Abstract
Receptor tyrosine kinases Flt-1 and Flk-1/KDR, and their ligand, the vascular endothelial growth factor (VEGF), were shown to be essential for angiogenesis in the mouse embryo by gene targeting. Flk-1/KDR null mutant mice exhibited impaired endothelial and hematopoietic cell development. On the other hand, Flt-1 null mutation resulted in early embryonic death at embryonic day 8.5, showing disorganization of blood vessels, such as overgrowth of endothelial cells. Flt-1 differs from Flk-1 in that it displays a higher affinity for VEGF but lower kinase activity, suggesting the importance of its extracellular domain. To examine the biological role of Flt-1 in embryonic development and vascular formation, we deleted the kinase domain without affecting the ligand binding region. Flt-1 tyrosine kinase-deficient homozygous mice (flt-1TK−/−) developed normal vessels and survived. However, VEGF-induced macrophage migration was strongly suppressed in flt-1TK−/− mice. These results indicate that Flt-1 without tyrosine kinase domain is sufficient to allow embryonic development with normal angiogenesis, and that a receptor tyrosine kinase plays a main biological role as a ligand-binding molecule.
Angiogenesis is an essential process in normal embryonic development and is closely involved in a variety of diseases such as diabetic retinopathy, rheumatoid arthritis, and tumor growth in vivo (1). Vascular endothelial growth factor (VEGF) and its receptors, Flt-1 and Flk-1/KDR, recently have been shown to be a very important regulator system for normal and pathological angiogenesis (2–8). Flt-1, as well as Flk-1/KDR, belongs to the tyrosine kinase family and has seven Ig-like domains in the extracellular domain, a transmembrane domain, and a tyrosine kinase domain with a long kinase insert (9–11). Although the structure of VEGF receptor family is related to that of the five-Ig type receptor family, which includes Fms/Kit/platelet-derived growth factor (PDGF) receptors, the signal transduction pathways in these families are suggested to be different because of the difference of signaling motif on the intracellular domains. Fms/Kit/PDGF receptors conserve the Tyr-X-X-Met motif in the kinase insert region and use a signal transduction pathway through phosphatidylinositol 3 kinase (PI3 kinase) (12, 13). On the other hand, Flt-1 and Flk-1/KDR lack this motif in the kinase insert region, and the tyrosine phosphorylation of PI3 kinase p85 subunit and the activation of PI3 kinase are very low, or nonexistent, in primary endothelial cells as well as in the Flt-1 or Flk-1/KDR-overexpressed NIH 3T3 cells after stimulation with VEGF (14, 15).
VEGF receptors, Flt-1 and Flk-1/KDR, are in most cases specifically expressed on vascular endothelial cells (16–20), and as an exception, only the Flt-1 is expressed on monocytes/macrophages (21, 22). These two VEGF receptors are structurally highly homologous; however, their biochemical features are quite distinct. Flt-1 shows at least a 10-fold higher affinity to VEGF even in the soluble form of only the extracellular domain but about a 10-fold lower kinase activity than Flk-1/KDR (2–4, 23, 24). Furthermore, Flt-1 overexpressed in NIH 3T3 or bovine endothelial cells did not induce cell proliferation in the presence of VEGF (23, 25), whereas Flk-1/KDR overexpressed in the above two types of cells showed cell growth after stimulation with VEGF (26). PlGF, which binds Flt-1 but not Flk-1/KDR, gives very weak, if any, biological activities such as endothelial cell growth-stimulatory activity and vascular permeability activity in most assay conditions (24, 27). These results strongly suggest that the biological functions of these receptors are different or independent from each other in the angiogenic processes in vivo. However, the differences between the two receptors and particularly the function of the Flt-1 in vivo remain to be clarified.
Recently, gene targeting studies were carried out on flt-1 and flk-1/KDR genes in mice (7, 8). The flk-1/KDR−/− homozygous mice showed a severe deficiency in vascular formation in association with hematopoietic impairment resulting in death at embryonic day (E) 8.5 (7). On the other hand, the flt-1−/− homozygotes showed a disorganization of blood vessels, particularly an overgrowth of endothelial-like abnormal cells within vascular lumens and died at E8.5 to E9.0 (8). Thus, Flk-1/KDR is considered to be a positive regulator for the commitment and proliferation of endothelial cells, whereas the flt-1 gene appears to be a negative regulator for proliferation of endothelial cells. This genetic study together with the biochemical characteristics of Flt-1 described above raises the possibility that the Flt-1 molecule functions as a trapping protein for VEGF to negatively regulate the levels of VEGF around the endothelial cells in embryogenesis, not as a strong signal transducer such as classical receptor tyrosine kinases.
