Abstract
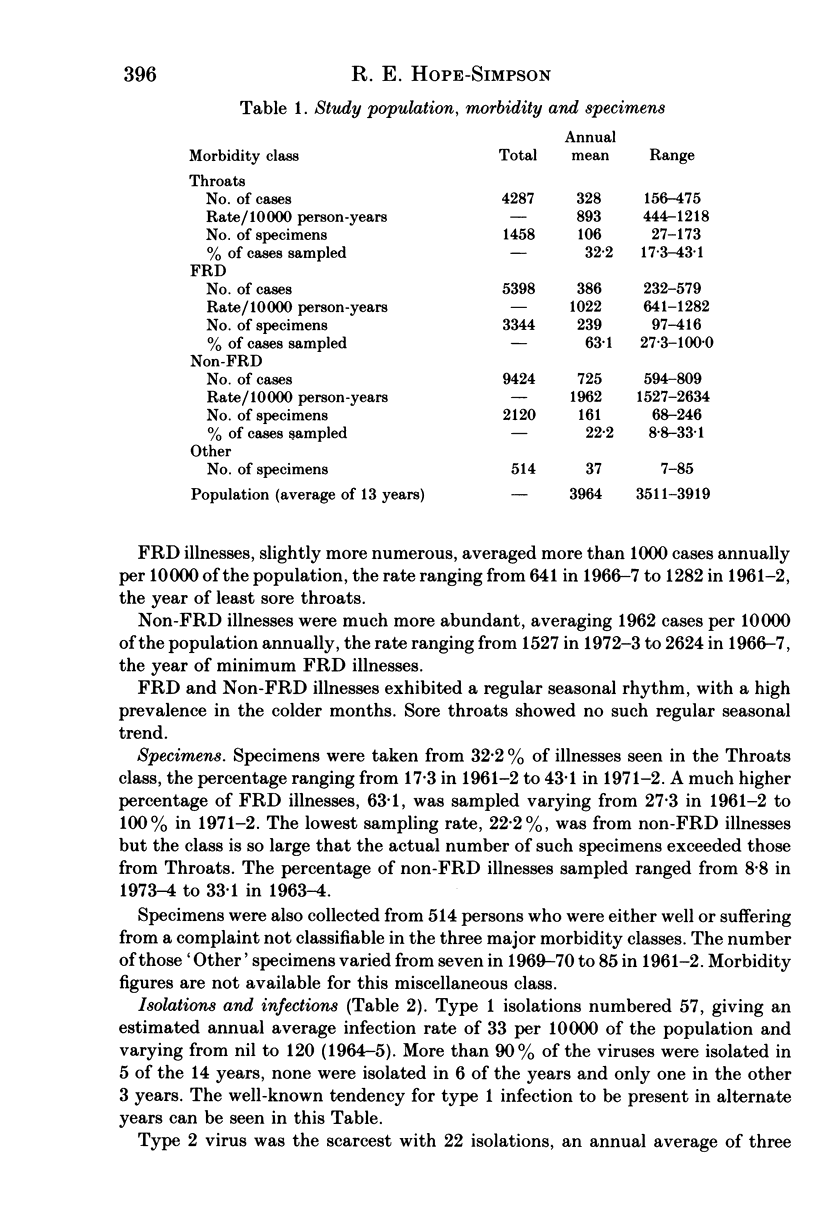
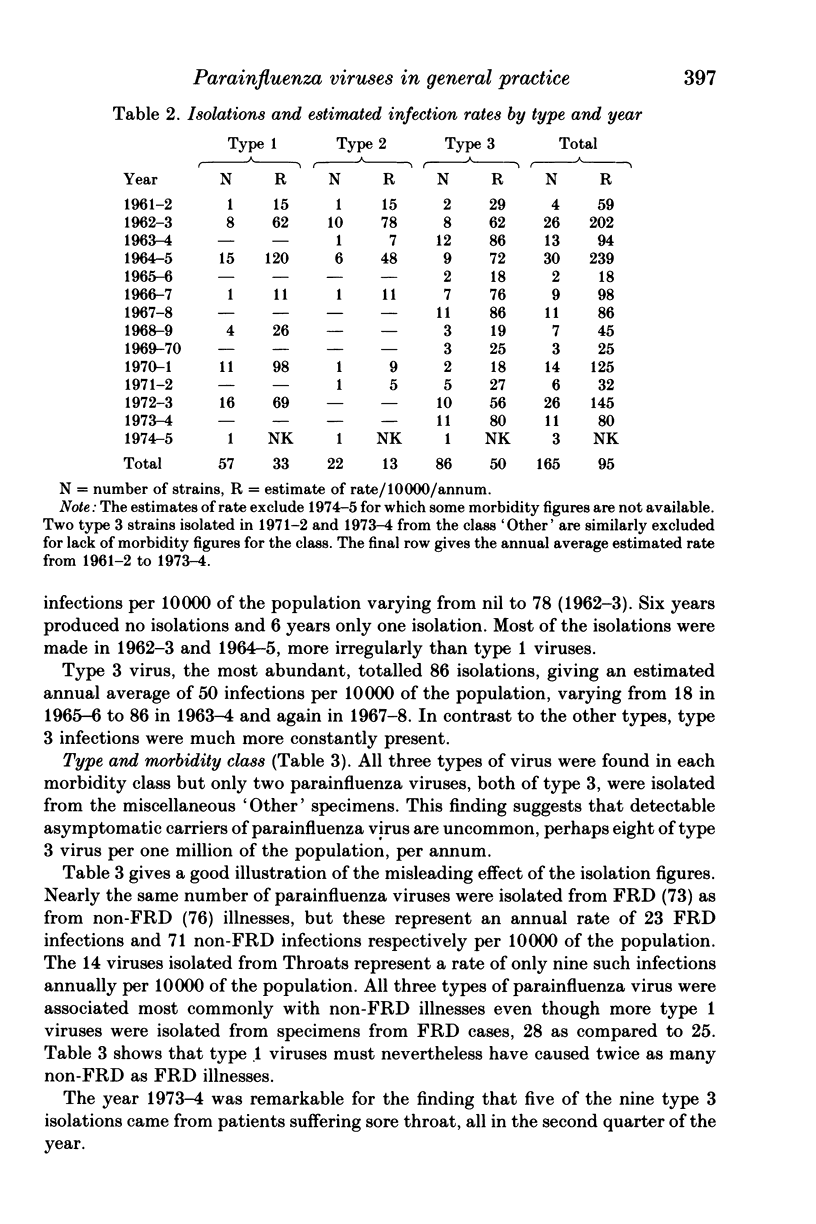
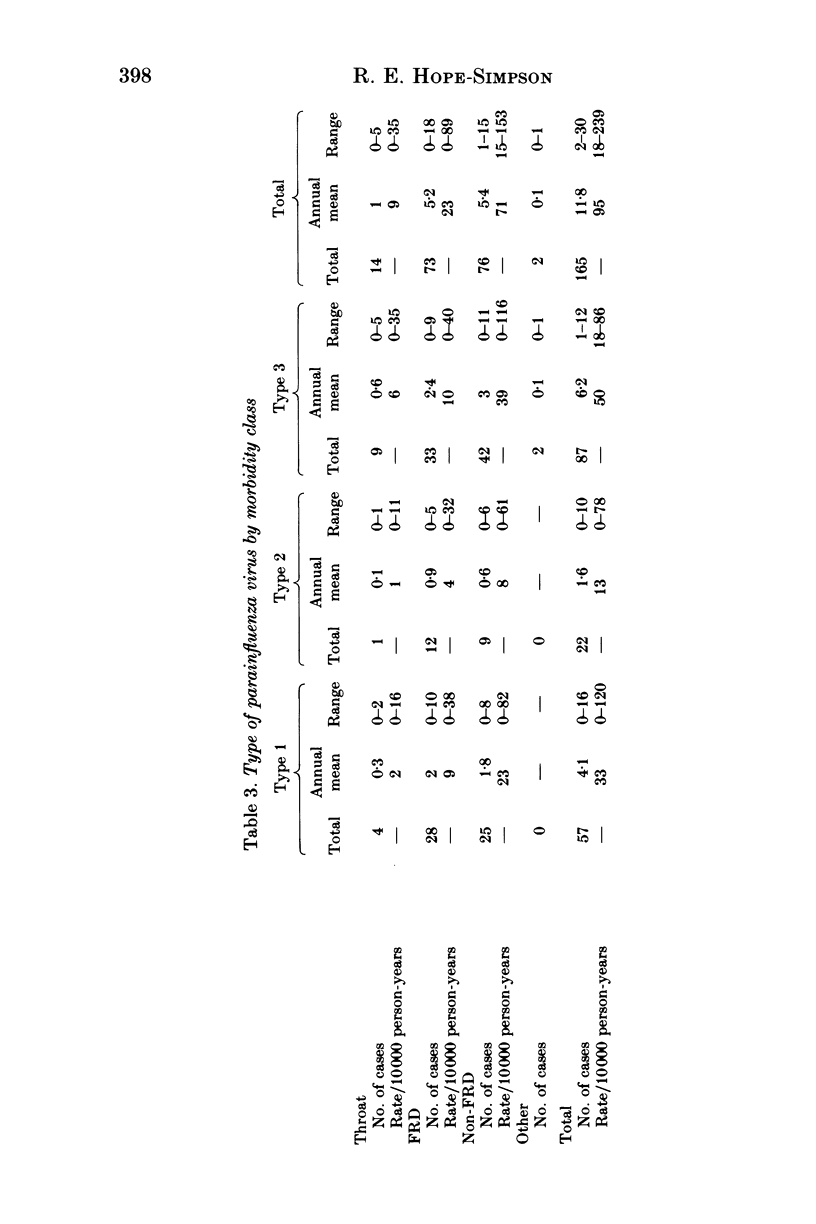
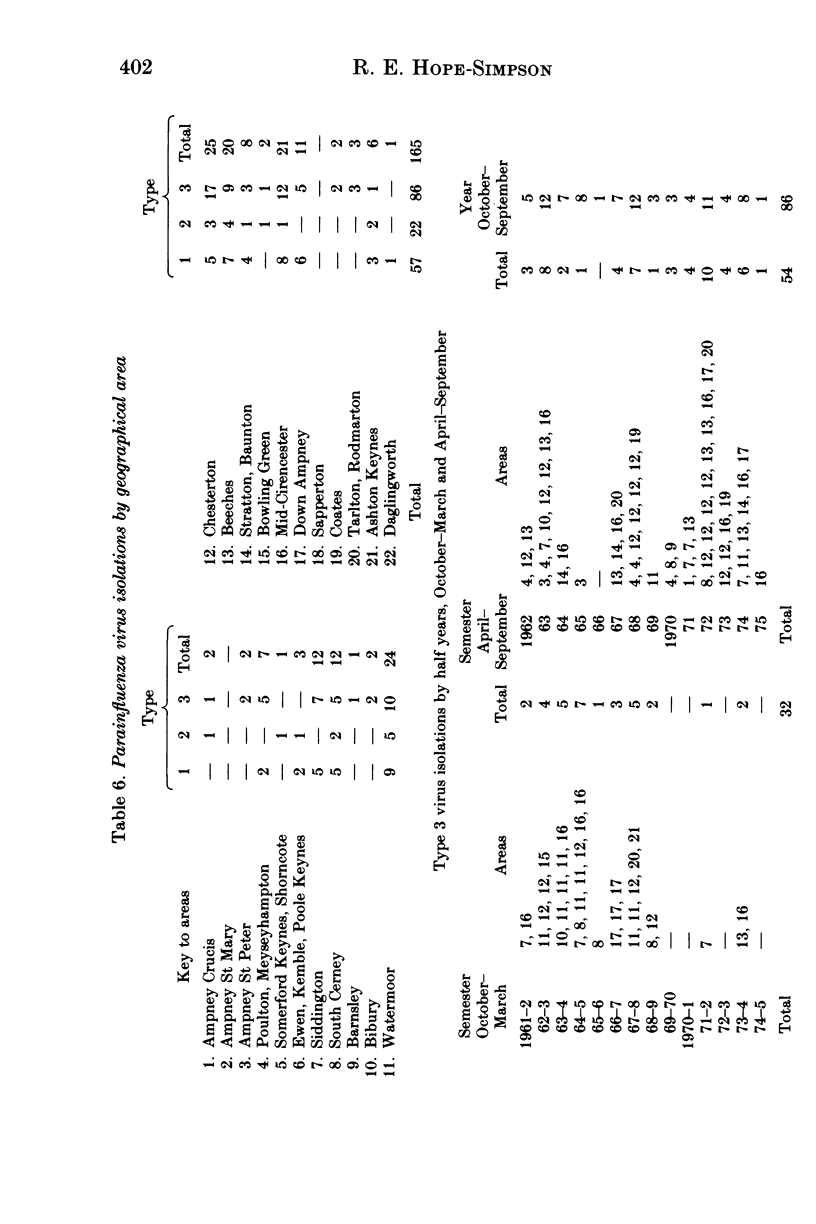
Parainfluenza viruses were isolated 165 times during 14 years surveillance of the illnesses of a general practice population of around 3700. Type 1 isolations numbered 57, type 2 isolations 22 and type 3 isolations 86, representing annual rates of 33, 13 and 50 infections respectively per 10000 of population. Type 4 parainfluenza virus was not isolated. Three major classes of illness gave the following rates: sore throats (Throats) nine, acute febrile respiratory diseases (FRD) 23, acute non-febrile respiratory diseases (non-FRD) 71. The illnesses caused by the three types isolated were similar. Type 1 infections were most abundant in November and type 2 infections in December, and only 11.4% of these types were isolated in the warm semester April