Abstract
High-affinity IL2 receptors consist of three components, the α, β, and γ chains that are associated in a noncovalent manner. Both the β and γ chains belong to the cytokine receptor superfamily. Interleukin 2 (IL2) binds to high-affinity receptors on the cell surface and IL2-receptor complexes are internalized. After endocytosis, the components of this multimolecular receptor have different intracellular fates: one of the chains, α, recycles to the plasma membrane, while the others, β and γ, are routed towards late endocytic compartments and are degraded. We show here that the cytosolic domain of the β chain contains a 10–amino acid sequence which codes for a sorting signal. When transferred to a normally recycling receptor, this sequence diverts it from recycling. The structure of a 17–amino acid segment of the β chain including this sequence has been studied by nuclear magnetic resonance and circular dichroism spectroscopy, which revealed that the 10 amino acids corresponding to the sorting signal form an amphipathic α helix. This work thus describes a novel, highly structured signal, which is sufficient for sorting towards degradation compartments after endocytosis.
During receptor-mediated endocytosis, receptors are transported from one membrane compartment to another. From each compartment, they can be routed to different destinations, and intracellular transport therefore requires sorting (reviewed in references 18, 54). The best documented sorting is the one which functions at the plasma membrane to concentrate receptors in clathrin-coated pits. It involves internalization signals, short peptides in the cytosolic part of receptors which have been classified in two groups, a tyrosine based motif and a di-leucine based motif (reviewed in reference 49).
After internalization from the plasma membrane, receptors rapidly reach early/recycling endosomes. From there, some receptors recycle back to the cell surface while others are sorted to different destinations (46). Recycling to the plasma membrane is generally considered as the default pathway (34, 54). For receptors to exit from the recycling pathway and move to other intracellular destinations (e.g., in most cases towards late endosomes and lysosomes, or to the trans-Golgi network or major histocompatibility complex class II compartments) or to new domains on the cell surface (as in transcytosis) requires selective routing and depends upon the possession of additional, specific sorting signals.
Although many internalization signals have now been described, much less is known about sorting steps from intracellular endocytic organelles. Receptors which transport nutrients to the cells, such as transferrin or low density lipoprotein receptors, recycle to the plasma membrane, while receptors for hormones and growth factors are often degraded after internalization. Degradation causes receptor down modulation and is important for arresting the cell response to growth factors and hormones. The mechanism by which these receptors are directed towards degradation is unknown.
Sequences which are necessary for diverting lysosomal membrane glycoproteins to lysosomes have been described by mutation analysis (reviewed in reference 49). They belong to the tyrosine or di-leucine families of signals described for clathrin-coated pit endocytosis (20, 24, 33, 43, 59). After synthesis, lysosomal proteins are sorted from the trans-Golgi network to endosomes and lysosomes (20, 24). Alternatively, some lysosomal proteins are exocytosed and then internalized by receptor-mediated endocytosis (33, 43, 59); they are then sorted from endosomes to lysosomes. In these cases, the same mutations usually affect both endocytosis and lysosomal targeting, and the distance between the signal and the plasma membrane may also be important for sorting (47). By mutational analysis, sequences necessary for internalization and degradation of nonlysosomal membrane proteins have been reported (2, 29, 32, 41, 55). However, in all of these cases, it has not been shown that a short sequence is sufficient to serve as a degradation signal by transferring it to a normally recycling receptor, which would then become degraded as a result. Only one case of a membrane molecule with a short sequence sufficient for lysosomal degradation after endocytosis has been reported, that of P-selectin. The cytosolic tail of this cell adhesion molecule contains a degradation signal which does not match any of the signals described so far (15).
The cytokine interleukin 2 (IL2)1 is produced by activated helper T lymphocytes and stimulates proliferation and effector functions of a variety of cells of the immune system (36). High-affinity IL2 receptors (kD ≈ 10 − 100 pM) consist of three distinct components, the α, β, and γ chains, that are associated in a noncovalent manner (36). Both the β and γ chains, but not the α chain, belong to the cytokine receptor superfamily (5). This hematopoietic cytokine receptor family includes receptors such as the IL3, IL4, IL5, IL6, IL7, IL9, and IL15 receptors, the erythropoietin receptor, the granulocyte colony-stimulating factor receptor, the granulocyte-macrophage colony-stimulating factor receptor, and the leukemia inhibitory factor receptor. This family also includes receptor proteins for factors that are believed to function normally outside the immune and hematopoietic system, i.e., growth hormone, prolactin, and ciliary neurotrophic factor. Many receptor subfamily members share at least one component: thus, the receptors for IL2, 4, 7, 9, and 15 have a common γ chain, and the receptors for IL2 and IL15 share the β chain (reviewed in reference 52). Patients suffering from X-linked severe combined immunodeficiency have mutations in the gene encoding the IL2 receptor γ chain (9, 10, 40).
