Abstract
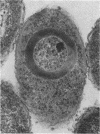
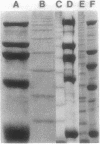
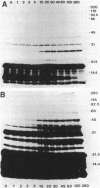
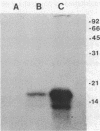
Type 51 R bodies are produced by all bacterial endosymbionts (Caedibacter taeniospiralis) of Paramecium tetraurelia that confer the hump-killer trait upon their hosts. Type 51 R-body synthesis by C. taeniospiralis is required for expression of the hump-killer trait. The genetic determinants for type 51 R-body synthesis by C. taeniospiralis 47 have been cloned and expressed in Escherichia coli. In this communication we describe three species of polypeptides required for R-body synthesis and the organization of their genetic determinants. Each polypeptide species is controlled by a separate gene that is expressed as an independent transcriptional unit possessing regulatory signals that are recognized by E. coli. Two polypeptide species of 10 and 18 kilodaltons are required for R-body synthesis but apparently are not structural subunits. The third polypeptide species (13 kilodaltons) is the major structural subunit. R-body assembly involves polymerization reactions that result in high-molecular-mass polypeptide complexes, primarily composed of the 13-kilodalton polypeptide species, that appear to be the result of covalent cross-linking between structural subunits. The results presented here have been suggested to apply to the assembly and structure of all type 51 R bodies, but not necessarily to other R-body types.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilts J. A., Quackenbush R. L. A mutation in the R body-coding sequence destroys expression of the killer trait in P. tetraurelia. Science. 1986 May 2;232(4750):641–643. doi: 10.1126/science.3008334. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., Wijfjes A., de Graaf F. K. Identification and characterization of precursors in the biosynthesis of the K88ab fimbria of Escherichia coli. J Bacteriol. 1983 Apr;154(1):41–49. doi: 10.1128/jb.154.1.41-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt C. Kinetics and regulation of cell-free alkaline phosphatase synthesis. J Bacteriol. 1980 Sep;143(3):1265–1274. doi: 10.1128/jb.143.3.1265-1274.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer J. R., Jr, Preer L. B., Jurand A. Kappa and other endosymbionts in Paramecium aurelia. Bacteriol Rev. 1974 Jun;38(2):113–163. doi: 10.1128/br.38.2.113-163.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer J. R., Jr, Preer L. B. Virus-like bodies in killer paramecia. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1774–1781. doi: 10.1073/pnas.58.4.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush R. L., Burbach J. A. Cloning and expression of DNA sequences associated with the killer trait of Paramecium tetraurelia stock 47. Proc Natl Acad Sci U S A. 1983 Jan;80(1):250–254. doi: 10.1073/pnas.80.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush R. L., Dilts J. A., Cox B. J. Transposonlike elements in Caedibacter taeniospiralis. J Bacteriol. 1986 Apr;166(1):349–352. doi: 10.1128/jb.166.1.349-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush R. L. Plasmids from bacterial endosymbionts of hump-killer paramecia. Plasmid. 1983 May;9(3):298–306. doi: 10.1016/0147-619x(83)90007-0. [DOI] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Gilberg W. Construction of plasmid vectors with unique PstI cloning sites in a signal sequence coding region. Gene. 1980 Dec;12(3-4):235–241. doi: 10.1016/0378-1119(80)90105-5. [DOI] [PubMed] [Google Scholar]
- WIDMAYER D. J. A NONKILLER RESISTANT KAPPA AND ITS BEARING ON THE INTERPRETATION OF KAPPA IN PARAMECIUM AURELIA. Genetics. 1965 Apr;51:613–623. doi: 10.1093/genetics/51.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]