To examine this possibility, we carried out a partial gene targeting study on the flt-1 gene in which the flt-1 tyrosine kinase domain was deleted and the exon encoding the 3′-juxtamembrane region and the amino terminal part of the tyrosine kinase domain was replaced with a neomycin (neo)-resistant gene cassette. The exon encoding the transmembrane domain remained without mutation, and thus, the truncated Flt-1 was expected to be localized on cell surface as a membrane protein. Here we report that the tyrosine kinase-negative flt-1 gene is sufficient for embryonic angiogenesis and rescues the lethality.
MATERIALS AND METHODS
Targeting Vector.
A mouse flt-1 cDNA of 300 bp carrying exons 17–19 (nucleotides 2411–2705) was used as a probe to isolate a 24-kb mouse flt-1 DNA clone from a genomic library of the 129Sv strain (Stratagene). The targeting vector contains a neo cassette (pPGKneopA) and the diphtheria toxin A gene (pMC1 DT-A) (28).
Embryonic Stem (ES) Cell Culture, DNA Transfection, and Mice.
J1 ES cells (29) were transfected by electroporation with targeting vector DNA (Fig. 1A), linearized by NotI, and then selected with drug applications (175 μg/ml of G418). EcoRI-digested DNA prepared from the resulting ES clones was screened by Southern blot analysis by using the 0.7-kb SpeI–HindIII genomic DNA fragment as a probe. To further confirm the construct obtained, Southern blot analysis was performed with other restriction enzymes (SalI and ApaI) by using exon 18 cDNA as a probe. ES cell clones containing the targeted gene were injected into C57B6/J blastocysts, and the male chimeric mice obtained were crossed with female C57B6/J mice to yield mice heterozygous for the flt-1TKmutation. The genotypes of the pups obtained by crosses between flt-1TK+/− mice were determined by Southern blot analysis and/or PCR analysis. The forward primer was ACCCTCTGTACCTGGTCAATTGATGCAAAG. The reverse primer was TGCAAACTCCCACTTGCTGGCATCATAG. The reverse neo primer was GCTAAAGCGCATGCTCCAGACTGCCTTG. Two lines of mutant mice were established from the ES cell clones.
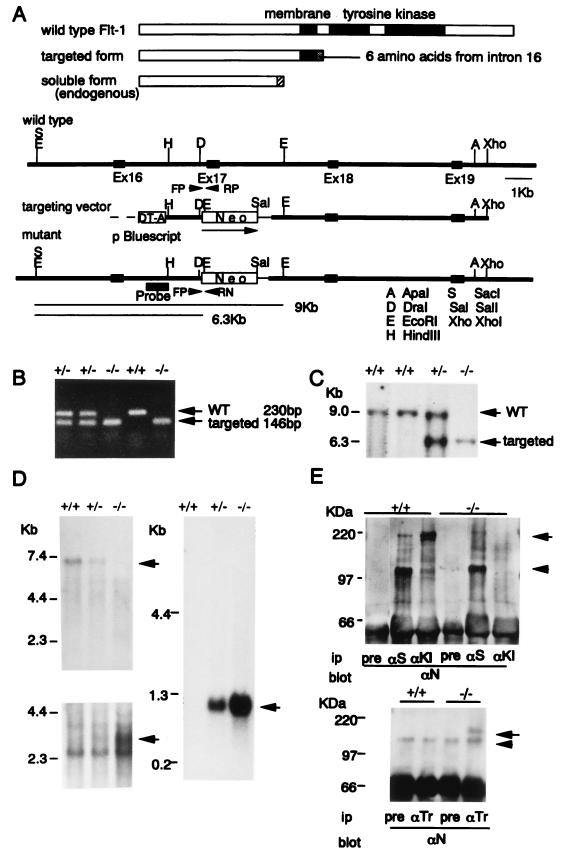
Figure 1.
