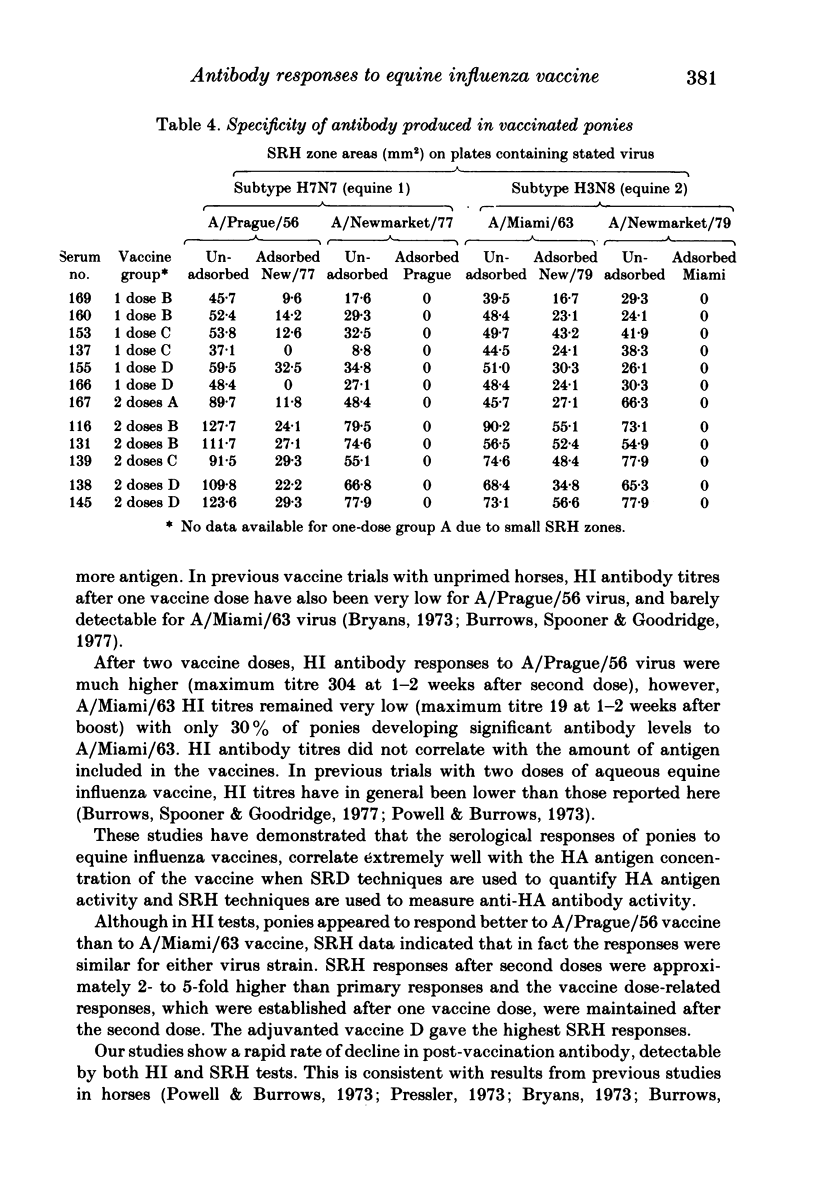
Abstract
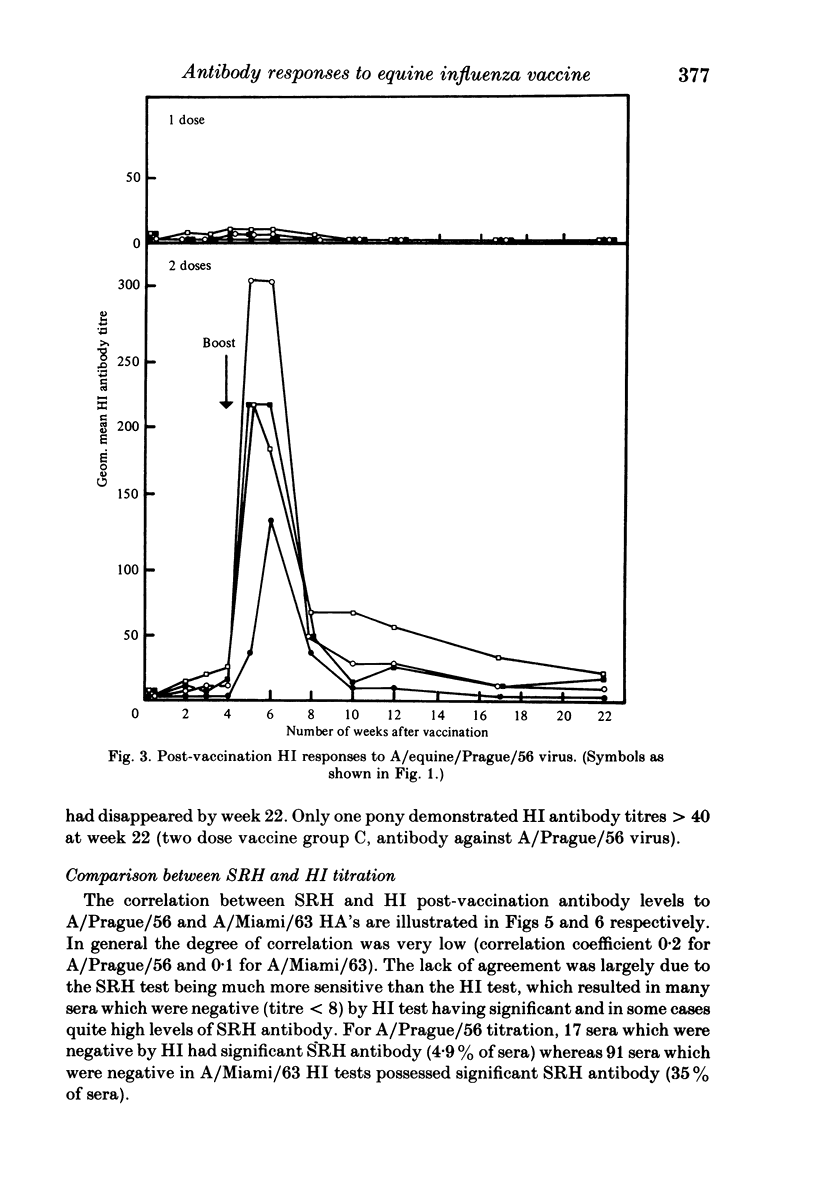
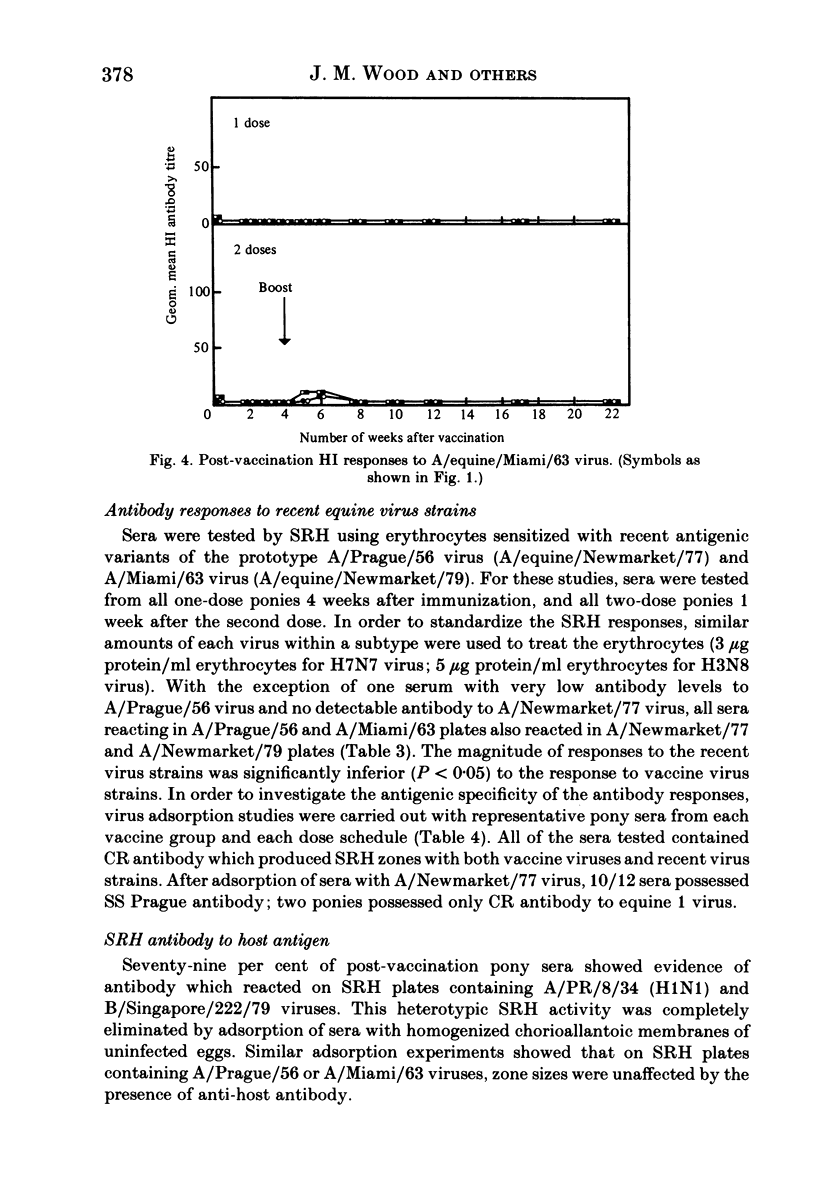
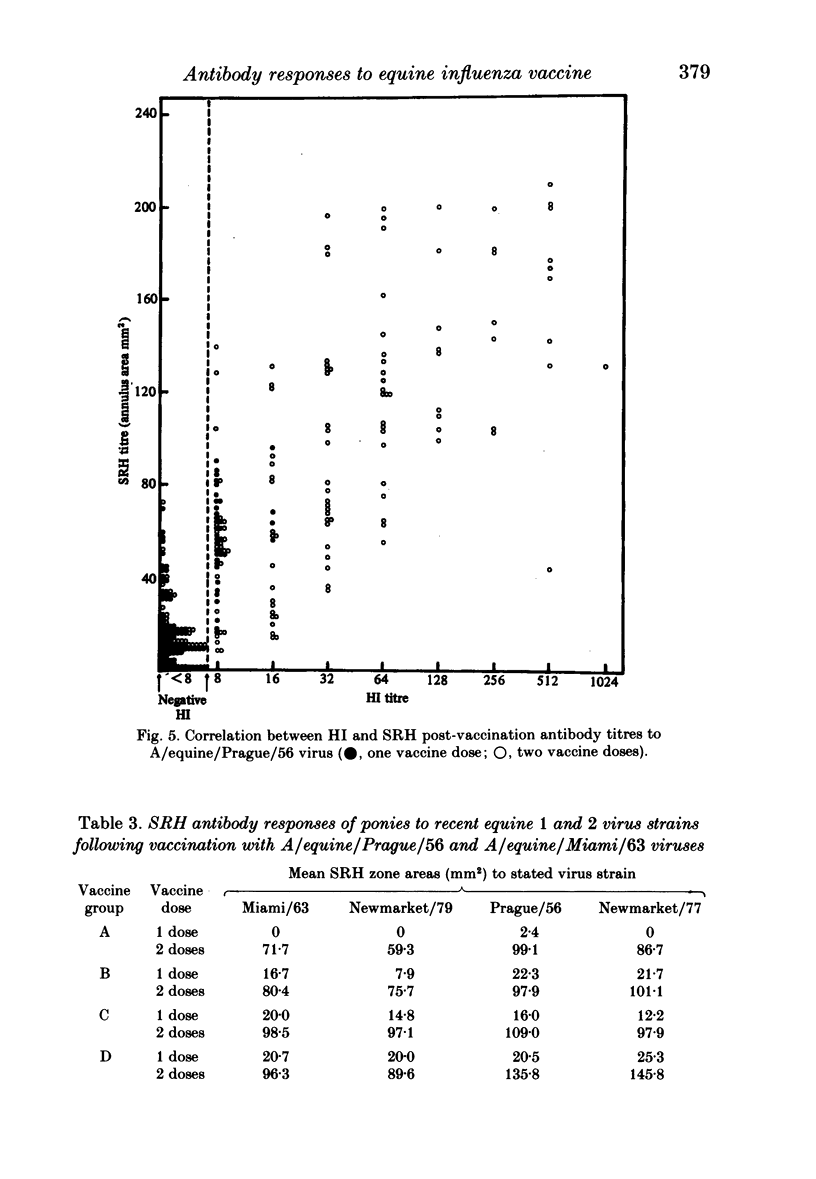
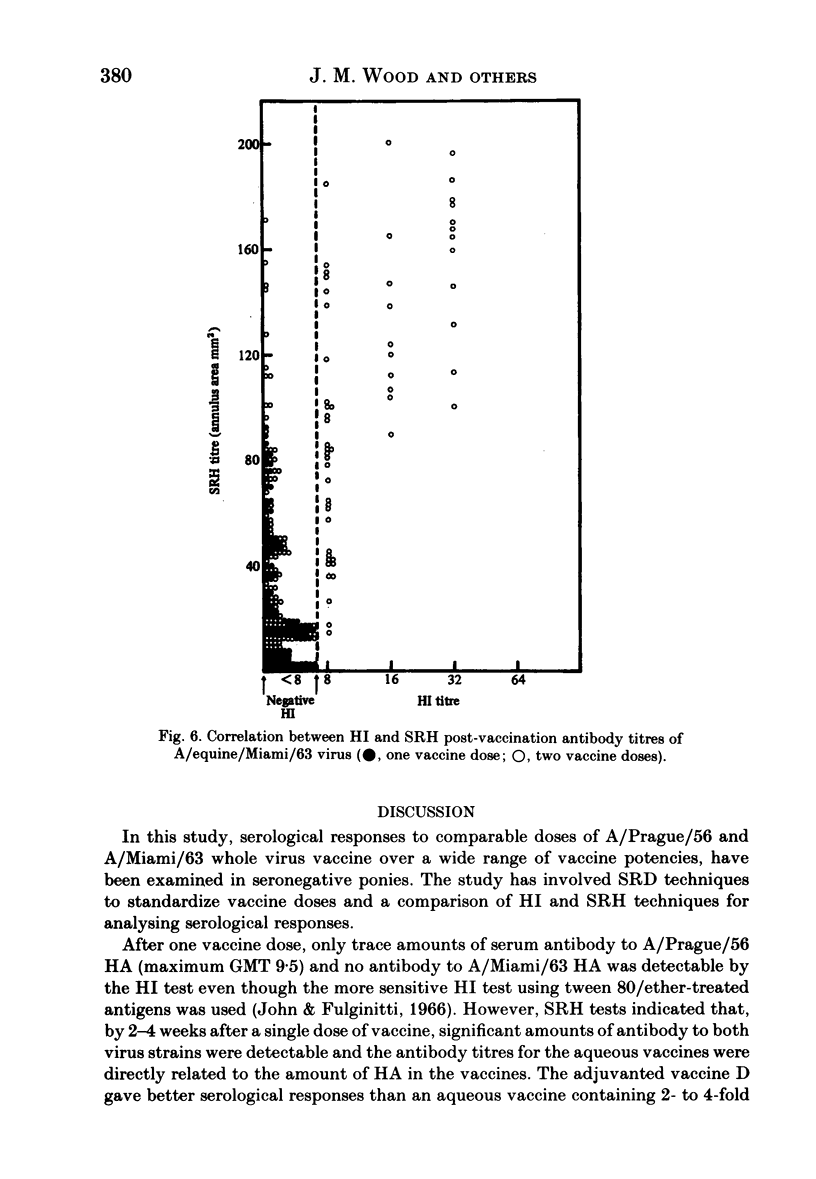
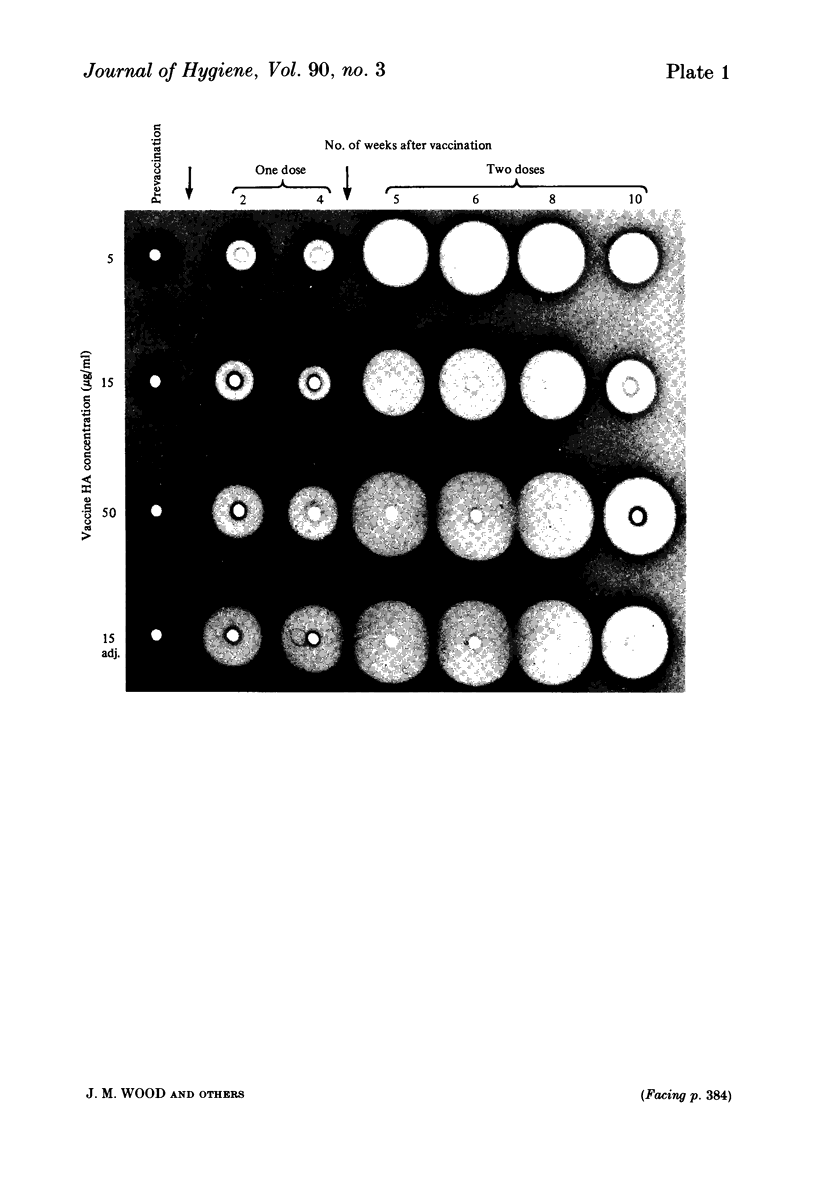
Serological responses to three bivalent aqueous equine influenza vaccines of different potency and an adjuvanted bivalent vaccine containing inactivated A/equine/Prague/56 (H7N7) and A/equine/Miami/63 (H3N8) viruses, were examined in seronegative ponies. Potencies of the vaccines, measured by single-radial-diffusion tests, ranged from 4 to 56 micrograms of haemagglutinin (HA) antigen activity/virus strain per dose. Serological responses to vaccination were examined by haemagglutination-inhibition (HI) and single-radial-haemolysis (SRH) tests. Four weeks after a primary dose, HI responses to both vaccine viruses were barely detectable; after a second dose the HI responses to