Abstract
The white pock variant of cowpox virus shows limited growth in chick embryo fibroblasts maintained in arginine-deprived culture medium. Since these conditions inhibit the growth of parental virus, there is a marked increase in the frequency of the white variant in the virus population recovered after passage in the absence of arginine. The variants generated in this system have been characterized by restriction endonuclease analysis of virus DNA in the total DNA recovered from infected cell cultures. Such analysis shows that the white variants arise as deletion mutants of the parental virus, but there was considerable heterogeneity in the restriction patterns of different isolates examined shortly after their generation. Further passage selected white cowpox virus populations with a stable genome configuration comparable with the DNA of pock-purified white variants.
Full text
PDF






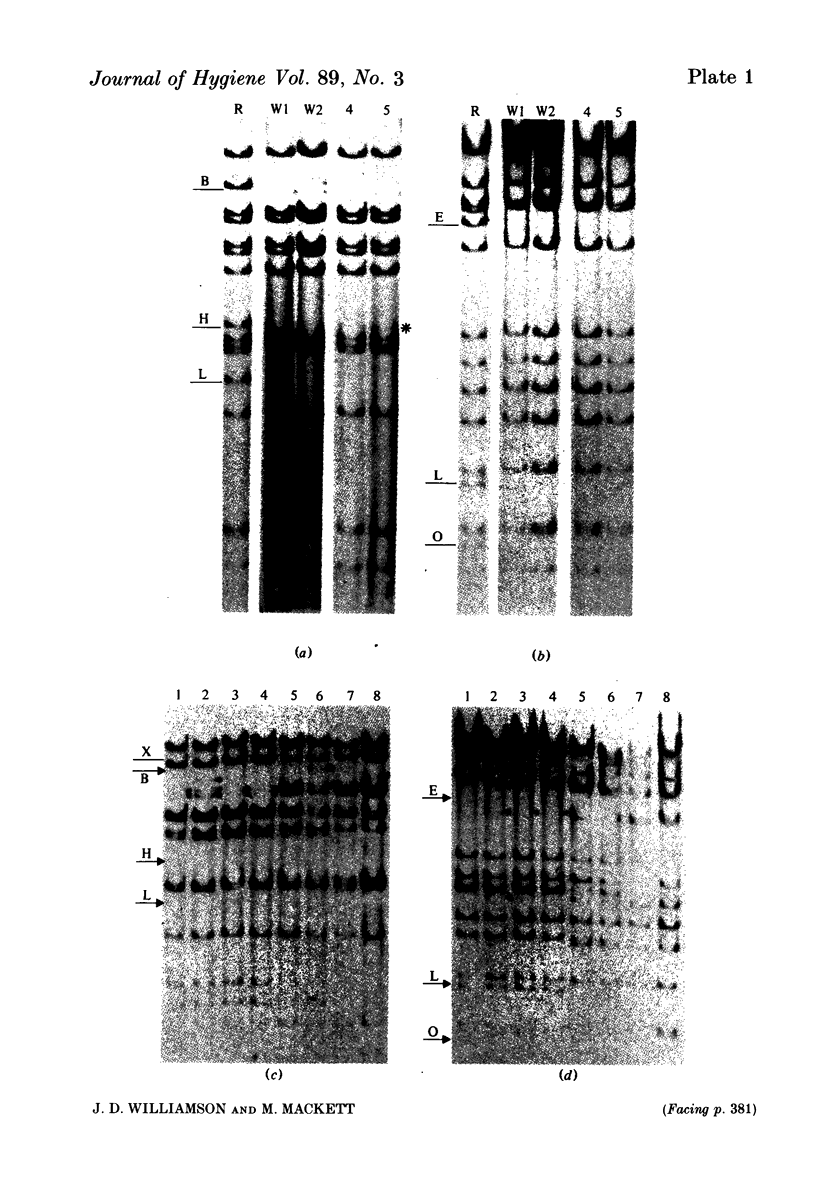

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archard L. C., Mackett M. Restriction endonuclease analysis of red cowpox virus and its white pock variant. J Gen Virol. 1979 Oct;45(1):51–63. doi: 10.1099/0022-1317-45-1-51. [DOI] [PubMed] [Google Scholar]
- Baxby D., Rondle C. J. The inhibition of growth of vaccinia and cowpox viruses in RK 13 cells. J Hyg (Lond) 1968 Jun;66(2):191–205. doi: 10.1017/s0022172400041073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B. C., Williamson J. D. Enhanced utilization of citrulline in rabbitpox virus-infected mouse sarcoma 180 cells. J Gen Virol. 1973 Nov;21(2):339–348. doi: 10.1099/0022-1317-21-2-339. [DOI] [PubMed] [Google Scholar]
- DOWNIE A. W., DUMBELL K. R. Pox viruses. Annu Rev Microbiol. 1956;10:237–252. doi: 10.1146/annurev.mi.10.100156.001321. [DOI] [PubMed] [Google Scholar]
- DOWNIE A. W., HADDOCK D. W. A variant of cowpox virus. Lancet. 1952 May 24;1(6717):1049–1050. doi: 10.1016/s0140-6736(52)90698-3. [DOI] [PubMed] [Google Scholar]
- Dumbell K. R., Archard L. C. Comparison of white pock (h) mutants of monkeypox virus with parental monkeypox and with variola-like viruses isolated from animals. Nature. 1980 Jul 3;286(5768):29–32. doi: 10.1038/286029a0. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Holtermann O. A. Amino acid requirement for the propagation of vaccinia virus in Earle's L cells. J Gen Virol. 1969 Jun;4(4):585–591. doi: 10.1099/0022-1317-4-4-585. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M. A rapid technique for distinguishing herpes-simplex virus type 1 from type 2 by restriction-enzyme technology. Lancet. 1979 Apr 21;1(8121):849–852. doi: 10.1016/s0140-6736(79)91265-0. [DOI] [PubMed] [Google Scholar]
- Mackett M., Archard L. C. Conservation and variation in Orthopoxvirus genome structure. J Gen Virol. 1979 Dec;45(3):683–701. doi: 10.1099/0022-1317-45-3-683. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Rothe C. T. The white pock mutants of rabbit poxvirus. I. Spontaneous host range mutants contain deletions. Virology. 1980 Apr 15;102(1):119–132. doi: 10.1016/0042-6822(80)90075-6. [DOI] [PubMed] [Google Scholar]
- RONDLE C. J., DUMBELL K. R. Antigens of cowpox virus. J Hyg (Lond) 1962 Mar;60:41–49. doi: 10.1017/s0022172400039292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN TONGEREN H. A. E. Spontaneous mutation of cowpox-virus by means of egg-passage. Arch Gesamte Virusforsch. 1952;5(1):35–52. doi: 10.1007/BF01245138. [DOI] [PubMed] [Google Scholar]
- Williamson J. D., Cooke B. C. Argininosuccinate synthetase-lyase activity in vaccinia virus-infected HeLa and mouse L cells. J Gen Virol. 1973 Nov;21(2):349–357. doi: 10.1099/0022-1317-21-2-349. [DOI] [PubMed] [Google Scholar]