Abstract
We have recently found that the erythroid ankyrin gene, Ank1, expresses isoforms in mouse skeletal muscle, several of which share COOH-terminal sequence with previously known Ank1 isoforms but have a novel, highly hydrophobic 72–amino acid segment at their NH2 termini. Here, through the use of domainspecific peptide antibodies, we report the presence of the small ankyrins in rat and rabbit skeletal muscle and demonstrate their selective association with the sarcoplasmic reticulum. In frozen sections of rat skeletal muscle, antibodies to the spectrin-binding domain (anti-p65) react only with a 210-kD Ank1 and label the sarcolemma and nuclei, while antibodies to the COOH terminus of the small ankyrin (anti-p6) react with peptides of 20 to 26 kD on immunoblots and decorate the myoplasm in a reticular pattern. Mice homozygous for the normoblastosis mutation (gene symbol nb) are deficient in the 210-kD ankyrin but contain normal levels of the small ankyrins in the myoplasm. In nb/nb skeletal muscle, anti-p65 label is absent from the sarcolemma, whereas anti-p6 label shows the same distribution as in control skeletal muscle. In normal skeletal muscle of the rat, anti-p6 decorates Z lines, as defined by antidesmin distribution, and is also present at M lines where it surrounds the thick myosin filaments. Immunoblots of the proteins isolated with rabbit sarcoplasmic reticulum indicate that the small ankyrins are highly enriched in this fraction. When expressed in transfected HEK 293 cells, the small ankyrins are distributed in a reticular pattern resembling the ER if the NH2-terminal hydrophobic domain is present, but they are uniformly distributed in the cytosol if this domain is absent. These results suggest that the small ankyrins are integral membrane proteins of the sarcoplasmic reticulum. We propose that, unlike the 210-kD form of Ank1, previously localized to the sarcolemma and believed to be a part of the supporting cytoskeleton, the small Ank1 isoforms may stabilize the sarcoplasmic reticulum by linking it to the contractile apparatus.
Ankyrin was first found to link integral membrane proteins to the underlying spectrin network in the human erythrocyte (Bennett, 1978). It was subsequently described in a variety of vertebrate cells and tissues, including brain (Davis and Bennett, 1984), epithelia (Drenckhahn and Bennett, 1987), and skeletal muscle (Nelson and Lazarides, 1984). In vertebrates, molecular cloning has identified at least three distinct genes encoding ankyrin proteins, termed Ank1, Ank2, and Ank3 in the mouse (ANK1, ANK2, and ANK3 in the human; Peters et al., 1995; for review see Peters and Lux, 1993). Although not restricted to these cell types, Ank1 is the major gene expressed in erythroid cells, Ank2 in brain, and Ank3 in epithelial cells. All three genes produce several alternatively spliced transcripts, some missing large segments that include whole functional domains (Lambert et al., 1990; Lux et al., 1990; Kuminoto et al., 1991; Otto et al., 1991; White et al., 1992; Birkenmeier et al., 1993; Kordeli et al., 1994; Peters et al., 1995). The diversity of the ankyrins suggest that, in addition to their well-known role in the membrane skeleton, ankyrins may serve other more specific roles in different cell types. Our investigation of the intracellular location of a group of unique, small isoforms of Ank1 supports this hypothesis.
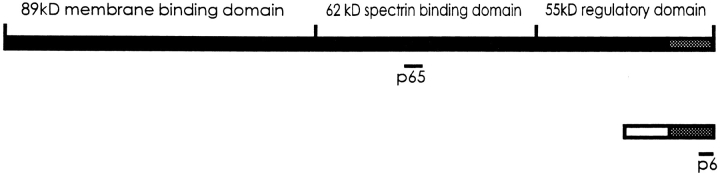
Most ankyrins contain three distinct structural domains (Lux et al., 1990). The NH2-terminal 89-kD domain, made up of 33 amino acid repeats, has binding activity for integral membrane proteins such as the anion exchanger (Davis et al., 1989; Davis and Bennett, 1990) and the voltage-gated sodium channel (Srinivasan et al., 1988, 1992), as well as for tubulin (Bennett and Davis, 1981; Davis and Bennett, 1984). Recently, a form of ankyrin without an NH2-terminal membrane-binding domain has been reported (Peters et al., 1995). The central 62-kD domain contains the binding site for the fifteenth repeat of the β-subunit of spectrin and fodrin (Weaver et al., 1984; Kennedy et al., 1991), and also for vimentin (Georgatos et al., 1985). This domain provides additional binding sites for the Na+, K+-ATPase (Nelson and Veshnock, 1987), although this transporter also binds at sites in the NH2-terminal 89-kD, membrane-binding domain (Davis and Bennett, 1990; Devarajan et al., 1994). The COOH-terminal 55-kD domain, termed the “regulatory” domain (Davis et al., 1992), is subject to extensive alternative splicing (Lambert et al., 1990; Lux et al., 1990; Kuminoto et al., 1991; Otto et al., 1991; White et al., 1992; Birkenmeier et al., 1993; Lambert and Bennett, 1993), which in the erythrocyte results in changes in binding affinities for β-spectrin and the anion transporter (Davis et al., 1992). An Ank1 transcript missing most of the regulatory domain has been found in mouse spleen (Birkenmeier et al., 1993).
Deficiences of erythroid ankyrin are responsible for some forms of human hereditary spherocytosis (Lux and Palek, 1995), as well as for an hereditary murine hemolytic anemia known as normoblastosis (nb/nb; Bodine et al., 1984; White et al., 1990). In the red blood cell precursors of affected mice, Ank1 transcripts are dramatically reduced, and only small amounts of the 210-kD and 150-kD ankyrin-like proteins are generated. Studies of the expression of Ank1 transcripts in tissues other than the bloodforming organs show that the consequences of the mutation are not limited to the erythroid lineage. In fact, Ank1 transcripts in nb/nb mice are reduced in the cerebellum, where a late onset neurological disorder is linked to the disappearance of a subset of Purkinje neurons (Peters et al., 1991).
Previous studies of skeletal muscle cells identified ankyrin immunologically and localized it to the neuromuscular junction (Flucher and Daniels, 1989), to triads (Flucher et al., 1990), and to domains at the sarcolemma (Nelson and Lazarides, 1984) known as costameres (Craig and Pardo, 1983; Pardo et al., 1983). In the course of our studies of the mouse Ank1 gene in skeletal muscle, we discovered three small transcripts of the Ank1 gene, in addition to the usual 9.0- and 7.5-kb transcripts of this gene (Birkenmeier et al., 1993). These small transcripts have now been sequenced. (These sequence data are available from GenBank/EMBL/DDB under accession number U73972.) The sequences predict that the major isoform encoded by these transcripts is 17.5 kD in mass, lacks both the membrane- and spectrin-binding domains, but retains, at its COOH terminus, the last 82 amino acids of the large Ank1 (Birkenmeier, C.S., J.J. Sharp, E.J. Hall, S.A. Deveau, and J.E. Barker, manuscript submitted for publication). The COOH terminus of full-length Ank1 is known to express four different sequences (A+C, B, A+B, and C) because of splicing of the mRNA (Birkenmeier et al., 1993; Gallagher, P., and B. Forget, personal communication). All ten of the cDNA clones for the small ankyrins carried the Btype alternative, suggesting that this form predominates in skeletal muscle (Birkenmeier, C.S., J.J. Sharp, E.J. Hall, S.A. Deveau, and J.E. Barker, manuscript submitted for publication). The NH2-terminal 72–amino acid segment is novel and is predicted to contain a single membrane-spanning helix, raising the possibility that the protein is membrane bound (Birkenmeier, C.S., J.J. Sharp, E.J. Hall, S.A. Deveau, and J.E. Barker, manuscript submitted for publication).
In the current paper, we report that, unlike the large, 210-kD form of ankyrin, which is present at the sarcolemma, the small ankyrins are concentrated at sites surrounding the Z lines and M lines of internal myofibrils, even when the sarcolemmal Ank1 is missing because of mutation. Subcellular fractionation indicates that these small, alternatively spliced ankyrins are highly enriched in the sarcoplasmic reticulum, suggesting a tight association with internal membranes. We propose a model in which the small Ank1 proteins in skeletal muscle link the sarcoplasmic reticulum to the contractile apparatus within each sarcomere.
Materials and Methods
Animals and Tissue
Adult female rats, purchased from Zivic Miller (Zelienople, PA), were anesthetized with Metofane (Pitman-Moore, Mundelein, IL) and sacrificed for removal of diaphragm, mixed hindlimb, or sternomastoid muscle. For the preparation of frozen sections, anesthetized rats were perfused through the left ventricle with buffered saline followed by 2% paraformaldehyde in buffered saline to fix muscle in situ. Diaphragm, sternomastoid, or hindlimb muscle was removed and plunged into a slush of liquid nitrogen. Tissue was stored at −70°C, for RNA isolation or protein extraction, or in liquid nitrogen, for preparation of frozen sections.
New Zealand White rabbits weighing over 400 g were purchased (Hazelton, Denver, PA) and used for isolation of sarcoplasmic reticulum from hindlimb muscles (see below).