Targeted inactivation of the tyrosine kinase domain of Flt-1 in ES cells and mice. (A) Predicted structures of wild-type, targeted, and endogenously expressed soluble Flt-1 (Upper), as well as the restriction maps of flt-1 wild-type allele, replacement-type targeting vector, and the targeted allele after homologous recombination (Lower). The targeting vector contains a PGK-neo cassette (pPGKneopA) and the diphtheria toxin A gene in the upstream region of the construct. (B and C) Genotype of a litter from flt-1TK+/− and flt-1TK+/− interbreeding. Tail DNA was isolated and analyzed by PCR (B) and Southern blotting (C) by using the probe shown in A after digestion with EcoRI. Primers used also are shown in A as FP (forward primer), RP (reverse primer), and RN (reverse neo primer). (D) Northern blot analysis of poly(A) RNAs (3 μg/lane) extracted from wild-type, flt-1TK+/−, and flt-1TK−/− mice of whole embryos. Probes used were the 3′ half of mouse flt-1 cDNA (nucleotide residue 2643–4008) containing the tyrosine kinase domain (Upper Left) and the 5′ half of flt-1 cDNA (69–1919) (Lower Left) and neo sequence (Right). The arrow in the upper left indicates the full-length mouse flt-1 mRNA of 7.0 kb long. (E) Immunoblot analysis of proteins obtained from mouse lung with various antibodies (Ab). pre, preimmune rabbit serum; aS, Ab against the soluble Flt-1 specific for the carboxyl terminal 31 amino acids (arrowhead, Upper); aKI, Ab against the kinase insert that is encoded from exon 21 (arrow); aN, Ab against the amino terminal region of the Flt-1 extracellular domain; aTr, Ab specific to the carboxyl terminal sequence of truncated Flt-1 (arrow, Lower). Arrowheads show nonspecific bands (Lower).
Preparation of Proteins.
Lung tissues derived from each of 10 wild-type or flt-1TK−/− homozygous mice were lysed in an ice-cold buffer containing 150 mM NaCl, 50 mM Hepes at pH 7.4, 10 mM EDTA, 1% Triton X-100, 10% glycerol, 2% aprotinin, and 1 mM phenylmethylsulfonyl fluoride (PMSF), and applied to a 1-ml HiTrap Heparin Affinity Column (Pharmacia). Proteins bound were eluted with a series of buffers from 0.6 to 0.9 M NaCl containing 50 mM Hepes at pH 7.4, 10 mM EDTA, 1% Triton X-100, 10% glycerol, 2% aprotinin, and 1 mM PMSF. The fractionated samples were analyzed by SDS/PAGE and Western blotting.
Immunohistochemistry.
Tissue sections were immunohistochemically stained with an antiserum against human von Willebrand factor (vWF) (Dako) as an endothelial cell specific marker. Tissue sections (6 μm thick) were fixed with acetone at −20°C for 5 min and rehydrated in PBS. For inhibition of endogenous peroxidase, sections were incubated in 0.1% H2O2 methanol solution for 15 min at room temperature and washed three times with PBS. Nonspecific binding of antibody was blocked by incubation with 10% normal goat serum-containing PBS for 30 min. Subsequently, sections were incubated with primary antibody against vWF for 1 hr, washed three times with PBS, incubated with biotinylated goat anti-rabbit Ig G (Vector), washed again with PBS, and then incubated with an ABC kit (Vector). After washing in PBS, vWF was detected by 3-amino-9-ethyl carbazole (Vector).
Culture of Primary Sinusoidal Endothelial Cells Obtained from Mouse Liver.
Sinusoidal endothelial cells were isolated from a mouse liver as previously described (20). These cells were grown in endothel basal medium (Kurabou, Osaka, Japan) with 0.5% fetal calf serum supplemented with or without 100 ng/ml of mouse recombinant VEGF (R&D Systems). After the cells were cultured in 96 wells of rat collagen I-coated plates for 4 days, MTS solution (Owen’s reagent; Becton Dickinson) was added and the absorbance at 490 nm was recorded by using a 96-well plate reader for the cell proliferation assay. A similar culture divided into 24 wells was fixed in 10% formaldehyde and stained with Crystal Violet for the detection of morphological changes.
Permeability Assay in Vivo.
About 5 min after the injection of 0.1% Evans Blue dye (400 μl) into mouse tail vein, 2 ml of 1% BSA-containing PBS with or without mouse VEGF (100 ng/ml) was injected to the peritoneal cavity. After 1 hr, 0.5 ml of peritoneal fluid was recovered. The samples were centrifuged to remove the cells, and the absorbance at 605 nm of these samples was monitored to measure the amount of dye released.
