Abstract
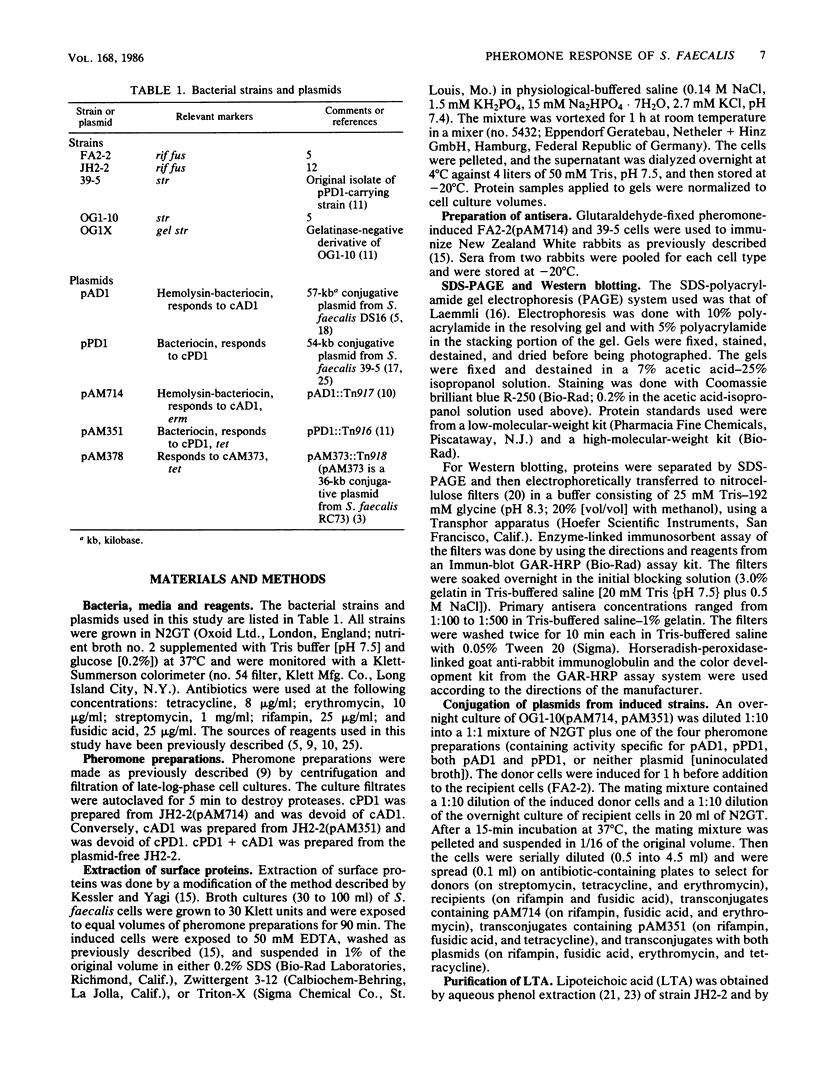
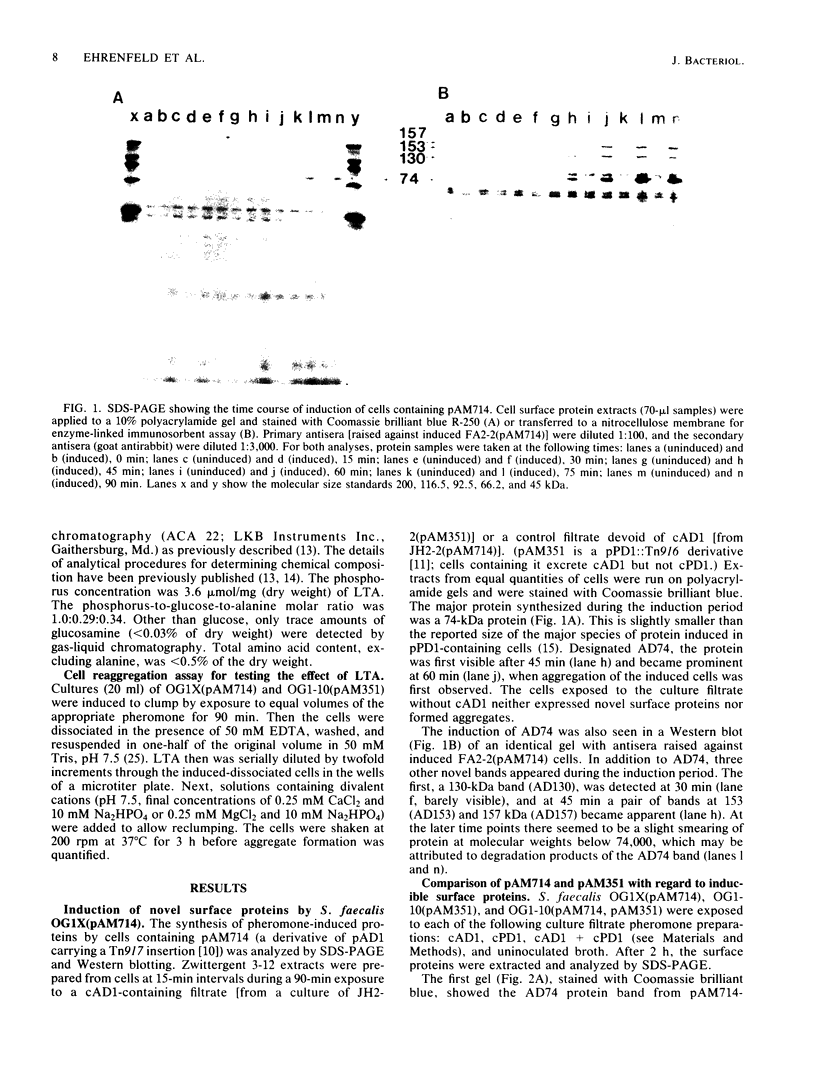
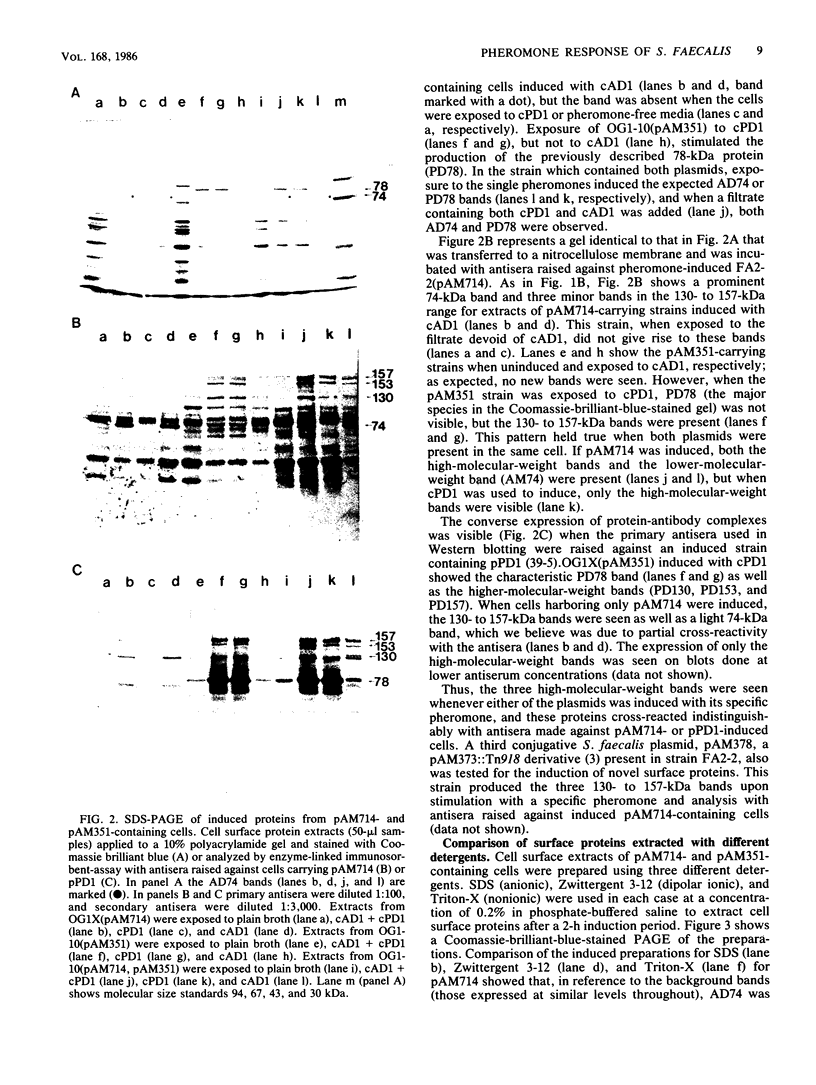
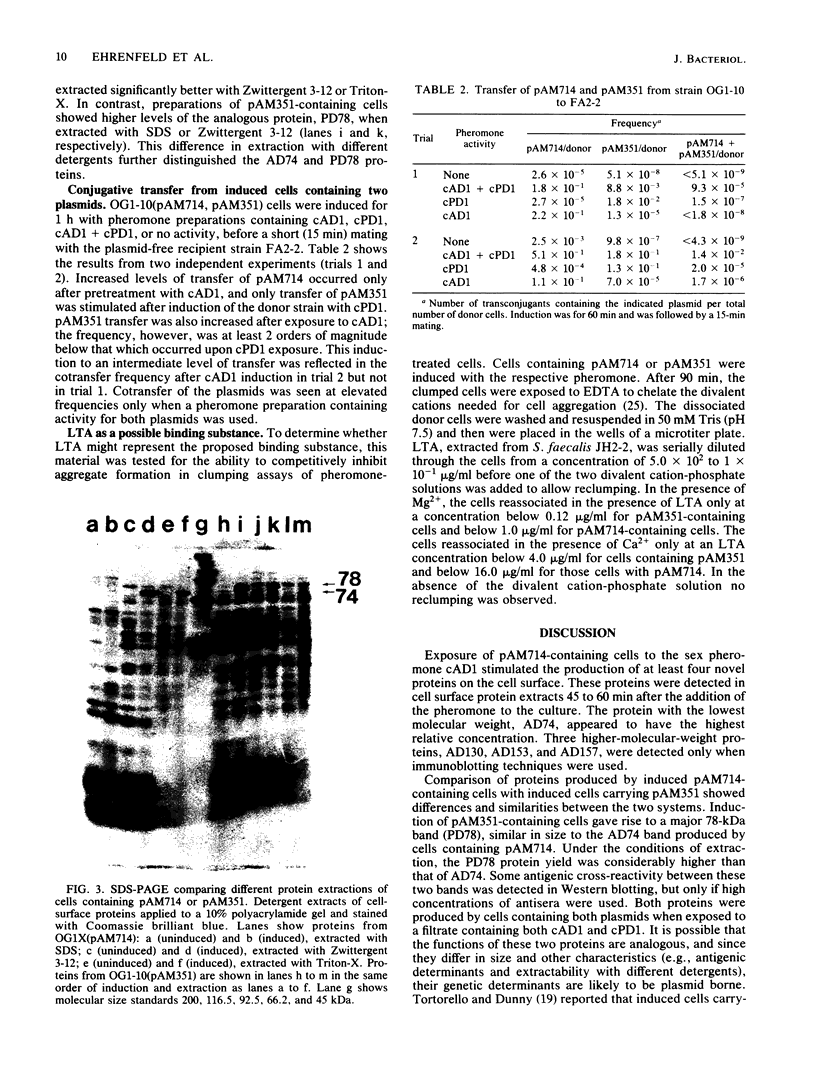
The conjugative transfer of the Streptococcus faecalis plasmid pAD1 is characterized by a 10,000-fold increase in frequency following sex pheromone (cAD1) induction. Before the increase in plasmid transfer, donor cells synthesize a proteinaceous adhesin that facilitates the formation of mating aggregates. Four novel surface proteins appearing after exposure of pAD1-containing cells to sex pheromone have been identified. Thirty minutes after induction, a 130-kilodalton (kDa) protein was detectable by Western blotting. A 74-kDa protein, the major species present, and a pair of bands at 153 and 157 kDa were evident 45 min after induction. Induced cells containing another conjugative S. faecalis plasmid, pPD1, gave rise to three high-molecular-weight proteins of the same size (130, 153, and 157 kDa) as those synthesized by pAD1-containing cells. These proteins cross-reacted with antisera raised against induced cells containing pAD1. However, the major protein species produced by pPD1-containing cells had a molecular