Control (WBB6F1 +/+) and nb/nb mice (WBB6F1 nb/nb) were from the Jackson Laboratory (Bar Harbor, ME). Mice were treated as above but without perfusion to harvest muscle samples.
Generation of Antibodies
Peptide-specific rabbit antibodies to sequences in the COOH-terminal and spectrin-binding domains of erythrocyte ankyrin (Ank1) were generated as described (Porter et al., 1992), except that the p65 peptide was coupled to keyhole limpet hemocyanin and that p6 peptide was coupled to hemocyanin or BSA before immunization. The sequences of the synthetic peptides were LGELEELEKKRV (residues 1051 to 1062 of Cb14/11; Birkenmeier et al., 1993) for the preparation of anti-p65, the antibody to the spectrin-binding domain, and ASLKRGKQ (residues 1840 to 1847 of Cb14/11; Birkenmeier et al., 1993) for the preparation of anti-p6, the antibody to the predicted COOH-terminal sequence of the small, alternatively spliced ankyrin (see Fig. 2). IgG was isolated from antisera by precipitation with 50% (NH4)2SO4, dialyzed against PBS (10 mM NaP, 145 mM NaCl, pH 7.4), and applied to affinity columns to which the appropriate synthetic peptides had been covalently linked. The columns were washed with several volumes of buffered saline and then eluted with 50 mM glycine, 500 mM NaCl, pH 2.7. Eluted fractions were collected into tubes containing sufficient 1 M Tris-HCl, pH 8.0, to bring their pH to 7.2. Affinity-purified antibodies and the antibody fractions that failed to bind to the affinity column were dialyzed against buffered saline containing 10 mM NaN3 and stored at 4°C.
Figure 2.
Diagrammatic representation of the small, alternatively spliced ankyrin of skeletal muscle relative to the large, erythroid ankyrin. The three major domains of a typical erythroid ankyrin are indicated. The region of the small ankyrin shared with the larger protein is shown by a shaded bar. The novel region containing a stretch of hydrophobic amino acids at the NH2 terminus is shown as an open bar. The locations of the epitopes of the two antibodies, anti-p65 and anti-p6, are underlined. The long isoform depicted in this figure is Cb14/11 from Birkenmeier et al., 1993.
The specificity of the antibodies was assessed by enzyme-linked immunoadsorption assays (ELISA), following the method of Engvall (1980), and by immunoblotting of the synthetic peptides separated by SDS-PAGE (see below). The results from ELISA confirmed the specificity of the antibodies for their corresponding antigens (data not shown), as did the immunoblotting (see Fig. 3).
Figure 3.

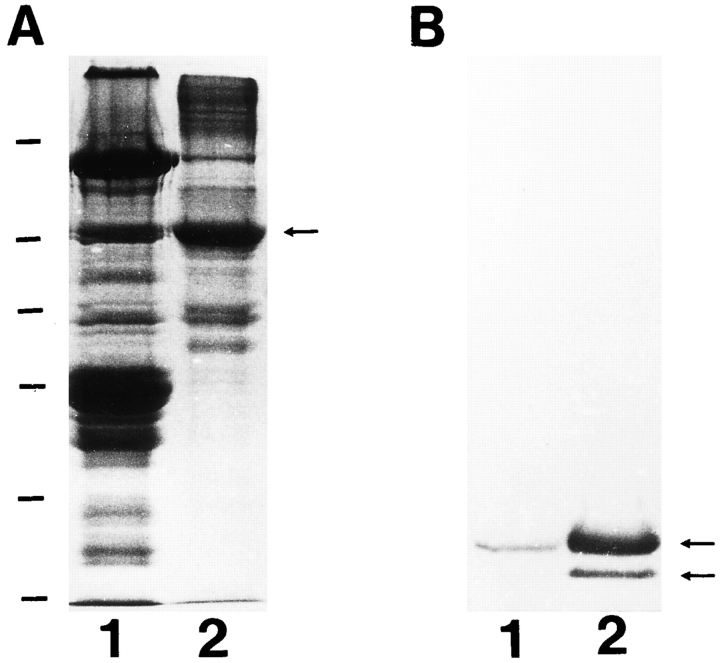
Imunoblotting of Ank1 in skeletal muscle by peptide-specific antibodies. Proteins in a homogenate of rat skeletal muscle were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Strips of the membrane from the same gel were incubated with antibodies to the spectrinbinding domain of Ank1 (anti-p65; lane 1) and to the COOH-terminal region of the small ankyrins (anti-p6; lane 2), followed by alkaline phosphatase-conjugated secondary antibodies. The molecular mass standards are shown to the right of lane 2 by dashes (from top to bottom, in kD): 200, 97, 68, 43, and 29.
Coupling of the synthetic peptides to Sepharose 4B (Pharmacia LKB Biotechnology, Piscataway, NJ) was done after activation of the matrix by cyanogen bromide (March et al., 1974). In some cases, activated resin was purchased from the manufacturer.
RNA Isolation and Northern Blot Analysis
RNA was isolated from frozen tissue by the guanidinium thiocyanate method described by Chomczynski and Sacchi (1987). Northern blot analysis was performed using the standard glyoxal/DMSO method. Briefly, mRNA (10 μg) was fractionated in a 1% agarose gel and transferred to a nylon membrane. After fixation by ultraviolet light (UV Crosslinker; Stratagene, La Jolla, CA), blots were prehybridized with quick-hybridization solution (Stratagene) for 15 min at 68°C. The 32P-labeled probe (106 cpm/ml) was then added and incubation was continued for 2 h. The blots were washed once for 30 min in 2× SSC, 0.1% SDS at room temperature and twice for 15 min in 0.1× SSC, 0.1% SDS at 60°C and then wrapped in Saran Wrap and exposed to x-ray film (X-OMAT; Eastman-Kodak, Rochester, NY). Prehybridization and hybridization were also performed in conventional hybridization solution containing 50% formamide, followed by washing at maximal stringency. The results from both were similar, but the quick hybridization method gave lower background.
Radioactive probes were generated using a random-primed labeling kit from GIBCO-BRL (Gaithersburg, MD). The cDNA probes were for the full-length mouse erythrocyte ankyrin (White et al., 1992), kindly provided by Dr. R.A. White (University of Kansas, Kansas City, KS) and the 5′ repeat regions of the human sequence of ANK2 (Otto et al., 1991), kindly provided by Dr. V. Bennett (Duke University, Durham, NC).
Analysis of Ank1 in Muscle Tissue
Frozen tissue was suspended in buffer containing 1% deoxycholate, 1% NP-40, 10 mM sodium phosphate, 0.5 M NaCl, 2 mM EDTA, pH 6.8 (Hoffman et al., 1989), supplemented with protease inhibitors (0.22 U/ml aprotinin, 1 mM benzamidine, 10 μg/ml leupeptin, 10 μg/ml antipain, 200 μg/ml soybean trypsin inhibitor). Tissue was homogenized (model 45; The Virtis Company, Gardiner, NY) at 0°C for 2 min (4 × 30 s) and centrifuged at 4,000 rpm in a rotor (model SS-34; DuPont-Sorvall, Wilmington, DE) to remove insoluble material. Aliquots of the supernatant containing 50 μg of protein were boiled in sample buffer (Laemmli, 1970), electrophoresed, and transferred electrophoretically to nitrocellulose membranes (Burnette, 1981). After blocking in buffered saline plus 3% (wt/ vol) nonfat dry milk for 2 h, strips of nitrocellulose were incubated with p65 or p6 antibodies for 2 h, washed, and incubated for 1 h with secondary antibody conjugated to alkaline phosphatase (Jackson ImmunoResearch, West Grove, PA). The chromogenic reaction to detect bound antibody was carried out using a kit from Kirkegaard and Perry Laboratories (Gaithersburg, MD). All reactions were done at room temperature. To enhance the signal of p65 labeling, bound antibody was in some cases detected by chemiluminescence with the Western-light kit from Tropix (Bedford, MA).
Immunofluorescence
Frozen diaphragm, hindlimb or extensor digitorum longus muscle was sectioned on a cryostat (Reichert-Jung, Cambridge Instruments, Deerfield, IL) at a thickness of 5–20 μm. Frozen sections were collected on slides pretreated with chrom-alum gelatin and stored dry at −70°C. For immunolabeling, samples were pretreated for 10 min in buffered saline (PBS) containing 1 mg/ml BSA (PBS/BSA) and then incubated for 1 h in the same solution with primary antibodies to the sequence within the spectrin-binding domain (anti-p65) or to the COOH-terminal sequence of the small ankyrins (anti-p6), each at 2 μg/ml. Monoclonal antibody to desmin (Boehringer-Mannheim Corp., Indianapolis, IN) was used at 4 μg/ml. Monoclonal antibody to a myosin of fast skeletal muscle fibers (Chemicon, Temecula, CA) was used at 1:10 dilutions, as recommended by the supplier. Monoclonal antibodies to the calcium ATPase of the sarcoplasmic reticulum (SERCA1)1 (MA3-911; Affinity Bioreagents Inc., Golden, Colorado) were diluted 1:100 in PBS/BSA containing 0.01% Triton X-100. A monoclonal antibody to syntrophin (1351E; Froehner et al., 1987) was used at 100 nM. Nonimmune rabbit serum (5 μg/ml) combined with antidesmin or MOPC was used as a control in every experiment. Additional controls included the use of the antibodies that failed to bind to the affinity columns and the use of the peptide antigens as haptenic inhibitors.