Migration Assay.
Three days after the i.p. injection of 1% glycogen (2 ml), mouse peritoneal macrophages were harvested by peritoneal lavage by using 10 ml of sterile PBS. Lavage fluids were centrifuged at 200 × g for 5 min. The cells were resuspended in RPMI 1640 medium and diluted to 2 × 106 cells/ml. Cell suspensions with a viability of more than 95% as assayed by Trypan Blue, Wright-Giemsa staining, and anti-CD11b antibody staining were used for experiments.
Cell migration was evaluated by using a chemotaxis Boyden chamber. Chemoattractant (VEGF or Zymosan-activated serum; ref. 30) in RPMI 1640 medium was added to the lower wells of the chamber, and 50 μl of cell suspension per well (105 cells) was seeded in the upper wells. The upper and lower wells were separated by a 5-μm pore size polyvinylpyrrolidone-free polycarbonate filter. The chamber was incubated at 37°C with 5% CO2 for 3 hr and 15 min. After incubation, filters were fixed and stained with Diff-Quik, and 10 high-power fields were counted for the migration assay. For examination, 4–5 8-week-old flt-1TK+/+ and flt-1TK−/− mice of the same litter from two independent lines were used. MTS and migration assays were performed in triplicate.
RESULTS AND DISCUSSION
A Partial Gene Targeting for Disruption of flt-1 Tyrosine Kinase Domain in Mice.
To make a targeting vector suitable for the construction of a tyrosine kinase-deficient flt-1 gene, we first examined the entire exon-intron structure of the flt-1 gene in mice and found that exon 17 encodes the amino terminal region of Flt-1 tyrosine kinase domain, amino acid residues 787–830 (31). This flt-1 exon 17 was replaced with a neo gene cassette, which carries its own promoter and polyadenylation signal (Fig. 1A). A rare possibility of an alternative splicing without neo gene to exon 18 causes an out-of-frame mutation. Chimeric male mice generated from injections with two different targeted ES cell lines transmitted this mutation through their germ line.
No Marked Abnormality of Blood Vessels in flt-1TK−/− Mice that Express a Truncated Flt-1 Receptor.
When we crossed flt-1TK+/− heterozygous mice and genotyped the litters of the newborns, Southern blot and PCR analyses (Fig. 1 B and C) revealed the presence of flt-1TK−/− homozygotes. As expected, the flt-1TK+/− heterozygotes expressed a lower level of the 7-kb, full-length flt-1 mRNA (Fig. 1D, Upper), and the flt-1TK−/− homozygotes had completely lost this 7-kb mRNA. On the other hand, both flt-1TK+/− and flt-1TK−/−mice expressed an artificial flt-1 mRNA of about 3 kb, which carries only the 5′ half of the flt-1 gene (Fig. 1D, Lower). This short flt-1 message did not contain the neo sequence, suggesting that these flt-1 mRNAs arose as a result of termination within intron 16. The neo message was detected to be 1 kb in size and was transcribed from its own promoter (Fig. 1D, Right).
To examine for Flt-1 proteins, lung tissues from wild-type and homozygous mice were lysed in buffer and partially purified by heparin affinity chromatography. As shown in Fig. 1E (Upper), wild-type mice contained both the 190-kDa, full-length molecule and the 110-kDa endogenous soluble form of Flt-1, which carries about 85% of the extracellular domain. This truncated form of flt-1 results from a termination within intron 13 associated with 31 amino acids derived from the 5′ sequence of this intron (31, 32). On the other hand, the flt-1TK−/− homozygotes had lost the full-length Flt-1, although the endogenous, soluble form of Flt-1 was expressed at a level almost equal to that of wild-type mice. Furthermore, flt-1TK−/− homozygotes expressed a truncated form of Flt-1 with a size of approximately 120 kDa. This form was detected by using antiserum raised against a 10-aa sequence, KLKRVRKQKI, the latter six amino acids of which are derived from read-through of intron 16 (Fig. 1E, Lower). Therefore, flt-1TK−/− homozygotes expressed both the novel truncated form of Flt-1, which lacks the cytoplasmic region, and soluble Flt-1, but not the full-length Flt-1 tyrosine kinase.