A/Miami/63 virus were low or undetectable but HI responses to A/Prague/56 virus were higher (17/20 ponies with titres greater than or equal to 1:16). In contrast SRH tests revealed dose-related antibody responses to both virus strains after one and two vaccine doses; levels after the second dose were 2- to 5-fold higher than after the primary dose. Highest post-vaccination antibody titres were obtained with the adjuvanted vaccine which contained 2- to 4-fold less antigen (13-23 micrograms HA) than the most potent aqueous vaccine. Post-vaccination antibody reacted well in SRH tests with recent antigenic variants of equine influenza virus. A remarkable finding was the high rate of decline in antibody, detected by HI or SRH tests, following one or two doses of vaccine. Even in animals with the highest post-vaccine antibody levels 2-4 weeks after a booster dose, antibody levels had declined to low or indetectable levels 14 weeks later. The low antibody titres detected at 14-32 weeks after vaccination were nevertheless vaccine dose-related.
Full text
PDF


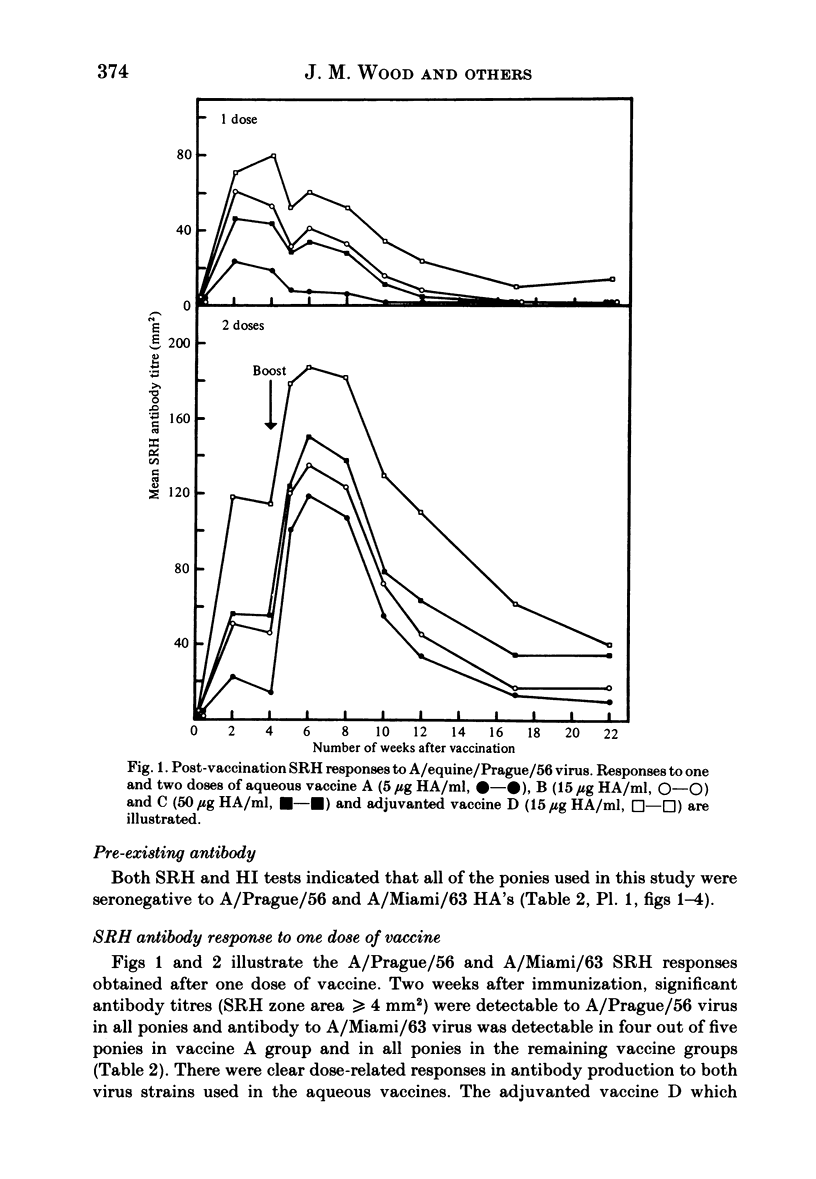
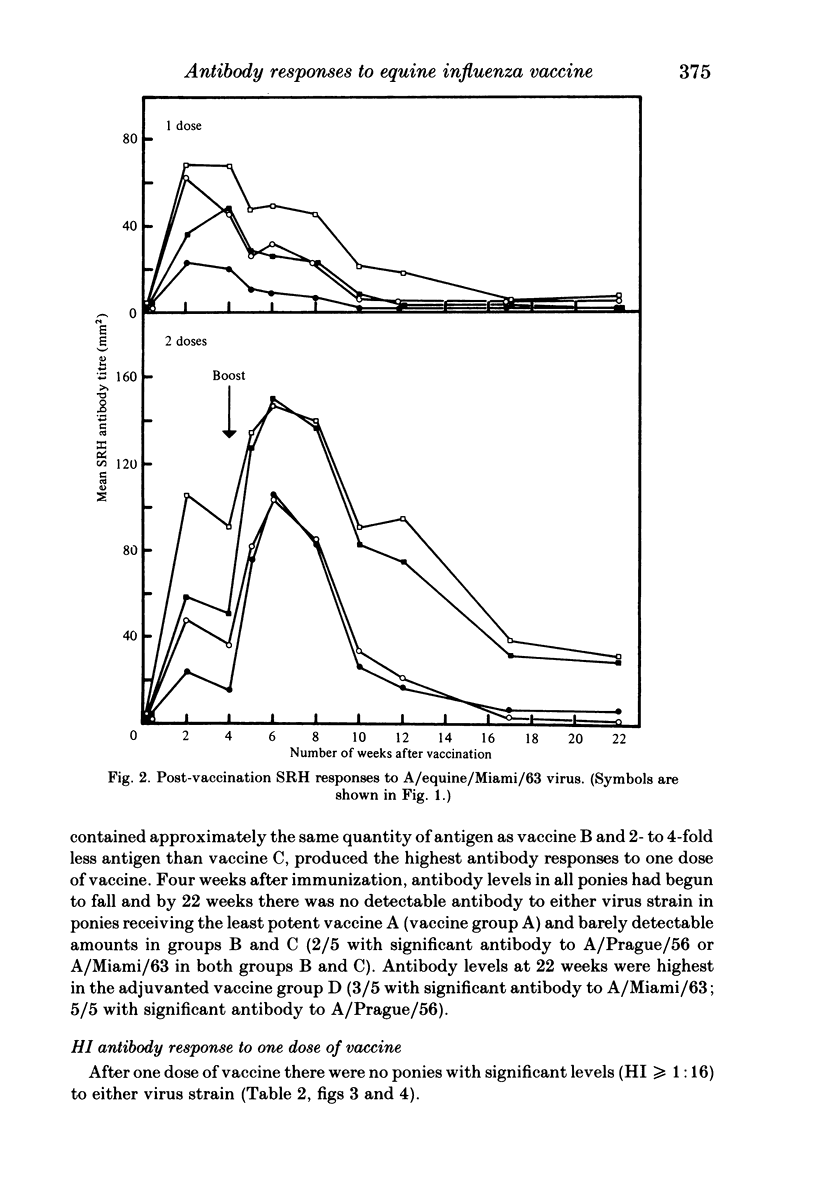
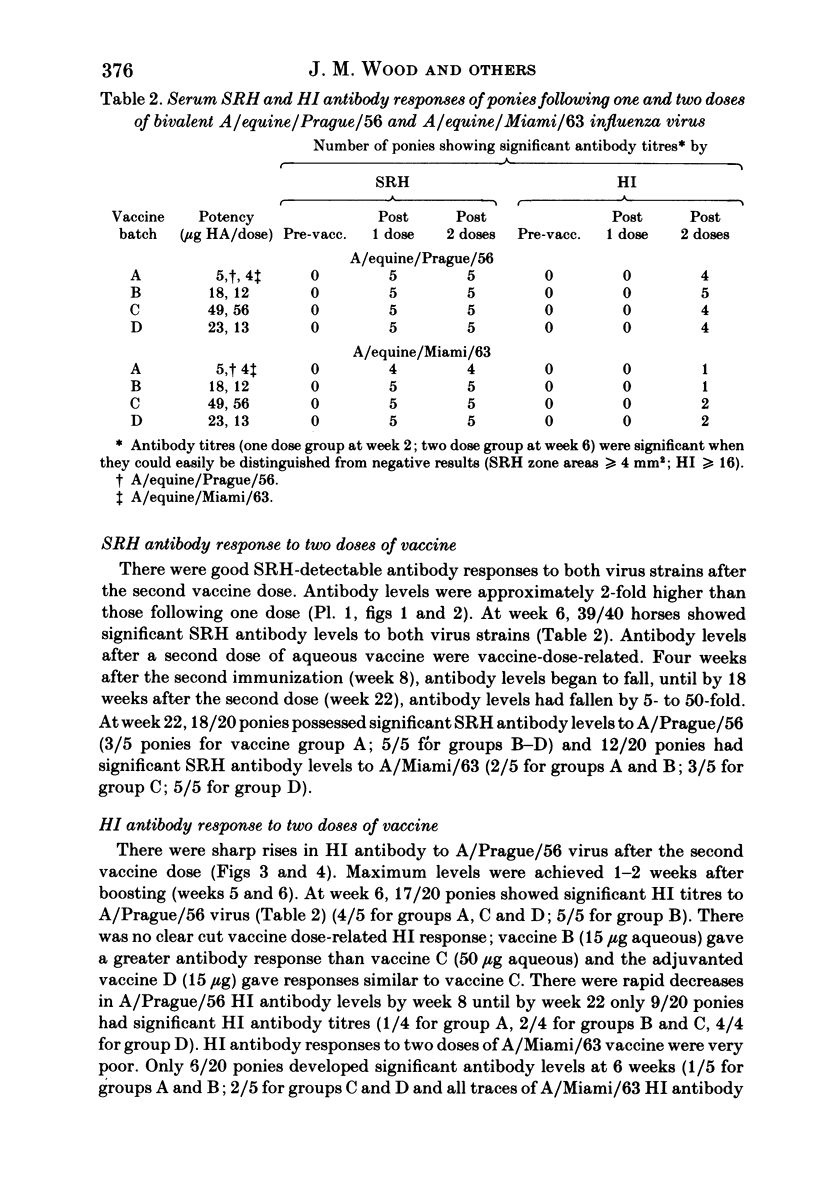



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burrows R., Denyer M., Goodridge D., Hamilton F. Field and laboratory studies of equine influenza viruses isolated in 1979. Vet Rec. 1981 Oct 17;109(16):353–356. doi: 10.1136/vr.109.16.353. [DOI] [PubMed] [Google Scholar]
- Burrows R., Spooner P. R., Goodridge D. A three-year evaluation of four commercial equine influenza vaccines in ponies maintained in isolation. Dev Biol Stand. 1977 Jun 1;39:341–346. [PubMed] [Google Scholar]
- HARBOE A. THE NORMAL ALLANTOIC ANTIGEN WHICH NEUTRALIZES THE INFLUENZA VIRUS HI-ANTIBODY TO HOST MATERIAL. Acta Pathol Microbiol Scand. 1963;57:488–492. doi: 10.1111/j.1699-0463.1963.tb05116.x. [DOI] [PubMed] [Google Scholar]