After extensive washing, slides were counterstained for 1 h with fluoresceinated goat anti–mouse IgG (FGAM, 10 μg/ml) and tetramethylrhodaminylated goat anti–rabbit IgG (RGAR, 10 μg/ml), both from Jackson ImmunoResearch. Controls established the specificity of these secondary antibodies for the appropriate IgG. All incubations were carried out at room temperature. Samples were washed extensively, mounted in 9 parts glycerol, 1 part 1 M Tris-HCl, pH 8.0, supplemented with 1 μg/ml p-phenylenediamine to reduce photobleaching (Johnson et al., 1982). Samples were first viewed under conventional epifluorescence optics and then under confocal optics with a confocal laser scanning microscope (model 410; Carl Zeiss, Inc., Thornwood, NY). Images were obtained at maximum resolution, sharpened using the MetaMorph image processing program (Universal Imaging, West Chester, PA), and printed on a photographic network printer (model NP-1600; Codonics, Middleburg Heights, OH).
Isolation of Sarcoplasmic Reticulum
Sarcoplasmic reticulum was purified according to the method of Eletr and Inesi (1972). Briefly, muscles were removed from the hindlimbs of an anesthetized rabbit and placed in cold, 0.1 mM EDTA. After blending in medium 1 (10 mM MOPS, 10% sucrose, 0.1 mM EDTA, pH 7.0) in the cold, the homogenate was centrifuged at 15,000 g for 20 min. The supernatant was collected and filtered through an 18.5-gauge needle attached to a 50 ml syringe. The filtrate was centrifuged at 40,000 g for 90 min. The pellet was suspended in medium 2 (10 mM MOPS, 0.6 M KCl, pH 7.0) and homogenized with a Dounce homogenizer. The homogenized suspension was centrifuged at exactly 11,250 rpm for 20 min in a rotor (model SS-34; DuPont-Sorvall). The middle layer of the supernatant was collected and centrifuged in the rotor for 90 min at 18,500 rpm. The supernatant was discarded and the pellet was suspended in medium 3 (10 mM MOPS, 30% sucrose, pH 7.0). This fraction, containing the light sarcoplasmic reticulum (SR), was homogenized twice with a Dounce apparatus, and the suspension was stored in aliquots at −70°C.
Cloning of Rat Small Ankyrin cDNA and Construction of the Plasmids
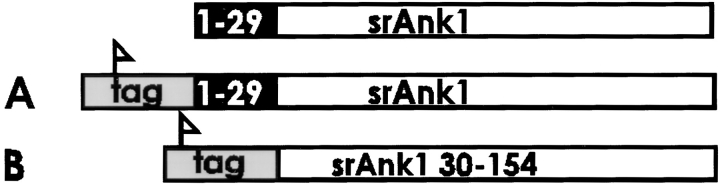
cDNA encoding the rat small ankyrins was obtained by reverse transcriptase–polymerase chain reaction (RT-PCR). Briefly, first strand cDNA was synthesized from 5 μg of total RNA from rat skeletal muscle using a cDNA synthesis kit from Pharmacia LKB Biotechnology, according to procedures provided by the manufacturer. The sense primer, 5′GTGAATTCCCATGTGGACCTTCATCACG3′, encoded the first six amino acids; the antisense primer, 3′TTTTCCCCGTTCGTCACTCCTTAAGTG5′, encoded the last five amino acids and the stop codon. An EcoRI site was included in each primer. The PCR was performed for 35 cycles as follows: denaturing at 95°C for 1 min, annealing at 55°C for 2 min, and extension at 72°C for 2 min. The PCR product containing the entire coding region of the small ankyrins was purified from an agarose gel, digested with EcoRI, cloned into pcDNA3.1HisA (InVitrogen, San Diego, CA), and sequenced. The rat amino acid sequence is identical to the mouse sequence with the exception of amino acid 52, for which a G replaces an E (data not shown). The cDNA sequence encoding amino acids 30 to 154 of the small ankyrins of rat skeletal muscle was also amplified by PCR and inserted in frame into the EcoRI site of the same vector. The sense primer for this smaller construct was 5′GTGAATTCCCGTCAAGGGTTCCCTGTGC3′; the antisense primer was the same. This construct, too, was verified by sequencing.
Transfection and Expression of the Small Ankyrins in HEK 293 Cells
HEK 293 cells (kindly provided by Dr. William Randall, University of Maryland, Baltimore, MD) were maintained in Dulbecco-Vogt–modified Eagle's medium plus 10% FBS. Transfection was achieved by a modified calcium phosphate precipitation method (Chen and Okayama, 1987). 1 d before the transfection, 105 cells were seeded onto a 25-mm coverslip in a 35-mm petri dish. Cells were transfected with 1 μg of the plasmids described above. Cells were fixed 36 h after transfection with 2% paraformaldehyde in PBS for 10 min, treated with 0.5% Triton X-100 in TBS (50 mM Tris, pH 7.4, 150 mM NaCl) for 5 min, incubated with 0.1 M glycine in PBS, and then immunolabeled as above with monoclonal antibodies to the FLAG-tag encoded by the vector (InVitrogen) and anti-p6.
Materials
Unless otherwise indicated, all materials were purchased from Sigma Chemical Co. (St. Louis, MO) and were the highest grade available.
Results
Rat Skeletal Muscle Contains Alternatively Spliced Ank1
Mouse skeletal muscle contains Ank1 mRNA at sizes of 1.6, 2.0, and 3.5 kb (Birkenmeier et al., 1993). We confirmed the presence of these small Ank1 transcripts in Northern blots of mRNA from rat skeletal muscle. The sizes we measured, 2.0, 2.4, and 3.5 kb, were slightly larger, perhaps because of our use of different standards or gel systems, or to species differences. Northern blots of rat muscle mRNA also revealed small amounts of longer Ank1 transcripts (7.5 and 9.0 kb; Fig. 1), as also seen in mouse (Birkenmeier, C.S., J.J. Sharp, E.J. Hall, S.A. Deveau, and J.E. Barker, manuscript submitted for publication). The larger transcripts are not due to contamination of muscle tissue with bone marrow or residual reticulocytes because the same blot did not contain significant amounts of the erythrocyte-specific isoform of β-spectrin (Zhou, D., J. Ursitti and R.J. Bloch, manuscript submitted for publication). We could not detect any Ank2 mRNA in these samples (data not shown). Thus, the most abundant ankyrin transcripts we detected in rat skeletal muscle tissue are the small, alternatively spliced transcripts of the Ank1 gene.
Figure 1.
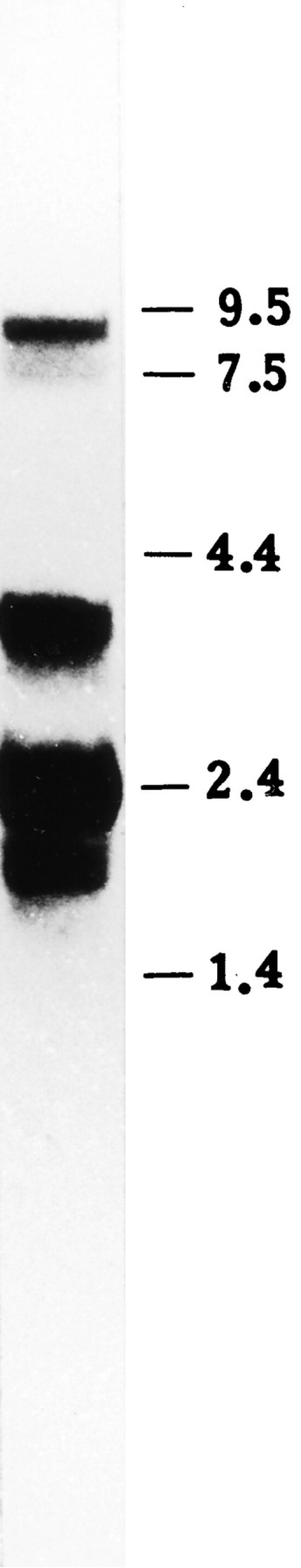
Northern blot analysis of erythroid ankyrin in rat skeletal muscle. Aliquots containing 10 μg of skeletal muscle mRNA were fractionated on a 1% agarose gel and transferred to a nylon membrane. The membrane was hybridized with full-length cDNA probes for erythroid ankyrin. The standards (in kb) are shown to the right. The results show the presence of three predominant transcripts at 2.0, 2.4, and 3.5 kb, all of which are too small to encode the typical ankyrin containing membrane-binding, spectrin-binding, and regulatory domains. An additional transcript at 9.0 kb and a minor transcript at 7.5 kb, also detected in the blots, are long enough to encode such an ankyrin.