Homozygous (flt-1TK−/−) mice survived and were essentially normal with respect to fertility. Genotyping of offspring obtained from a cross between heterozygotes gave a ratio of almost 1:2:1 (80:166:77 from a total of 323 F2 mice) for wild-type, flt-1TK+/− heterozygous, and flt-1TK−/−homozygous mice, respectively. This finding indicates that the loss of the tyrosine kinase domain in Flt-1 did not strongly disadvantage the second generation.
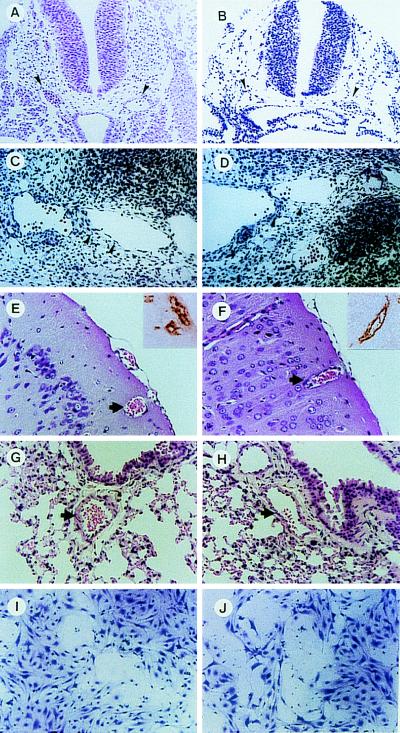
Histologically, there were no marked differences in the blood vessels between flt-1TK−/− homozygous embryos and adult mice when using hematoxylin-eosin and anti-von Willebrand factor antibody staining (Fig. 2 A–H).
Figure 2.
No detectable abnormalities in the endothelial cells and blood vessels in vivo and in vitro of flt-1TK−/− mice. Wild-type (A, C, E, G, and I) and flt-1TK−/− (B, D, F, H , and J) mice were used. Arrowheads show the structure of dosal aorta at E9.0 (A and B) and endothelial cell layers of the dosal aorta at E10.5 (C and D) in embryos. Arrows show blood vessels in brain (E and F) and lung (G and H) from adult mice. Embryos and organs were fixed with 10% formaldehyde, and sections were stained with hematoxylin-eosin. (E and F, Upper) Endothelial cells stained with antibody against von Willebrand factor in frozen sections of brain. (I and J) Spindle-like sinusoidal endothelial cells of liver were cultured with 100 ng/ml of VEGF.
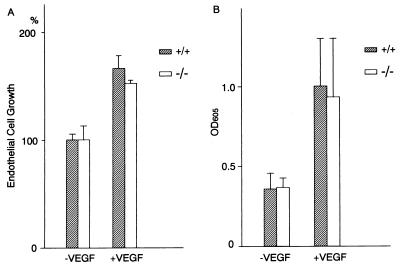
Endothelial Cell Growth and Vascular Permeability of flt-1TK−/− Mice in Response to VEGF.
VEGF plays the dual role of a growth factor and a vascular permeability factor for endothelial cells and vessels (2–4). As Flt-1 is a key element in the mediation of VEGF responses, we investigated whether VEGF still elicits the above effects in the flt-1TK−/− mutant background. In Figs. 2 I and J and 3A, we show that the mitogenic and morphological responses induced by VEGF in the sinusoidal endothelial cells derived from the liver of flt-1TK−/− mice were not significantly affected. To examine vascular permeability in vivo, we established an assay system. After an injection of dye into the tail vein of the mouse, VEGF was immediately injected into the intraperitoneal cavity, and 1 hr later, the amount of dye permeating through the peritoneal blood vessels into the abdominal cavity was measured. Almost equal levels of permeability were observed for both the wild-type and flt-1TK−/− mice (Fig. 3B). The VEGF-related ligand, PlGF (33), which binds only to Flt-1 and not to Flk-1/KDR (24, 27, 34–36), induced very low levels of permeability in both wild-type and flt-1TK−/−mice (not shown). This finding may indicate that the rapid response of blood vessels to VEGF-induced permeability is mostly related to Flk-1/KDR.
Figure 3.