One of the early events following IL2 binding to high-affinity receptors on the cell surface is the internalization of IL2 receptor complexes (12, 51). After endocytosis, the components of this multimolecular receptor have different intracellular fates: one of the chains, α, recycles to the plasma membrane, while the others, β and γ, are routed to late endocytic compartments (23).
This multimeric receptor represents a particularly interesting model for studying sorting signals during receptormediated endocytosis. We have previously shown that the β chain is internalized and not recycled when expressed without the α or γ chain (22). In this paper, we show that the 27 amino acids adjacent to the membrane in the cytosolic tail of the β chain are sufficient for its endocytosis and degradation. In this sequence, a 10–amino acid peptide encodes a signal for sorting towards degradation compartments.
Materials and Methods
Cells, Monoclonal Antibodies, and Reagents
K562, a human erythroleukemia cell line, was grown in suspension in RPMI 1640, 10% decomplemented FCS, supplemented with 2 mM l-glutamine. Stably transfected K562 cells were grown in the same medium supplemented with 1.5 mg/ml G418. HeLa cells were grown in DMEM, 10% decomplemented FCS, supplemented with 2 mM l-glutamine.
Monoclonal antibodies 7G7B6 (IgG2a) and OKT9 (IgG1), directed against the α chain of the IL2 receptor and the transferrin receptor, respectively, were obtained from the Amer. Tissue Culture Collection (Rockville, MD) (48, 53). Monoclonal antibodies 341 and 561 were kind gifts from Dr. R. Robb (Dupont Merck Pharmaceutical Co., Wilmington, DE) (56). FITC-conjugated anti-murine IgG1 antibodies and Texas red– conjugated anti-murine IgG2a antibodies were obtained from Southern Biotechnology (Birmingham, AL).
Saponin and cycloheximide were obtained from Sigma Chem. Co. (St. Louis, MO). 2,2,2-Trifluoroethyl alcohol-d2,OH (99 atom %D) and deuterium oxide (99.9 atom %) were purchased from Solvants Documentation Synthèse (S.D.S., France).
Plasmids
All constructs were prepared in the NT vector, a kind gift of Dr. C. Bonnerot (Institut Curie, Paris, France). The plasmid pdKCRβ, coding for the IL2R β chain was kindly provided by Dr. T. Kono (Osaka University, Japan) (37). The truncated forms of βn were generated by PCR by insertion of a stop codon after the n th amino acid of the cytosolic part of the protein (assuming that Asparagine is the first cytosolic amino acid), and were cloned into the Not1/Sac1 sites of NT by standard techniques.
The plasmid T-XO, a kind gift of Dr. P. Cosson (Basel, Switzerland), is a modified version of IL2Rα cDNA in the pCDM8 vector. In this plasmid, the 3′ side of the sequence is modified to create a HindIII/XbaI cloning cassette (ACAATCTAG to ACAATCCAAGCTTCCTCCTGAGTAGCGTCTAGA). This modification adds four amino acids (QASS) to the wild-type IL2Rα chain, but its cellular behavior is not modified, and therefore we will refer to this protein as “α.”
This plasmid was further modified by inserting a sequence coding for the transferrin receptor YTRF internalization signal, in the cytosolic part of α. In this T-XOY construct, the 3′ side of the sequence AAGAGTAGAAGAACAATCCAAGCTTCCTCCTGA was modified to AAGAGTGAACCATTGTCATATACCCGGTTCCAAGCTTCC T CC - TGA by PCR. The COOH terminus of the T-XOY protein is KSEPLSYTRFQASS, instead of KSRRTIQASS for T-XO.
All T-XOβnm and T-XOYβnm constructs were subcloned in the Not1/ Sac1 cloning sites of NT. The corresponding constructs were named αβnm and αYβnm, and were used for stable transfections in K562 cells.