Immunoblots Detect Ank1 Proteins in Rat Skeletal Muscle
To characterize the ankyrins in rat skeletal muscle further, we used two peptide-specific polyclonal antibodies: antip65, which recognizes a short, highly charged sequence in the middle of the spectrin-binding domain of Ank1, and anti-p6, which recognizes a COOH-terminal sequence predicted to be present in the small, alternatively spliced ankyrins (Fig. 2). In immunoblots of human erythrocyte ghosts, anti-p65 reacted strongly with a band at 210 kD, consistent with its recognizing sequences in the spectrinbinding domain of erythroid ankyrin (band 2.1; data not shown). In homogenates of rat skeletal muscle, anti-p65 detected a similarly sized band at 210 kD, and in addition reacted with a band at 70 kD (Fig. 3, lane 1). By contrast, anti-p6 detected two bands with apparent molecular masses of 20.5 and 26 kD (Fig. 3, lane 2) as well as a faint band of intermediate size. Anti-p6 would be expected to detect two, B and A+B, of the four possible alternatives (Birkenmeier et al., 1993) at the COOH terminus of the small Ank1 proteins. The predicted masses of these two alternatives would be 17.5 and 20.5 kD. The sizes of the forms detected are in reasonable agreement with this prediction. It is possible that other as yet undetected splicing events occur within the coding region for the small ankyrins, and this could explain the intermediate size detected on the immunoblots. Since anti-p6 does not detect a 210-kD isoform in skeletal muscle, it is probable that the full-length forms detected by anti-p65 contain the C or the A+C alternatively spliced sequences. A polyclonal antibody against purified erythroid ankyrin, kindly provided by Dr. J.S. Morrow (Yale University School of Medicine, New Haven, CT), recognized all the bands detected both by p6 and p65 (data not shown), confirming that they are products of the Ank1 gene.
In skeletal muscle, therefore, the p6 antibodies detect the small, alternatively spliced products of the Ank1 gene, while the p65 antibodies recognize larger forms containing the spectrin-binding domain, presumably products of the 7.5- and 9-kb transcripts. Whether the 70-kD band is a proteolytic product or is encoded by one of the larger transcripts is currently under investigation in our laboratories.
Subcellular Distribution of Ankyrins in Skeletal Muscle
Immunofluorescence labeling of rat skeletal muscle with p65 antibody revealed that ankyrin containing the β-spectrin–binding domain was located at the sarcolemma (Fig. 4, A and B). In agreement with the Northern blot analysis, which showed relatively low levels of the 7.5- and 9.0-kb transcripts, the labeling of skeletal muscle fibers by antip65 was not bright. The p65 antibody did, however, label red blood cells in unperfused samples very brightly (not shown, but see Fig. 6). In addition to labeling the sarcolemma, anti-p65 also labeled spots near the membrane or, occasionally, in the center of muscle fibers (Fig. 4 A). Subsequent studies have revealed these spots to be nuclei (Zhou, D., and R.J. Bloch, manuscript in preparation), in agreement with an earlier report (Bennett and Davis, 1981). Nonimmune rabbit serum, as well as the IgG fraction from immune serum that failed to bind to the p65 affinity column, did not label the sarcolemma or myonuclei (e.g., Fig. 5 A). Labeling by anti-p65 was therefore specific.
Figure 4.
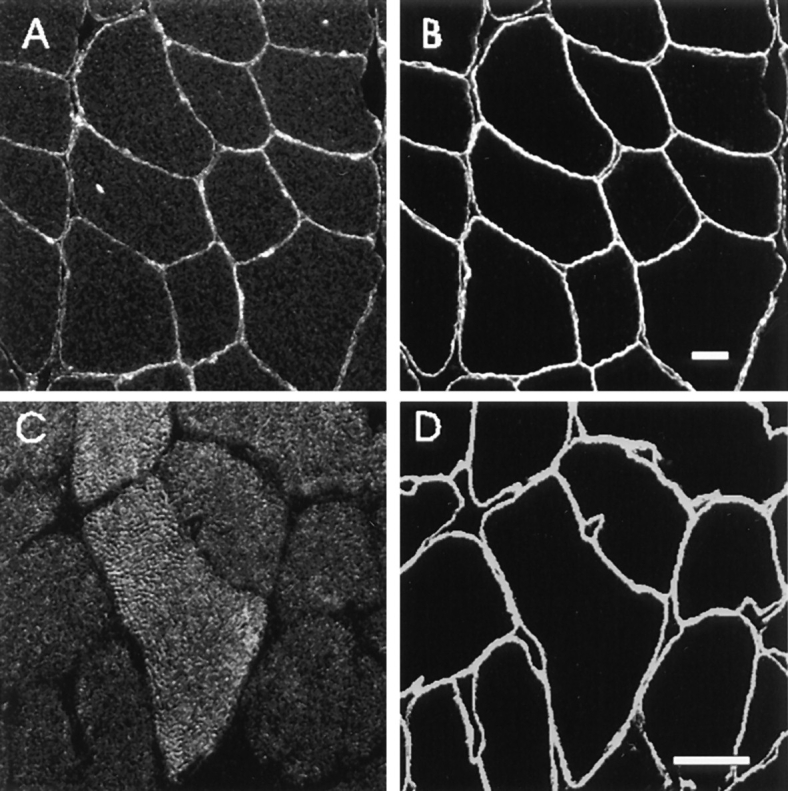
Distribution of Ank1 proteins in skeletal muscle. Cross sections of adult rat diaphragm were labeled with the polyclonal p65 antibody to the spectrin-binding domain of Ank1 (A) and the p6 antibody to the small ankyrins (C). The sections were doublelabeled with monoclonal antibodies to syntrophin to mark the sarcolemma (B and D). The bound antibodies were detected with rhodamine-conjugated goat anti–rabbit IgG and FITC-conjugated goat anti–mouse IgG secondary antibodies. The results show that the 210-kD (and perhaps the 70-kD) ankyrin of skeletal muscle is distributed at the sarcolemma and nuclei while the small ankyrins are inside the muscle fibers. Bars, 20 μm.
Figure 6.
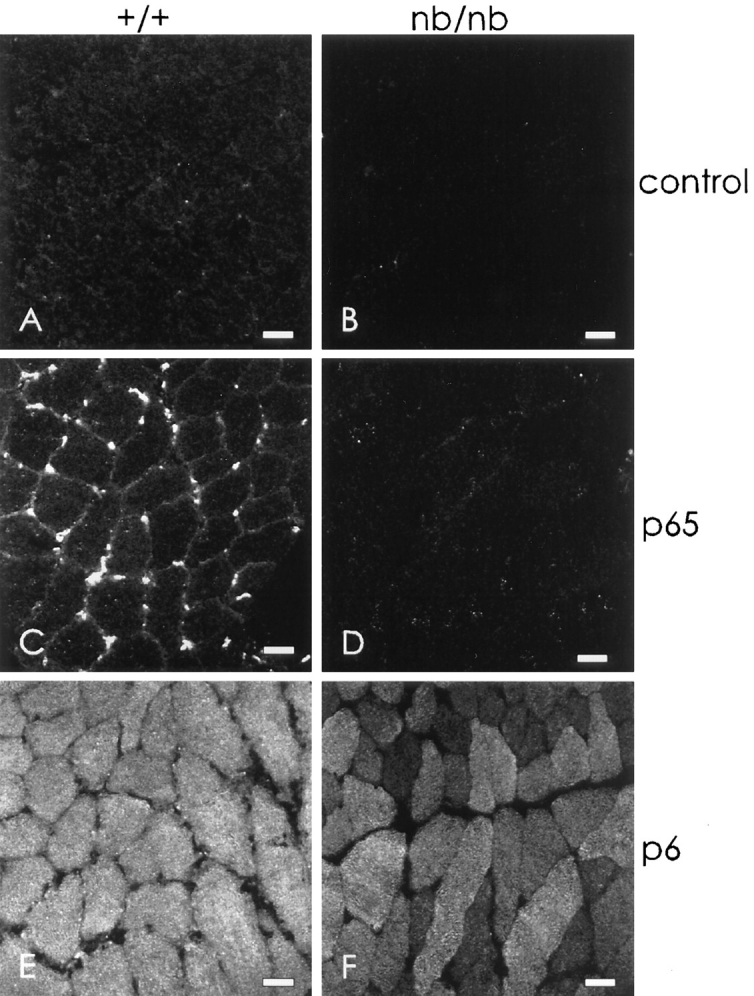
The small Ank1 isoforms in skeletal muscle are unaffected by the nb/nb mutation. Cross sections of diaphragms from wild-type (A, C, and E) and nb/nb (B, D, and F) mice were labeled with nonimmune rabbit serum (A and B), p65 antibodies to the spectrin-binding domain of ankyrin 1 (C and D), and p6 antibodies to the small ankyrins (E and F), followed by rhodamineconjugated secondary antibodies. Samples were taken from unperfused animals, so erythrocytes remaining in the capillaries could react with the antibodies if they contained the appropriate antigens. The results show that skeletal muscle fibers in the nb/nb mouse selectively lack the 210-kD Ank1 at the sarcolemma but are not deficient in the small myoplasmic forms. Bars, 20 μm.