Endothelial cell growth and vascular permeability in flt-1TK−/− homozygous mice. (A) Growth stimulatory activity of 100 ng/ml of mouse VEGF on primary sinusoidal endothelial cells derived from wild-type and flt-1TK−/− mice by using the MTS assay. The assay was performed in triplicate for one mouse, and four or five mice for each type were used. The relative mean absorbances at 490 nm after stimulation with VEGF were shown in comparison with the mean absorbances without VEGF as control (100%). The average value for wild-type mice without VEGF was 0.31 OD490, and that for flt-1TK−/− homozygous mice was 0.45 OD490. This slight difference in the basal levels of two types of mice appears mostly because of the technical difficulty for the preparation of mouse liver sinusoidal endothelial cells. (B) VEGF-dependent increase in vascular permeability of flt-1TK−/− mice. −VEGF, with 2 ml of 1% BSA-PBS solution; +VEGF, with 200 ng of mouse VEGF in 2 ml of 1% BSA-PBS solution. The absorbances at 605 nm after stimulation with VEGF or with BSA alone (control) were indicated (see Materials and Methods).
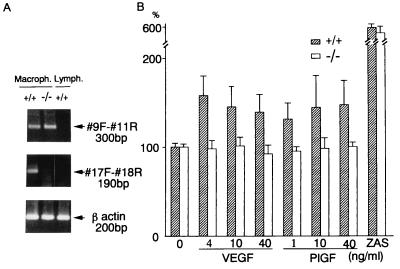
Suppression of VEGF-Dependent Macrophage Migration in flt-1TK−/− Mice.
The whole structure of the flt-1 gene is highly conserved among mammalian species (9, 20, 37). Recently, the flt-1 gene, but not the flk-1/KDR gene, was found to be well expressed in human peripheral monocytes and to play an important role in VEGF-dependent cell migration (21, 22). Thus, we examined whether or not the tyrosine kinase of Flt-1 is dispensable for this function of Flt-1. The wild-type, flt-1TK+/− heterozygous, and flt-1TK−/− homozygous mice macrophages in the abdominal cavity were used for the cell migration assay. Only the macrophages, and not the lymphocytes, expressed flt-1 mRNA as detected by reverse transcription–PCR (Fig. 4A). The macrophages from wild-type mice had the full-length flt-1 mRNA, whereas those from flt-1TK−/− homozygotes carried the truncated form of flt-1.
Figure 4.
Loss of VEGF- and PlGF-dependent macrophage migration in flt-1TK−/− homozygous mice. (A) Reverse transcription–PCR used for the detection of flt-1 mRNA in mouse macrophages. Primers: #9F (exon 9 forward primer) and #11R (exon 11 reverse primer) for the extracellular domain (Top), #17F (exon 17 forward primer) and #18R (exon 18 reverse primer) for kinase domain (Middle), and mouse β actin primers (Bottom). (B) VEGF165 or PlGF-2 at different concentrations, as well as Zymosan-activated serum (ZAS), were added to the lower chamber of the Boyden chamber apparatus. The results are mean percentages (±SD) of migrated cells in 10 high-power fields. For each experiment, 4–5 wild-type or flt-1TK−/− homozygous mice were used, and each experiment was carried out in triplicate. The mean numbers of migrated macrophages without chemoattractant were shown as 100 and compared with those with chemoattractants. (The real mean numbers of migrated macrophages without chemoattractant were 120 in the wild-type and 112 in the flt-1TK−/− homozygous mice.)
As shown in Fig. 4B, VEGF-induced macrophage migration was markedly decreased to almost an undetectable level in flt-1TK−/− homozygotes, although these macrophages responded well to the general migration inducer, Zymosan-activated serum (30), irrespective of whether they carried the full-length or truncated form of Flt-1. PlGF gave essentially the same results (Fig. 4B). These observations indicate that the Flt-1 tyrosine kinase domain is required for its secondary physiological function, namely, the induction of monocyte/macrophage migration in the presence of VEGF.
Possible Models for the Biological Function of Tyrosine Kinase-Deficient Flt-1 in Mice.
Thus, we conclude that the tyrosine kinase activity of Flt-1 is dispensable for embryonic angiogenesis but necessary for macrophage migration mediated by VEGF. As previously reported, the flt-1 null mutant mice were embryonic lethal because of abnormal overgrowth of endothelial cells (8), suggesting a negative regulatory function of Flt-1 in VEGF-induced angiogenesis. Consistent with this finding, we and others already had shown that Flt-1 has a greater (more than 10-fold) affinity for VEGF than Flk-1/KDR but only a very weak tyrosine kinase activity, and that the extracellular domain of Flt-1 has a potent suppressor activity on VEGF both in vivo and in vitro (23, 24, 27, 32–36).