- Jackson D. C., Brown L. E., White D. O. Antigenic determinants of influenza virus haemagglutinin. VI. Antigenic characterization of the oligosaccharide sidechains form HA1 of influenza virus haemagglutinins. J Gen Virol. 1981 Jan;52(Pt 1):163–168. doi: 10.1099/0022-1317-52-1-163. [DOI] [PubMed] [Google Scholar]
- John T. J., Fulginiti V. A. Parainfluenza 2 virus: increase in hemagglutinin titer on treatment with Tween-80 and ether. Proc Soc Exp Biol Med. 1966 Jan;121(1):109–111. doi: 10.3181/00379727-121-30711. [DOI] [PubMed] [Google Scholar]
- Mumford J., Wood J. M., Scott A. M., Folkers C., Schild G. C. Studies with inactivated equine influenza vaccine. 2. Protection against experimental infection with influenza virus A/equine/Newmarket/79 (H3N8). J Hyg (Lond) 1983 Jun;90(3):385–395. doi: 10.1017/s0022172400029016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble G. R., Kaye H. S., O'Brien R. J., Kendal A. P., Bregman D. J., Wright P. F., Amler R. W., Dowdle W. R. Persistence of influenza A/New Jersey/76 (Hsw1N1) antibody one year after vaccination. Dev Biol Stand. 1977 Jun 1;39:253–260. [PubMed] [Google Scholar]
- Oxford J. S., Schild G. C., Potter C. W., Jennings R. The specificity of the anti-haemagglutinin antibody response induced in man by inactivated influenza vaccines and by natural infection. J Hyg (Lond) 1979 Feb;82(1):51–61. doi: 10.1017/s0022172400025468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. G., Takimoto S., Piegas N. S., do Valle L. A. Antigenic variation of equine (Heq2Neq2) influenzavirus. Bull World Health Organ. 1972;47(4):465–469. [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Clark A., Jennings R., Schild G. C., Wood J. M., McWilliams P. K. Reactogenicity and immunogenicity of inactivated influenza A (H1N1) virus vaccine in unprimed children. Report to the Medical Research Council Committee on influenza and other respiratory virus vaccines. J Biol Stand. 