Figure 5.
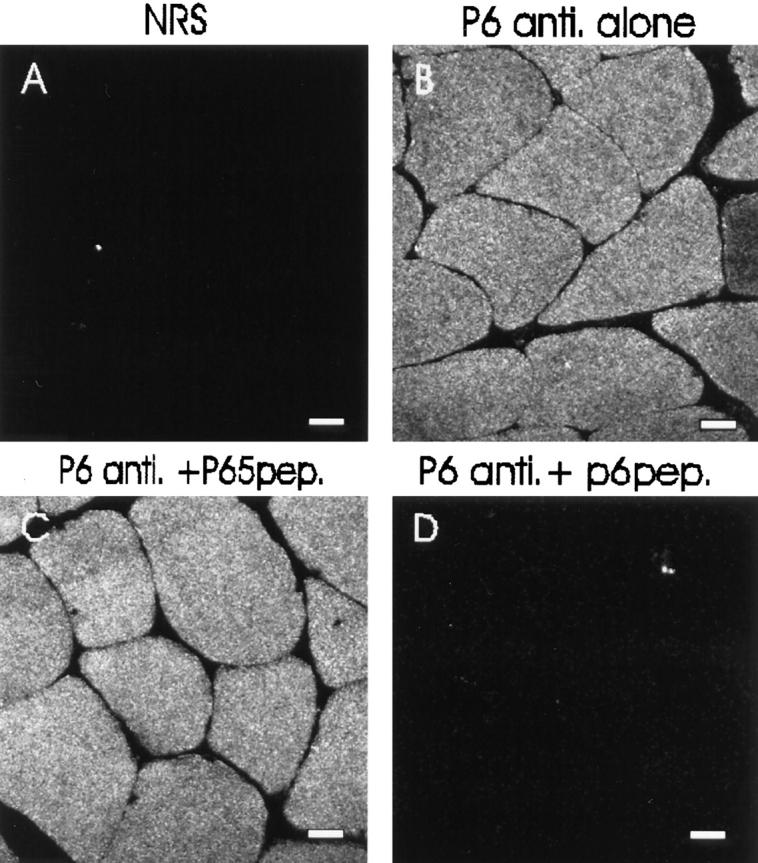
Specificity of antibodies to the small ankyrins assessed by immunofluorescence. Cross sections of rat diaphragm were labeled with nonimmune rabbit serum (A), p6 antibody to the small ankyrins (B), p6 antibodies preincubated with a 100-fold molar excess of irrelevant peptide (C), and the antibodies preincubated with a 100-fold molar excess of the antigenic peptide (D), followed by rhodamine-conjugated secondary antibodies. All the settings used to obtain these images on the laser scanning confocal microscope were identical. C and D show adjacent serial sections. Bars, 20 μm.
In contrast to anti-p65, the p6 antibody failed to label the sarcolemma and instead labeled the myoplasm of the muscle fibers in a reticular pattern (Fig. 4, C and D). The labeling was specific, as it was not mimicked by nonimmune rabbit antibodies (Fig. 5 A), antibodies from immunized animals that failed to bind to the p6 peptide affinity column, or other affinity-purified rabbit antibodies. The specificity of labeling was further established by preincubating the p6 antibody with a 100-fold molar excess of the peptide antigen, which almost completely abolished the labeling (Fig. 5 D). Preincubation with an excess of the p65 peptide antigen had no effect (Fig. 5 C).
These results indicate that the larger isoforms of Ank1 or a closely related protein are present at the sarcolemma of rat skeletal muscle, while the small Ank1 isoforms or closely related proteins are present primarily in the myoplasm.
The Small Ankyrins in Ankyrin-deficient Mice
The nb/nb mouse expresses only limited amounts of the 210-kD erythroid ankyrin (C.S. Birkenmeier, unpublished observations), together with a product at 150 kD (White et al., 1990). The deficiency of erythroid ankyrin causes a severe hemolytic anemia, as well as pathological consequences in other tissues (Peters et al., 1991). To determine if skeletal muscle of nb/nb mice expresses normal Ank1 products, we examined frozen cross sections of diaphragm muscle that had been labeled with anti-p65 and anti-p6 antibodies.
Labeling of diaphragm from wild-type mice confirmed our results with rat muscle. Labeling by anti-p65 was quite bright in the capillaries, where it reacted with erythrocytes in unperfused samples, and it was readily detectable at the sarcolemma (Fig. 6 C). In nb/nb muscle, however, antip65 showed no labeling of either sarcolemma or capillaries (Fig. 6 D), consistent with a severe depletion of the 210-kD form of Ank1 in the mutant. By contrast, the expression of the small ankyrins was not affected (Fig. 6, E and F). In both wild type and nb/nb samples, anti-p6 labeled a reticular pattern in the myoplasm at similar intensities. Variations in the intensity of labeling of different muscle fibers (e.g., Fig. 6 F) were due to differences in the amount of the small ankyrins in different muscle fiber types (Williams, M., N. Porter, D. Zhou, C.S. Birkenmeier, J.E. Barker, and R.J. Bloch, manuscript in preparation).
These results were confirmed by immunoblotting (data not shown). In agreement with previous reports (Bodine et al., 1984; Peters et al., 1991), skeletal muscle from nb/nb mouse contained no 210-kD ankyrin detectable with antip65, but anti-p6 detected control amounts of the small Ank1 isoforms in the same blot.
Association of the Small Ankyrins with the M and Z Lines
We compared the labeling of anti-p6 with labeling by monoclonal antibodies to desmin to try to determine the basis for the reticular distribution of the small ankyrins in the myoplasm. We observed a reticular pattern in cross sections labeled with antidesmin (Fig. 7 E), in agreement with published results (Granger and Lazarides, 1979). Confocal microscopic comparisons of double labeled samples (Fig. 7, D–F) revealed that much of the reticular pattern visualized with antidesmin was also seen with antibody to p6 (Fig. 7 F; overlapping label appears yellow; desmin alone appears green). Unlike labeling for desmin, anti-p6 labeling was absent from regions at the periphery of the myofibers. Also, some areas of the interior reticulum labeled with anti-p6 but not with antidesmin (Fig. 7 F; antip6 alone appears red). Thus, the p6 epitope is likely to be concentrated with desmin at the Z line surrounding the Z disk, but it is probably also present at other sites in the myoplasm. We confirmed this by examining longitudinal sections, in which both anti-p6 and antidesmin labeled the Z lines, but anti-p6 alone labeled structures in the middle of the A bands, at the position of M lines (Fig. 7, A–C).
Figure 7.
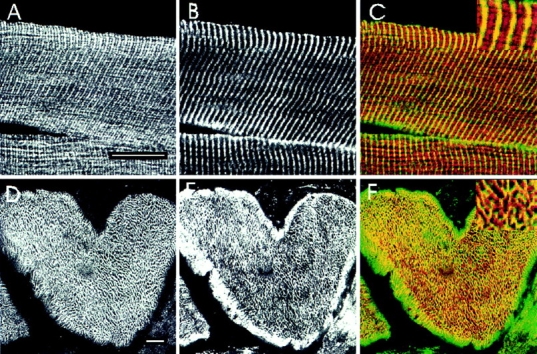
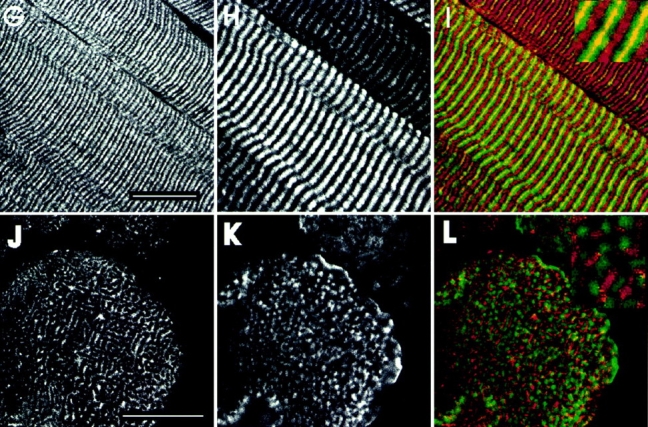
The small skeletal muscle ankyrins surround contractile structures at the Z and M lines. Cross sections (D–F and J–L) and longitudinal sections (A–C and G–I) of rat diaphragm were double labeled with polyclonal p6 antibodies to the small ankyrins (A, D, G, and J) and monoclonal antibodies to desmin (B and E) or myosin (H and K), followed by rhodamine-conjugated goat anti–rabbit IgG and FITC-conjugated goat anti–mouse IgG. To compare the paired antibodies, confocal microscopic images from rhodamine and fluorescein channels were overlain (C, F, I, and L). In the overlays, desmin and myosin are shown in green and the small ankyrins in red; regions containing both the small ankyrins and myosin or desmin are shown in yellow. Higher magnification views of selected regions are shown as inserts. The small ankyrins were found not only at Z lines (depicted in yellow in C and F) but also at the M lines (depicted by yellow in I), where it surrounds myosin in the thick filaments (L). Bars, 20 μm.