Therefore, a reasonable explanation for the rescue of angiogenesis with the kinase-deficient Flt-1 shown here is that the extracellular domain of Flt-1 is necessary and sufficient to absorb a significant amount of VEGF in vivo, resulting in a negative regulation of endothelial growth in embryonic angiogenesis.
Another possibility is that the kinase-deficient Flt-1 still binds to some unidentified negative-signal transducer, which also might function under normal conditions with wild-type Flt-1. However, this model seems unlikely because only 11 amino acid residues, RKLKRVRKQKI, are left downstream of the transmembrane domain of the truncated Flt-1 derived from exon 16 and intron 16.
In general, receptor tyrosine kinases are believed to exert their function through activation and inactivation of their kinase activity (38, 39). Among gene targeting studies, the deletion of tyrosine kinase domain of receptors such as c-met and MuSK resulted in inactivation of their biological activities and embryonic or perinatal death (40, 41). There are a few cases in which the null mutant mice survived, and some phenotypic differences were observed between null mutant and tyrosine kinase-deficient mutant mice. For example, the extent of reduction of the sensory ganglia in the trkC null mutant mice was more severe than that in the truncated, kinase-negative trkC mutant mice (42, 43).
In addition, in the case of the Nuk receptor gene, which is a member of the eph tyrosine kinase family, a mutant without the tyrosine kinase domain prevented nonlethal abnormality in the central nervous system of Nuk null mutant mice (44). The results described here for the Flt-1 receptor (VEGFR-1) demonstrate a tyrosine kinase receptor without the active participation of its tyrosine kinase domain to overcome a lethal abnormality.
Acknowledgments
We thank Drs. Kiyoshi Ohura (Osaka Dental University) and Tsuneo Takahashi (Institute of Medical Science, University of Tokyo) for equipment and helpful discussions regarding macrophage migration assay. This work was supported by a grant-in-aid for Special Project Research on Cancer-Bioscience 04253204 and for Scientific Research (B) from the Ministry of Education, Science, Sports and Culture of Japan and by a research grant from the Mitsubishi Foundation.
ABBREVIATIONS
- VEGF
vascular endothelial growth factor
- E
embryonic day
- ES
embryonic stem
References
- 1.Risau W. Nature (London) 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Davis-Smith T. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 3.Shibuya M. Adv Cancer Res. 1995;67:281–316. doi: 10.1016/s0065-230x(08)60716-2. [DOI] [PubMed] [Google Scholar]
- 4.Mustonen T, Alitalo K. J Cell Biol. 1995;129:895–898. doi: 10.1083/jcb.129.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Nature (London) 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Moore K C, Chen H, Dowd M, Lu L, O’Shea K S, Braxton L P, Hillan K J, Moore M W. Nature (London) 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 7.Shalaby F, Rossant J, Yamaguchi T P, Gertsenstein M, Wu X F, Breitman M L, Schuh A C. Nature (London) 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 8.Fong G-H, Rossant J, Gertsenstein M, Breitman M L. Nature (London) 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya M, Yamaguch S, Yamane A, Ikeda T, Tojo A, Matushime H, Sato M. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- 10.Matthews W, Jordan C T, Gavin M, Jenkins N A, Copeland N G, Lemischka I R. Proc Natl Acad Sci USA. 1991;88:9026–9030. doi: 10.1073/pnas.88.20.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terman B I, Dougher-Vermazen, Carrion M E, Dimitrov D, Armellino D C, Gospodarowicz D, Bohlen P. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 12.Fanti W J, Escobedo J A, Martin G A, Turck C W, del Rosario M, McCormick F, Williams L T. Cell. 1992;69:413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- 13.Valius M, Kazlauskas A. Cell. 1993;73:321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- 14.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin C-H. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 15.Guo D, Jia Q, Song H-Y, Warren R S, Donner D B. J Biol Chem. 1995;270:6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- 16.Jakeman L B, Winer J, Bennett G L, Altar C A, Ferrara N. J Clin Invest. 1993;89:244–253. doi: 10.1172/JCI115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichmann A, Marcella C, Bréant C, Le Douarin N M. Mech Dev. 1993;42:33–48. doi: 10.1016/0925-4773(93)90096-g. [DOI] [PubMed] [Google Scholar]