Cell Transfection
All kinetics and half-life analyses described here have been performed in stably transfected K562 cells. To generate stable transfectants, 7 × 106 K562 cells were washed once in DMEM, 4.5 g/l glucose and resuspended in 800 μl of the same medium, with 20 μg of the plasmid of interest. Electroporation was performed using the Easyject electroporator (Eurogentec, Seraing, Belgium) with simple pulse, 240 V, 1,500 μF. Selection with 1.5 mg/ml G418 (Geneticin, Gibco/BRL, Gaithersburg, MD) was initiated 2 d after transfection, and the cells were cloned in 96-well dishes. G418-resistant clones were assayed for expression by flow cytometry using anti-α (7G7B6) or anti-β (341) antibodies. The expression levels of recombinant proteins in all clones tested were the same or less than their normal level in activated lymphocytes.
HeLa cells were used for transient expression of the constructs. Transfections were performed using the calcium phosphate method as described in reference 1 using 5 μg of DNA per transfection.
Endocytosis of Radiolabeled Antibodies
7G7B6 and 561 antibodies were radiolabeled with 125I by the chloramine T method to a specific activity of 2–10 μCi/μg. For endocytosis experiments, 2 × 106 cells were incubated in 100 μl RPMI-Hepes, pH 7.2, 1 mg/ml BSA, at 37°C, and 1–5 nM 125I-labeled antibody was added. After incubation at 37°C for the indicated times, the cells were rapidly cooled to 4°C and washed twice. Cell surface–associated radioactive ligand was then removed by two successive acid pH treatments (2 min at pH 2.0) at 4°C as previously described (11). Nonspecific binding, measured for each ligand by adding a 100-fold excess of the same unlabeled ligand, was <5% and was substracted. The efficiency of removal of cell surface–associated ligands by acid pH washes was measured for each ligand and was >95%.
In all figures, the ratio of intracellular to total associated 125I-labeled antibody is represented. All experiments were done with different clones expressing the same construct. One representative experiment is shown.
Cell Surface Half-life Measurement
To measure the half-life at the cell surface of the different βn, αβnm or αYβnm constructs, cells were incubated with cycloheximide to prevent the synthesis of new receptors. After different times of incubation at 37°C in culture medium with 50 μM cycloheximide, the cells were cooled to 4°C, and cell surface expression of the constructs was assayed by flow cytometry as described (21). Time zero on the graph corresponds to a 30-min incubation in cycloheximide, which is the time required for a newly synthesized IL2 receptor to reach the cell surface (11). All experiments were done in triplicate with different clones expressing the same construct. One representative experiment is shown.
Immunofluorescence and Confocal Microscopy
Either stably transfected K562 cells, or HeLa cells grown on coverslips and transfected 2 d before with the plasmid of interest, were used. The cells were incubated for the indicated times at 37°C with 7G7B6 mAb, before being washed in PBS at 4°C and fixed in 3.7% paraformaldehyde and 0.03 M sucrose for 30 min at 4°C. Subsequent steps were performed at room temperature. The cells were washed once in PBS, and after quenching for 10 min in 50 mM NH4Cl in PBS, the cells were washed once in PBS supplemented with 1 mg/ml BSA. Cells were then incubated, when indicated, with OKT9 mAb in permeabilization buffer (PBS with 1 mg/ml BSA and 0.05% saponin) for 20 min at 37°C. After two washes in the permeabilization buffer, the presence of antibodies was revealed by incubating the cells for 20 min at 37°C in permeabilization buffer containing labeled second antibodies. For 7G7B6 and OKT9, the second antibodies were Texas red–labeled anti-IgG2a (1/250) and FITC-labeled anti-IgG1 (1/ 100), respectively. Washes, sample mounting, and confocal microscopy were performed as described in reference 23.
No immunofluorescence staining was ever observed when second antibodies were used without the first antibody or with an irrelevant first antibody.
Metabolic Labeling
For pulse metabolic labeling with 35S-amino acids, 35 × 106 cells were washed with PBS, then incubated for 1 h in DMEM lacking methionine and cysteine and containing 2% dialyzed FCS. Cells were labeled for 2 h in the same medium containing 200 μCi/ml 35S-amino acid mixture (Promix, Amersham, England), then washed in PBS. After 75 min incubation at 37°C in complete medium, cells were harvested at 30-min intervals, washed in PBS, pelleted by centrifugation, and kept frozen at −20°C before analysis by immunoprecipitation.