Double labeling immunofluorescence studies with the p6 antibody and monoclonal antibodies to myosin confirmed the presence of the small ankyrins in the middle of the A band (Fig. 7, G–I; in I, antimyosin is shown in green, anti-p6 is in red, and areas of overlap are in yellow). In cross sections, labeling by anti-p6 was distinct from the labeling by antimyosin (Fig. 7, J–L; note the relative paucity of yellow regions in L). Thus, the small ankyrins are not integral to the M lines but instead appear to surround the myosin filaments at the level of the M lines. Skelemin has been reported to show a similar relationship to the myofilament at M lines, but skelemin is not present at Z lines (Price, 1987).
The Small Ank1 Proteins Are Highly Enriched in Sarcoplasmic Reticulum
The small Ank1 isoforms of ankyrin may associate with membranes through a stretch of hydrophobic amino acids at the unique NH2 terminus (Birkenmeier, C.S., J.J. Sharp, E.J. Hall, S.A. Deveau, and J.E. Barker, manuscript submitted for publication). Our immunofluorescence results indicate that these small ankyrins surround the myofibrils at the levels of the M and Z lines, consistent with an association with the sarcoplasmic reticulum. (The t-tubular system in mammalian skeletal muscle is localized to the A–I junction, but the sarcoplasmic reticulum surrounds the entire sarcomere; Franzini-Armstrong, 1994). We examined purified fractions of sarcoplasmic reticulum from rabbit skeletal muscle to test this possibility.
In immunoblots of homogenates of rabbit muscle, the p6 antibody detected bands similar in mass to those observed in rat skeletal muscle (Fig. 8, B, lane 1). Fractions of purified sarcoplasmic reticulum protein were highly enriched in these bands (Fig. 8 B, lane 2), but not in myosin or desmin (data not shown). As expected for purified sarcoplasmic reticulum, these fractions were also highly enriched in the Ca2+-ATPase (c.f., Fig. 8 A). These results indicate that the small ankyrins associate tightly and preferentially with the sarcoplasmic reticulum of mammalian skeletal muscle. Thus, the small ankyrins are probably membrane proteins.
Figure 8.
The small ankyrins of skeletal muscle are highly enriched in purified sarcoplasmic reticulum. Protein (50 μg) from total homogenate of rabbit skeletal muscle (lanes 1) and from purified sarcoplasmic reticulum (lanes 2) were separated by SDSPAGE (17% acrylamide gel) and either stained with Coomassie blue (A) or transferred to nitrocellulose filters and blotted with p6 antibodies to the small ankyrins (B). (A) Coomassie blue staining of the gel. The arrow indicates the position of the Ca2+-ATPase. (B) Immunoblotting. Arrows indicate the small ankyrins. Molecular mass standards, shown to the left of A, are, from top to bottom, (in kD): 200, 97, 68, 43, 29, and 18.
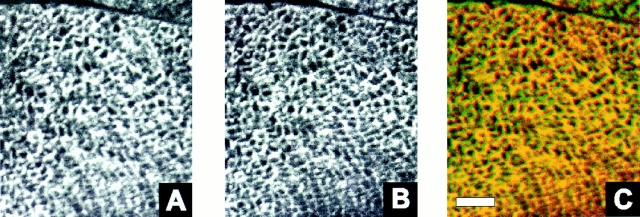
To confirm the association of the small ankyrins with the SR, we compared its distribution with that of the SERCA1 in fast twitch muscles of the rat (Fig. 9). As previously reported (Franzini-Armstrong, 1994), the SERCA1 is present in a reticular pattern (Fig. 9 A) that resembles the distribution of the small ankyrins (Fig. 9 B). The similarity is confirmed in overlays, which suggest extensive codistribution (Fig. 9 C) consistent with the localization of the small ankyrins in the SR.
Figure 9.
Comparison of the distribution of the small ankyrins and the SERCA1 ATPase in rat skeletal muscle. Frozen cross sections (5 μm) of the extensor digitorum longus muscle of the rat were prepared and double-labeled by immunofluorescence with anti-p6 antibodies to the small ankyrins and monoclonal antibodies to the SERCA1 ATPase of fast twitch muscle fibers, as described in Materials and Methods. Images were obtained by confocal microscopy. (A) SERCA ATPase, visualized with fluoresceinated anti-mouse IgG. (B) Small ankyrins, visualized with tetramethylrhodamine-conjugated anti-rabbit IgG. (C) Computer overlay of the images in A and B, in which structures labeled by both antibodies appear yellow. There is extensive coincidence of the two labels. Bar, 6 μm.
The Hydrophobic Domain Is Sufficient to Target Small Ankyrin to ER in Transfected Cells
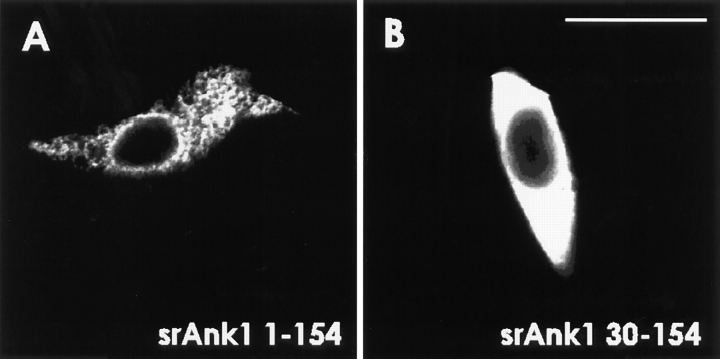
Their hydrophobic NH2-terminal sequences and their close association with the SR suggest that the small ankyrins may be integral proteins of the SR membrane. As a preliminary step in addressing this question, we transfected HEK 293 cells with cDNAs encoding the small ankyrins with or without the hydrophobic sequence (Fig. 10). When transfected 293 cells were stained with anti-p6 antibodies, the small ankyrins containing the hydrophobic NH2-terminal sequence were distributed in a reticular pattern resembling the pattern observed in skeletal muscle (Fig. 10 A). The reticulum in HEK 293 cells is probably the endoplasmic reticulum (e.g., Frangioni et al., 1992; Mitoma and Ito, 1992; Kutay et al., 1995; see also Villa et al., 1993; Foletti et al., 1995). When cells were transfected with the cDNA lacking the hydrophobic NH2 terminus, we observed a homogeneous distribution of the expressed proteins in the cytosol (Fig. 10 B). These results strongly suggest that NH2-terminal hydrophobic sequence of the small ankyrins is sufficient to target these proteins to a membrane compartment in transfected cells, and they support the notion that the small ankyrins are integral proteins of the SR of skeletal muscle.
Figure 10.
Small ankyrins expressed in transfected HEK 293 cells with or without the NH2-terminal hydrophobic domain. cDNA sequences encoding amino acids 1–154 or 30– 154 of the small ankyrins were obtained by RT-PCR and cloned into pcDNA3.1 HisA, which introduces an NH2-terminal FLAG tag. Plasmid DNA was introduced as a calcium phosphate precipitate into HEK 293 cells. 1 d later, cells were fixed, permeabilized, and labeled with anti-p6. Cells expressing the fulllength, tagged protein (srAnk1 1–154) were labeled in a reticular pattern in the cytoplasm (A), whereas cells expressing the truncated version of the protein that lacked the hydrophobic NH2-terminal sequence (srAnk1 30–154) were labeled uniformly in the cytoplasm (B). Identical results were obtained when transfected cells were labeled with anti-FLAG (data not shown). Bar, 20 μm.
Discussion
Ankyrin was first identified in the human erythrocyte as the protein responsible for linking spectrin to the membrane, and until recently, studies of its structure were consistent with its playing a similar role in other tissues. All three ankyrins that have been cloned and sequenced have a large NH2-terminal domain responsible for binding to integral membrane proteins, a central domain responsible for binding spectrin, and a COOH-terminal domain with regulatory functions (Bennett and Gilligan, 1993). In the last year, however, unusual ankyrin variants have been reported that do not share this structure (Peters et al., 1995; Devarajan et al., 1996). In the course of investigating the ankyrins of skeletal muscle, we detected three transcripts of the Ank1 gene that, unlike the 7.5- and 9.0-kb transcripts that are also present, are too short to encode a protein containing the three domains typical of ankyrin (Birkenmeier et al., 1993). Characterization of cDNA clones representing these small transcripts predicted the presence in skeletal muscle of small proteins that share sequence with the regulatory domain of Ank1 but that are distinguished by a unique, hydrophobic NH2-terminal sequence (Birkenmeier, C.S., J.J. Sharp, E.J. Hall, S.A. Deveau, and J.E. Barker, manuscript submitted for publication). Here we show that the proteins encoded by these alternatively spliced transcripts are expressed at significant levels in rat skeletal muscle, where they are concentrated together with the SERCA ATPase in the sarcoplasmic reticulum that surrounds the M and Z lines in each sarcomere. Furthermore, their localization is different from that of the larger ankyrins, which, unlike the small ankyrin products, are concentrated at the sarcolemma.
