions. J Biol Stand. 1979 Apr;7(2):123–136. doi: 10.1016/s0092-1157(79)80044-x. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Clark A., Jennings R., Schild G. C., Wood J. M., McWilliams P. K. Reactogenicity and immunogenicity of inactivated influenza A (H1N1) virus vaccine in unprimed children. Report to the Medical Research Council Committee on influenza and other respiratory virus vaccines. J Biol Stand. 1980;8(1):35–48. doi: 10.1016/s0092-1157(80)80045-x. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Jennings R., McLaren C., Edey D., Stuart-Harris C. H., Brady M. A new surface-antigen-adsorbed influenza virus vaccine. II. Studies in a volunteer group. J Hyg (Lond) 1975 Dec;75(3):353–362. doi: 10.1017/s0022172400024414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Jennings R., Phair J. P., Clarke A., Stuart-Harris C. H. Dose-response relationship after immunization of volunteers with a new, surface-antigen-adsorbed influenza virus vaccine. J Infect Dis. 1977 Mar;135(3):423–431. doi: 10.1093/infdis/135.3.423. [DOI] [PubMed] [Google Scholar]
- Ruben F. L., Jackson G. G. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis. 1972 Jun;125(6):656–664. doi: 10.1093/infdis/125.6.656. [DOI] [PubMed] [Google Scholar]
- Welty P. B., Jr, Epstein B., O'Brien J., Brackett R. G., Brandon F. B., Shillis J. L. Reactions and serologic responses after administration of inactivated monovalent influenza A/swine virus vaccines. I. Immunization of children and adults with influenza A/Shope virus vaccines. J Infect Dis. 1977 Dec;136 (Suppl):S604–S608. doi: 10.1093/infdis/136.supplement_3.s604. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Schild G. C., Newman R. W., Seagroatt V. An improved single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen: application for potency determinations of inactivated whole virus and subunit vaccines. J Biol Stand. 1977;5(3):237–247. doi: 10.1016/s0092-1157(77)80008-5. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Bryant J. D., Karzon D. T. Comparison of influenza B/Hong Kong virus infections among infants, children, and young adults. J Infect Dis. 1980 Apr;141(4):430–435. doi: 10.1093/infdis/141.4.430. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Dolin R., La Montagne J. R. From the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the Center for Disease Control, and the Bureau of Biologics of the Food and Drug Administration. Summary of clinical trials of influenza vaccines--II. J Infect Dis. 1976 Dec;134(6):633–638. doi: 10.1093/infdis/134.6.633. [DOI] [PubMed] [Google Scholar]