through September. Type 3 infections were seasonally bi-modal, with a winter peak in January and an even greater prevalence (66% of the total) in the warm semester. Type 3 infections in the warmer months and in the later years of the Survey were usually more severe. Type 3 virus may therefore be heterogeneous, one subtype possessing and the other lacking the genetic mechanism of "cold-season' prevalence. Geographical discontinuity between summer and winter isolation strengthens the case for the existence of the two subtypes of type 3 parainfluenza virus. Type 3 infections caused the majority of the infections in very young infants. Type 2 infections were widely distributed at all ages. Females were attacked more often than males: type 1, 68.4%; type 2, 63.6%; type 3, 53.5%. Type 3 infections in males outnumbered those in females up to 60 years of age, whereas female predominance became apparent in types 1 and 2 before 10 years of age. All types were widely and sparsely distributed, areas of prevalence changing from year to year. Recurrences occurred only twice, both with type 3 infections. Six persons suffered both a type 1 and a type 3 infection, and one person suffered both a type 2 and a type 3 infection.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gavrilov V. I., Asher D. M., Vyalushkina S. D., Ratushkina L. S., Zmieva R. G., Tumyan B. G. Persistent infection of continuous line of pig kidney cells with a variant of the WSN strain of influenza A 0 virus. Proc Soc Exp Biol Med. 1972 May;140(1):109–117. doi: 10.3181/00379727-140-36405. [DOI] [PubMed] [Google Scholar]
- Golubev D. B., Paramonova M. S., Medvedeva M. N., Poljakov Y. M. Influenza A2 neuraminidases and their changes during persistance of viruses in tissue cultures. Z Gesamte Hyg. 1975 Apr;21(4):324–329. [PubMed] [Google Scholar]
- Hope-Simpson R. E. Epidemic mechanisms of type A influenza. J Hyg (Lond) 1979 Aug;83(1):11–26. doi: 10.1017/s002217240002578x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope-Simpson R. E. Streptococcus pyogenes in the throat: a study in a small population, 1962-1975. J Hyg (Lond) 1981 Aug;87(1):109–129. doi: 10.1017/s0022172400069291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore H. G., Parkinson A. J., Humphries J. E., Scott E. N., McIntosh D. A., Scott L. V., Cooney M. K., Miles J. A. Persistent parainfluenza virus shedding during isolation at the South Pole. Nature. 1981 Jan 15;289(5794):187–189. doi: 10.1038/289187a0. [DOI] [PubMed] [Google Scholar]
- Parkinson A. J., Muchmore H. G., McConnell T. A., Scott L. V., Miles J. A. Serologic evidence for parainfluenzavirus infection during isolation at South Pole Station, Antarctica. Am J Epidemiol. 1980 Sep;112(3):334–340. doi: 10.1093/oxfordjournals.aje.a112999. [DOI] [PubMed] [Google Scholar]