Data from the nb/nb mouse confirm the fact that the products of the 9.0- and 7.5-kb Ank1 transcripts are associated with the sarcolemma. It is known that the larger transcripts (9.0 and 7.5 kb) are deficient in the muscle of the mutant mice, as is the 210-kD protein (Bodine et al., 1984; Peters et al., 1993), whereas the small transcripts are present in normal amounts (Birkenmeier, C.S., J.P. Sharp, H.A. Field, and J.E. Barker. 1993. Blood. 82:5a). Consequently, anti-p65 antibodies, directed against the spectrinbinding domain encoded by these transcripts, fail to label the sarcolemma of nb/nb muscle. By contrast, anti-p6 antibodies, directed against the small Ank1 proteins, label the internal reticulum of muscle fibers normally, and normal amounts of the small ankyrins can be detected by immunoblotting (not shown). Thus, the synthesis in skeletal myofibers of the small ankyrins is not linked to that of the larger isoforms.
Although the nb mutation has no apparent consequences for muscle function, subtle differences may eventually be detected by more rigorous tests. Such differences may be difficult to ascribe to deficiencies in ankyrin, however, because of the severe hemolytic anemia, and the changes in motor control associated with cerebellar neuropathy, that accompany the nb mutation (Peters et al., 1991). The task will be further complicated by the fact that there are multiple Ank1 transcripts at 9.0- and 7.5-kb in erythroid precursors (White et al., 1992) and in the brain (Birkenmeier et al., 1993), suggesting similar complexity in skeletal muscle.
Considering the prominence in skeletal muscle of the small ankyrins and the mRNAs that encode them, it is surprising that they have not been characterized before. Several laboratories have identified proteins in skeletal muscle that are localized at the Z or M lines, but most are either large cytoskeletal proteins, like the M protein (185 kD; Grove et al., 1985), or intermediate filament proteins, like desmin (53 kD; Granger and Lazarides, 1979) and vimentin (57 kD; Granger and Lazarides, 1979). With the exception of zeelins (23 and 35 kD; Ferguson et al., 1994), none of the proteins is similar in size to the small ankyrins. The small ankyrins are not zeelins, however, both because they share no obvious homology (Sainsbury and Bullard, 1980; Vigoreaux et al., 1993) and because the zeelins probably do not associate preferentially with either the Z or the M line (Ferguson et al., 1994). It appears, therefore, that the small ankyrins belong to a novel class of proteins that associate with the sarcoplasmic reticulum and surround both Z and M lines.
The presence in the small ankyrins of a highly hydrophobic NH2-terminal sequence (Birkenmeier, C.S., J.J. Sharp, E.J. Hall, S.A. Deveau, and J.E. Barker, manuscript submitted for publication), the ability of this sequence in transfection experiments to target tagged, chimaeric variants of the small ankyrins to intracellular membranes, and the fact that they copurify with the light sarcoplasmic reticulum suggest that they are integral proteins of the sarcoplasmic reticulum membrane. Other integral membrane variants of ankyrin have been identified (Otsuka, A.J., P. Boontrakulpoontawee, and D. Otsuka. 1995. Mol. Biol. Cell. 6:269a), but none have been reported to associate preferentially with intracellular membranes. A form of ankyrin has recently been localized to the Golgi apparatus (Devarajan et al., 1996), but this isoform of Ank3 is significantly larger than the small ankyrins characterized here and does not have a distinct hydrophobic domain.
The ease with which the anti-p6 antibodies label the COOH-terminal sequence of the small ankyrins suggests that, if the hydrophobic NH2-terminal tail anchors these proteins to the sarcoplasmic reticulum membrane, then the hydrophilic COOH terminus extends into the myoplasm, where it could interact with other proteins. Interactions with protein ligands that are already assembled at the M and Z lines may help to localize the small ankyrins to nearby sites in the sarcoplasmic reticulum, thereby creating distinct membrane domains. Skelemin and desmin, for example, surround the myofibril at the M and Z lines, respectively (Granger and Lazarides, 1979; Price, 1987). Binding of the small ankyrins to these or other proteins at the M and Z lines would also serve to link the sarcoplasmic reticulum to the contractile apparatus, just as the membrane skeleton at the sarcolemma helps to link that membrane to the M and Z lines of superficial myofibrils (Porter et al., 1992; Vybiral et al., 1992). As a result, the sarcoplasmic reticulum would contain domains responsible for anchoring it to the contractile apparatus at distinct sites within each of the sarcomeres it surrounds. Such a linkage has obvious consequences for myofibrillar assembly and organization and for the stability of the SR during the contractile cycle. We are currently using cellular transfection with the hydrophilic portion of the p6 peptide to test this possibility.
Acknowledgments
We thank Andrea O'Neill and Wendy Resneck for their excellent technical support, and Dr. Paul Luther for his help in confocal microscopy. We also thank Dr. S. Hua and Dr. G. Inesi (University of Maryland at Baltimore) for their help with the purification of sarcoplasmic reticulum. Our research was supported by grants from the National Institutes of Health (NS17282 and HD16596 to R.J. Bloch; HL29305 to J.E. Barker) and from the Muscular Dystrophy Association (to R.J. Bloch).
Abbreviations used in this paper
- SERCA
sarcoplasmic-endoplasmic reticulum calcium ATPase
- SR
sarcoplasmic reticulum
Footnotes
Address all correspondence to Robert J. Bloch, Department of Physiology, University of Maryland School of Medicine, 660 W. Redwood St., Baltimore, MD 21201. Tel.: (410) 706-3020. Fax: (410) 706-2894. E-mail: rbloch@umabnet.ab.umd.edu
References
- Bennett V. Purification of an active proteolytic fragment of the membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1978;253:2292–2299. [PubMed] [Google Scholar]
- Bennett V, Davis J. Erythrocyte ankyrin: immunoreactive analogues are associated with mitotic structures in cultured cells and with the microtubules in brain. Proc Natl Acad Sci USA. 1981;78:7550–7554. doi: 10.1073/pnas.78.12.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Birkenmeier CS, White RA, Peters LL, Hall EJ, Lux SE, Barker JE. Complex patterns of sequence variation and multiple 5′ and 3′ ends are found among transcripts of the erythroid ankyrin gene. J Biol Chem. 1993;268:9533–9540. [PubMed] [Google Scholar]
- Bodine DM, Birkenmeier CS, Barker JE. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984;37:721–729. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Burnette WN. “Western Blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transfection of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Craig SW, Pardo JV. γ actin, spectrin, and intermediate filament proteins colocalize with vinculin at costameres, myofibril-to-sarcolemma attachment sites. Cell Motil. 1983;3:445–462. doi: 10.1002/cm.970030513. [DOI] [PubMed] [Google Scholar]
- Davis JH, Bennett V. Brain ankyrin, a membrane-associated protein with binding sites for spectrin, tubulin, and the cytoplasmic domain of the erythrocyte anion channel. J Biol Chem. 1984;259:13550–13559. [PubMed] [Google Scholar]
- Davis JQ, Bennett V. The anion exchanger and Na+,K+- ATPase interact with distinct sites on ankyrin in vitro assays. J Biol Chem. 1990;265:17252–17256. [PubMed] [Google Scholar]
- Davis LH, Lux SE, Bennett V. Mapping the ankyrin-binding site of the human erythrocyte anion exchanger. J Biol Chem. 1989;264:9665–9672. [PubMed] [Google Scholar]
- Davis LH, Davis JQ, Bennett V. Ankyrin regulation: an alternatively spliced segment of the regulatory domain functions as an intramolecular modulator. J Biol Chem. 1992;267:18966–18972. [PubMed] [Google Scholar]
- Devarajan P, Scaramunzzino DA, Morrow JS. Ankyrin binds to two distinct cytoplasmic domains of Na, K-ATPase α subunit. Proc Natl Acad Sci USA. 1994;91:2965–2969. doi: 10.1073/pnas.91.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P, Stabach PR, Mann AS, Ardito T, Kashgarian M, Morrow JS. Identification of a small cytoplasmic ankyrin (AnkG119) in kidney and muscle that binds β I σ * spectrin and associates with the Golgi apparatus. J Cell Biol. 1996;133:819–830. doi: 10.1083/jcb.133.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D, Bennett V. Polarized distribution of Mr210,000 and 190,000 analogs of erythrocyte ankyrin along the plasma membrane of transporting epithelia, neurons and photoreceptors. Eur J Cell Biol. 1987;43:479–486. [PubMed] [Google Scholar]
- Eletr S, Inesi G. Phase changes in the lipid moieties of sarcoplasmic reticulum membranes induced by temperature and protein conformational changes. Biochem Biophys Acta. 1972;282:174–179. doi: 10.1016/0005-2736(72)90062-4. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70:419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Ferguson C, Lakey A, Hutching SA, Butcher GW, Leonard KR, Bullard B. Cytoskeletal proteins of insect muscle: location of zeelins in Lethocerusflight and leg muscle. J Cell Sci. 1994;107:1115–1129. doi: 10.1242/jcs.107.5.1115. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Daniels MP. Distribution of Na+channels and ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43 kd protein. Neuron. 1989;3:163–175. doi: 10.1016/0896-6273(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Morton ME, Froehner SC, Daniels MP. Localization of the α1 and α2 subunits of the dihydropyridine receptor and ankyrin in skeletal muscle triads. Neuron. 1990;5:339–351. doi: 10.1016/0896-6273(90)90170-k. [DOI] [PubMed] [Google Scholar]
- Foletti D, Guerini D, Carafoli E. Subcellular targeting of the endoplasmic reticulum and plasma membrane Ca2+pumps: a study using recombinant chimeras. Fed Amer Soc Exper Biol J. 1995;9:670–680. doi: 10.1096/fasebj.9.8.7768360. [DOI] [PubMed] [Google Scholar]
- Frangioni JV, Beahm PH, Shifrin V, Jost CA, Neel BG. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68:545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong, C. 1994. The sarcoplasmic reticulum and the transverse tubules. In Myology. 2nd Ed. Vol. 1. A.G. Engel and C. Franzini-Armstrong, editors. 176–199.