Cell Iodination
YT cells (2 × 107) washed in PBS and resuspended in PBS, pH 7.3, 1 mM Ca2+, 1 mM Mg2+, were surface labeled using the lactoperoxidase method with 1 mCi Na125I (21). After iodination, the cells were washed two times in culture medium and then kept in a 37°C incubator. After 30 min incubation, cells were harvested at 30-min intervals, washed in PBS, pelleted by centrifugation and kept frozen at −20°C before analysis by immunoprecipitation.
Immunoprecipitation and Gel Analysis
Cells were lysed for 30 min at 4°C in lysis buffer (1% NP-40, 150 mM NaCl, 10 mM Tris HCl, pH 8.0) complemented with 1 mM EDTA, 50 mM NaF, 1 mM NaVO4, 10 μg/ml leupeptin, 20 μg/ml aprotinin, and 2 mM PMSF. Insoluble material was pelleted at 15,000 g for 30 min, and the supernatant was then precleared for 60 min at 4°C with protein A Sepharose CL-4B (Pharmacia, Sweden) before being immunoprecipitated overnight at 4°C with relevant first antibodies, and protein A–Sepharose coupled with anti–mouse antibody (Biosys, France). First antibodies were anti-IL2Rβ mAb 341 and 561 for the measurement of β half-life after metabolic labeling, and anti-IL2Rα mAb 7G7B6 and anti-transferrin receptor mAb OKT9 for the measurement of αβ18–27 half-life after iodination. The Sepharose beads were then washed three times in 1% NP-40, 0.5 M NaCl, 10 mM Tris HCl, pH 8.0, and once in 10 mM Tris HCl, pH 8.0. Bound proteins were eluted into electrophoresis sample buffer (60 mM Tris HCl, pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol) before analysis by SDS-PAGE. Gels were dried and radioactivity in the gel bands was quantitated using a phosphorimager and ImageQuaNT software (Molecular Dynamics, Inc., Sunnyvale, CA). Gels were subsequently exposed to Hyperfilm-MP (Amersham, England) at −80°C.
Peptide Synthesis
The peptides were synthesized by the Merrifield solid-phase method (35) using a 430 A synthesizer and a Pam (Boc, t-butoxycarbonyl; Pam, acetamidomethyl) resin (Applied Biosystems, Foster City, CA). The final purity (99.5%) of the peptides was checked by analytical reverse-phase HPLC.
Circular Dichroism Experiments
Circular dichroism (CD) spectra of the wild-type peptide were recorded at 25°C on a Jobin Yvon CD6 dichrograph with a 0.2-mm path-length cell. The peptide was first dissolved in water at pH 5.3. The solution was then diluted in trifluoroethanol (TFE)/water mixtures varying from 0 to 95% TFE (V/V). Peptide concentration was 0.32 mg/ml (0.2 mM) as determined from amino acid analysis.
Proton Nuclear Magnetic Resonance Experiments
The samples were prepared as 6 mM peptide solutions in water at pH 3.4 and 5.3 for BETApep and 5.3 for P20Lpep, respectively. The solutions were then lyophilized and the samples solubilized in either 90%H2O/ 10%D2O or in 25% and 40%TFE-d2/H2O.
All nuclear magnetic resonance (NMR) measurements were obtained on a Varian Unity 500 spectrometer operating at a proton frequency of 500 MHz and interfaced to a Sun Sparc 1+ station. The sweep width was 5,000 Hz. Spectra in water were recorded at 0°C and 25°C, while spectra in TFE:water mixtures were recorded at 25°C. Spectra in water are referenced to external trimethylsilyl-3-propionic acid-d4 2,2,3,3 sodium salt (TMSP) and in TFE to the internal methylene resonance of the solvent at 3.88 ppm relative to tetramethylsilane (TMS). The OH resonance of water or of TFE-d2 were suppressed by selective irradiation during the relaxation delay and, in the case of NOESY and ROESY spectra, during the mixing time as well. All 2D-data were collected in the phase-sensitive mode using the States-Haberkorn method (50). A total of 512 FIDs of 2K complex data points were collected in t2 with 32 scans per increment and zero-filling was applied in both dimensions before Fourier transformation to form a matrix of 4K × 2K. These data were then processed with shifted sine-bell window functions in both dimensions.
The phase-sensitive two-dimensional double-quantum-filtered correlated spectroscopy (DQF-COSY) (44) and total correlated spectroscopy (clean-TOCSY) (16) with 80-ms mixing time were used for spin systems assignment. Nuclear Overhauser two-dimensional experiments (NOESY) (30) and rotating frame nuclear Overhauser two-dimensional experiments (ROESY) (6) were recorded for sequential assignment and structure determination using several mixing times (100, 200, 300 ms) for the NOESY and 400 ms for the ROESY.