1980;8(1):35–48. doi: 10.1016/s0092-1157(80)80045-x. [DOI] [PubMed] [Google Scholar]
- Powell D. G., Thomson G. R., Spooner P., Plowright W., Burrows R., Schild G. C. The outbreak of equine influenza in England April-May 1973. Vet Rec. 1974 Mar 30;94(13):282–287. doi: 10.1136/vr.94.13.282. [DOI] [PubMed] [Google Scholar]
- Russell S. M., McCahon D., Beare A. S. A single radial haemolysis technique for the measurement of influenza antibody. J Gen Virol. 1975 Apr;27(1):1–10. doi: 10.1099/0022-1317-27-1-1. [DOI] [PubMed] [Google Scholar]
- SOVINOVA O., TUMOVA B., POUSKA F., NEMEC J. Isolation of a virus causing respiratory disease in horses. Acta Virol. 1958 Jan-Mar;2(1):52–61. [PubMed] [Google Scholar]
- Schild G. C., Pereira M. S., Chakraverty P. Single-radial-hemolysis: a new method for the assay of antibody to influenza haemagglutinin. Applications for diagnosis and seroepidemiologic surveillance of influenza. Bull World Health Organ. 1975;52(1):43–50. [PMC free article] [PubMed] [Google Scholar]
- Schild G. C., Smith J. W., Cretescu L., Newman R. W., Wood J. M. Strain-specificity of antibody to haemagglutinin following inactivated A/port chalmers/1/73 vaccine in man: evidence for a paradoxical strain-specific antibody response. Dev Biol Stand. 1977 Jun 1;39:273–281. [PubMed] [Google Scholar]
- Schild G. C., Wood J. M., Newman R. W. A single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen. Proposals for an assay method for the haemagglutinin content of influenza vaccines. Bull World Health Organ. 1975;52(2):223–231. [PMC free article] [PubMed] [Google Scholar]
- WADDELL G. H., TEIGLAND M. B., SIGEL M. M. A NEW INFLUENZA VIRUS ASSOCIATED WITH EQUINE RESPIRATORY DISEASE. J Am Vet Med Assoc. 1963 Sep 15;143:587–590. [PubMed] [Google Scholar]
- Wood J. M., Schild G. C., Newman R. W., Seagroatt V. An improved single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen: application for potency determinations of inactivated whole virus and subunit vaccines. J Biol Stand. 1977;5(3):237–247. doi: 10.1016/s0092-1157(77)80008-5. [DOI] [PubMed] [Google Scholar]
- Yamagishi H., Nagamine T., Shimoda K., Ide S., Igarashi Y., Yoshioka I., Matumoto M. Comparative measurement of equine influenza virus antibodies in horse sera by single radial hemolysis, neutralization, and hemagglutination inhibition tests. J Clin Microbiol. 1982 Apr;15(4):660–662. doi: 10.1128/jcm.15.4.660-662.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oirschot J. T., Masurel N., Huffels A. D., Anker W. J. Equine influenza in the Netherlands during the winter of 1978-1979; antigenic drift of the A-equi 2 virus. Vet Q. 1981 Apr;3(2):80–84. doi: 10.1080/01652176.1981.9693801. [DOI] [PubMed] [Google Scholar]