- 18.Kaipainen A, Korhonen J, Pajusola K, Aprelikova O, Persico M G, Terman B I, Alitalo K. J Exp Med. 1993;178:2077–2088. doi: 10.1084/jem.178.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn T P, Peters K G, De Vries C, Ferrara N, Williams L T. Proc Natl Acad Sci USA. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamane A, Seetharam L, Yamaguchi S, Gotoh N, Takahashi T, Neufeld G, Shibuya M. Oncogene. 1994;9:2683–2690. [PubMed] [Google Scholar]
- 21.Barleon B, Sozzani S, Zhou D, Weich H A, Mantovani A, Marmé D. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 22.Clauss M, Weich H, Breier G, Knies U, Röckl W, Waltenberger J, Risau W. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 23.Sawano A, Takahashi T, Yamaguchi S, Shibuya M. Biochem Biophys Res Commun. 1997;238:487–491. doi: 10.1006/bbrc.1997.7327. [DOI] [PubMed] [Google Scholar]
- 24.Sawano A, Takahashi T, Yamaguchi S, Aonuma M, Shibuya M. Cell Growth Diff. 1996;7:213–221. [PubMed] [Google Scholar]
- 25.Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M. Oncogene. 1995;10:135–147. [PubMed] [Google Scholar]
- 26.Takahashi T, Shibuya M. Oncogene. 1997;14:2079–2089. doi: 10.1038/sj.onc.1201047. [DOI] [PubMed] [Google Scholar]
- 27.Park J E, Chen H H, Winer J, Houck A A, Ferrara N. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 28.Matsumoto M, Nakagawa T, Inoue T, Nagata E, Tanaka K, Takano H, Minowa O, Kuno J, Sakakibara S, Yamada M, et al. Nature (London) 1996;379:168–171. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- 29.Li E, Bestor T H, Jaenisch R. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 30.Issekutz A C, Movat K W, Movat H Z. Clin Exp Immunol. 1980;41:512–520. [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo K, Hiratsuka S, Subbalakshmi E, Matsushime H, Shibuya M. Gene. 1998;208:297–305. doi: 10.1016/s0378-1119(98)00006-7. [DOI] [PubMed] [Google Scholar]
- 32.Kendall R L, Thomas K A. Proc Natl Acad Sci USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico M G. Proc Natl Acad Sci USA. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendall R L, Wang G, DiSalvo J, Thomas K A. Biochem Biophys Res Commun. 1994;201:326–330. doi: 10.1006/bbrc.1994.1705. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka K, Yamaguchi S, Sawano A, Shibuya M. Jpn J Cancer Res. 1997;88:867–876. doi: 10.1111/j.1349-7006.1997.tb00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barleon B, Totzke F, Herzog C, Blanke S, Kremmer E, Siemeister G, Marmé D, Baron G M. J Biol Chem. 1997;272:10382–10388. doi: 10.1074/jbc.272.16.10382. [DOI] [PubMed] [Google Scholar]
- 37.Finnerty H, Kelleher K, Morris G E, Bean K, Merberg D M, Kriz R, Morris J C, Sookdeo H, Turner K J, Wood C R. Oncogene. 1993;8:2293–2298. [PubMed] [Google Scholar]
- 38.Hunter T. Cell. 1997;88:333–346. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- 39.Schlessinger J, Ullrich A. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- 40.Maina F, Casagranda F, Audero E, Simeone A, Comoglio P M, Klein R, Ponzetto C. Cell. 1996;87:531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- 41.DeChiara T M, Bowen D C, Valenzuela D M, Simmons M V, Poueymirou W T, Thomas S, Kinetz E, Compton D L, Rojas E, Park J S, et al. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 42.Klein R, Silos-Santiago I, Smeyne R J, Lira S A, Brambilla R, Bryant S, Zhang L, Snider W D, Barbacid M. Nature (London) 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- 43.Liebl D J, Tessarollo L, Palko M E, Parada L F. J Neurosci. 1997;17:9113–9121. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henkemeyer M, Orioli D, Henderson J T, Saxton T M, Roder J, Pawson T, Klein R. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]