- Froehner SC, Murnane AA, Tobler M, Peng HB, Sealock R. A postsynaptic Mr58,000 (58K) protein concentrated at acetylcholine receptor–rich sites in Torpedo electroplaques and skeletal muscle. J Cell Biol. 1987;104:1633–1646. doi: 10.1083/jcb.104.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos SD, Marchesi VT. The binding of vimentin to human erythrocyte membrane: a model system for the study of intermediate filament–membrane interactions. J Cell Biol. 1985;100:1955–1961. doi: 10.1083/jcb.100.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger BL, Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell. 1979;18:1053–1063. doi: 10.1016/0092-8674(79)90218-6. [DOI] [PubMed] [Google Scholar]
- Grove BK, Cerny L, Periard JC, Eppenberger HM. Myomesin and M-protein: expression of two proteins in pectoral muscle and during heart development. J Cell Biol. 1985;101:1413–1421. doi: 10.1083/jcb.101.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Watkins SC, Slayter HS, Kunkel LM. Detection of a specific isoform of α-actinin with antisera directed against dystrophin. J Cell Biol. 1989;108:503–510. doi: 10.1083/jcb.108.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GD, Davis RS, McNamee KC, Russell G, Goodwin D, Holborow EJ. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982;43:349–350. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Kennedy SP, Warren SL, Forget BG, Morrow JS. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid β-spectrin. J Cell Biol. 1991;115:267–277. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. AnkyrinG, a new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1994;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Kuminoto M, Otto E, Bennett V. A new 440-kD isoform is the major ankyrin in neonatal rat brain. J Cell Biol. 1991;115:1319–1331. doi: 10.1083/jcb.115.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Ahnert-Hilger G, Hartmann E, Wiedenmann B, Rapoport T. Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum. Eur Mol Biol Org J. 1995;14:217–223. doi: 10.1002/j.1460-2075.1995.tb06994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structure proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert S, Bennett V. Postmitotic expression of ankyrinR and βRspectrin in discrete neuronal populations of the rat brain. J Neurosci. 1993;13:3725–3735. doi: 10.1523/JNEUROSCI.13-09-03725.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Yu H, Prchal JT, Lawler J, Ruff P, Speicher D, Cheung MC, Kan YW, Palek J. cDNA sequence for human erythrocyte ankyrin. Proc Natl Acad Sci USA. 1990;87:1730–1734. doi: 10.1073/pnas.87.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux, S.E., and J. Palek. 1995. Disorders of the red cell membrane. In Blood, Principles and Practice of Hematology. R.I. Handlin, S.E. Lux, and T.P. Stossel, editors. J.P. Lippincott Co., Philadelphia. 1701–1818.
- Lux SE, John KM, Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissuedifferentiation and cell-cycle control proteins. Nature (Lond) 1990;344:36–43. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- March SC, Patrikh L, Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974;60:149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Mitoma J-y, Ito A. The carboxy-terminal 10 amino acids of cytochrome b5 are necessary for its targeting to the endoplasmic reticulum. Eur Mol Biol Org J. 1992;11:4197–4203. doi: 10.1002/j.1460-2075.1992.tb05513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Lazarides E. Goblin (ankyrin) in striated muscle: identification of the potential membrane receptor for erythroid spectrin in muscle cells. Proc Natl Acad Sci USA. 1984;81:3292–3296. doi: 10.1073/pnas.81.11.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+/K+) ATPase and implication for the organization of membrane domains in polarized cells. Nature (Lond) 1987;328:533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- Otto E, Kunimoto M, McLaughlin T, Bennett V. Isolation and characterization of cDNAs encoding human brain ankyrins reveal a family of alternatively spliced genes. J Cell Biol. 1991;114:241–253. doi: 10.1083/jcb.114.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA. 1983;80:1008–1012. doi: 10.1073/pnas.80.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters LL, Lux SE. Ankyrins: structure and function in normal cells and hereditary spherocytes. Sem Hematol. 1993;30:85–118. [PubMed] [Google Scholar]
- Peters LL, Birkenmeier CS, Bronson RT, White RA, Lux SE, Otto E, Bennett V, Higgins A, Barker JE. Purkinje cell degeneration associated with erythroid ankyrin deficiency in nb/nbmice. J Cell Biol. 1991;114:1233–1241. doi: 10.1083/jcb.114.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters LL, Turtzo C, Birkenmeier CS, Barker JE. Distinct fetal Ank-1 and Ank-2 related proteins and mRNAs in normal and nb/nbmice. Blood. 1993;81:2144–2149. [PubMed] [Google Scholar]
- Peters LL, John KM, Lu FM, Eicher EM, Higgins A, Yialamas M, Turtzo LC, Otsuka AJ, Lux SE. Ank3(epithelial ankyrin), a widely distributed new member of the ankyrin gene family and the major ankyrin in kidney, is expressed in alternatively spliced forms, including forms that lack the repeat domain. J Cell Biol. 1995;130:313–330. doi: 10.1083/jcb.130.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter GA, Dmytrenko GM, Winkelmann JC, Bloch RJ. Dystrophin colocalizes with β-spectrin in distinct subsarcolemmal domains in mammalian skeletal muscle. J Cell Biol. 1992;117:997–1005. doi: 10.1083/jcb.117.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MG. Skelemins: cytoskeletal proteins located at the periphery of M-discs in mammalian striated muscle. J Cell Biol. 1987;104:1325–1336. doi: 10.1083/jcb.104.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury GM, Bullard B. New proline-rich proteins in isolated insect Z-discs. Biochem J. 1980;191:333–339. doi: 10.1042/bj1910333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan Y, Elmer L, Davis J. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature (Lond) 1988;333:177–180. doi: 10.1038/333177a0. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y, Lewallen M, Angelides KJ. Mapping the binding site on ankyrin for the voltage-dependent sodium channel from brain. J Biol Chem. 1992;267:7483–7489. [PubMed] [Google Scholar]
- Vigoreaux JO, Saide JD, Valgerisdottic K, Pardue ML. Flightin: a novel myofibrillar protein of Drosophilastretch-activated muscle. J Cell Biol. 1993;121:587–598. doi: 10.1083/jcb.121.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A, Podini P, Nori A, Panzeri MC, Martini A, Meldolesi J, Volpe P. The endoplasmic reticulum-sarcoplasmic reticulum connection. II. Postnatal differentiation of the sarcoplasmic reticulum in skeletal muscle fibers. Exp Cell Res. 1993;209:140–148. doi: 10.1006/excr.1993.1294. [DOI] [PubMed] [Google Scholar]
- Vybiral T, Winkelmann JC, Roberts R, Joe E-H, Casey DL, Williams JK, Epstein HF. Human cardiac and skeletal muscle spectrins: differential expression and localization. Cell Motil Cytoskel. 1992;21:293–304. doi: 10.1002/cm.970210405. [DOI] [PubMed] [Google Scholar]
- Weaver DC, Pasternack GR, Marchesi VT. The structural basis of ankyrin function. II. Identification of two functional domains. J Biol Chem. 1984;259:6170–6175. [PubMed] [Google Scholar]
- White RA, Birkenmeier CS, Lux SE, Barker JE. Ankyrin and the hemolytic anemia mutation, nb, map to mouse chromosome 8: presence of the nballele is associated with a truncated erythrocyte ankyrin. Proc Natl Acad Sci USA. 1990;87:3117–3121. doi: 10.1073/pnas.87.8.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RA, Birkenmeier CS, Peters LL, Barker JE, Lux SE. Murine erythrocyte ankyrin cDNA: highly conserved regions of the regulatory domain. Mammal Genome. 1992;3:281–285. doi: 10.1007/BF00292156. [DOI] [PubMed] [Google Scholar]