weight of 78,000 and did not cross-react significantly with the corresponding band of the pAD1 system. Pheromone-induced transfer of the two plasmids, when both were present in the same cell, was independent; induction was limited to the pheromone-specified plasmid. The possibility that lipoteichoic acid might act as a receptor (binding substance) for the induced adhesin protein was also explored. Free lipoteichoic acid (isolated from S. faecalis) inhibited clumping of induced cells, apparently by acting as a competitive inhibitor of the cellular binding substance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., An F. Y., White B. A., Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J Bacteriol. 1985 Jun;162(3):1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Brown B. L. Sex pheromone cAD1 in Streptococcus faecalis: induction of a function related to plasmid transfer. J Bacteriol. 1980 Aug;143(2):1063–1065. doi: 10.1128/jb.143.2.1063-1065.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Tomich P. K., Gawron-Burke M. C., Franke A. E., Yagi Y., An F. Y. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J Bacteriol. 1982 Dec;152(3):1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Craig R. A., Carron R. L., Clewell D. B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979 Jul;2(3):454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon Tn917 as an insertional mutagen. J Bacteriol. 1984 Jun;158(3):777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Craig R. A., White B. A., Yagi Y., Clewell D. B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Thivierge B. H. Effects of substitution on polyglycerol phosphate-specific antibody binding to lipoteichoic acids. Infect Immun. 1983 Aug;41(2):549–555. doi: 10.1128/iai.41.2.549-555.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Yagi Y. Identification and partial characterization of a pheromone-induced adhesive surface antigen of Streptococcus faecalis. J Bacteriol. 1983 Aug;155(2):714–721. doi: 10.1128/jb.155.2.714-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., van de Rijn I., McCarty M. Characterization and localization of the enzymatic deacylation of lipoteichoic acid in group A streptococci. J Exp Med. 1979 Dec 1;150(6):1498–1509. doi: 10.1084/jem.150.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- ROSAN B., WILLIAMS N. B. HYALURONIDASE PRODUCTION BY ORAL ENTEROCOCCI. Arch Oral Biol. 1964 May-Jun;9:291–298. doi: 10.1016/0003-9969(64)90061-5. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Damle S. P., Clewell D. B. Plasmid-related transmissibility and multiple drug resistance in Streptococcus faecalis subsp. zymogenes strain DS16. Antimicrob Agents Chemother. 1979 Jun;15(6):828–830. doi: 10.1128/aac.15.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorello M. L., Dunny G. M. Identification of multiple cell surface antigens associated with the sex pheromone response of Streptococcus faecalis. J Bacteriol. 1985 Apr;162(1):131–137. doi: 10.1128/jb.162.1.131-137.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKEN A. J., ELLIOTT S. D., BADDILEY J. The identity of streptococcal group D antigen with teichoic acid. J Gen Microbiol. 1963 May;31:231–239. doi: 10.1099/00221287-31-2-231. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Yagi Y., Kessler R. E., Shaw J. H., Lopatin D. E., An F., Clewell D. B. Plasmid content of Streptococcus faecalis strain 39-5 and identification of a pheromone (cPD1)-induced surface antigen. J Gen Microbiol. 1983 Apr;129(4):1207–1215. doi: 10.1099/00221287-129-4-1207. [DOI] [PubMed] [Google Scholar]