3JNH-Hα coupling constants were measured from one dimensional spectra recorded with a digital resolution of 0.15 Hz/point at 0°C and 25°C.
Results
The IL2Rβ Chain Is Degraded after Endocytosis
We have previously shown that the β chain of IL2 receptor is internalized and has a half-life at the cell surface of ∼110 min, in the absence of IL2, in three different lymphocytic cell lines (YT, IARC 301.5, CIAC) (22). At steady state, the total number of cell surface receptors results from the balance between receptor biosynthesis and endocytosis. After endocytosis, most membrane molecules are recycled back to the cell surface or degraded. The level of expression at the cell surface of a protein that is entirely recycled after internalization remains the same, even when protein synthesis is inhibited. On the other hand, the level of expression at the cell surface of a protein that is not recycled after internalization decreases with time when protein synthesis is inhibited. Therefore, the short half-life of IL2Rβ shows that it is not recycled after endocytosis. Its localization in late endocytic compartments suggests that it is degraded (23).
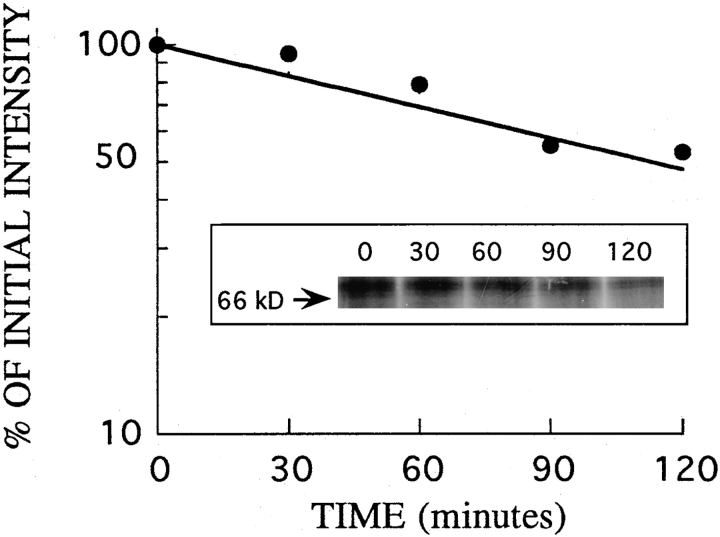
We studied the turnover of IL2Rβ in the YT cell line, which has been extensively used to study IL2 receptors. The rate of turnover of IL2Rβ was measured in these cells by pulse-chase metabolic labeling and immunoprecipitation. Cells were labeled with 35S-amino acids and incubated in chase medium at 37°C for different times as described in the Materials and Methods section. After a 75-min incubation to allow export to the plasma membrane, cells were harvested at 30-min intervals. Proteins were quantitatively immunoprecipitated from cell lysates, the precipitates resolved on SDS-polyacrylamide gels, and the radioactivity in the IL2Rβ band quantitated by phosphorimager analysis. An autoradiograph of a typical experiment is shown in Fig. 1. Analysis of the turnover of IL2Rβ showed that it was degraded with a half-life of 2 h. Immunoprecipitation of the transferrin receptor in these experiments showed that it was stable, as expected for this recycling receptor (not shown). The half-life of IL2Rβ measured here is the same as its half-life on the cell surface previously measured by flow cytometry (22). Therefore the loss of β chain from the cell surface can be equated with degradation. Since measuring the loss of receptors from the cell surface is a very sensitive, simple, and quantitative method, it was used to probe for receptor turnover in subsequent studies.
Figure 1.
Turnover of IL2Rβ in YT cells. YT cells were pulse labeled with 35S-amino acids for 2 h, and then washed and incubated in chase medium. Cells were harvested beginning 75 min after the end of the pulse (t = 0), then at the indicated intervals. IL2Rβ was immunoprecipitated from detergent lysates of the cells and separated by SDS-PAGE (inset). Radioactivity in the bands was quantitated by phosphorimager analysis. The values in the graph represent the percentage of IL2Rβ detected in the gel at different chase times relative to that at time zero.
The First 27 Cytosolic Amino Acids of the IL2Rβ Chain Are Sufficient for Its Endocytosis and for Its Short Half-Life on the Cell Surface
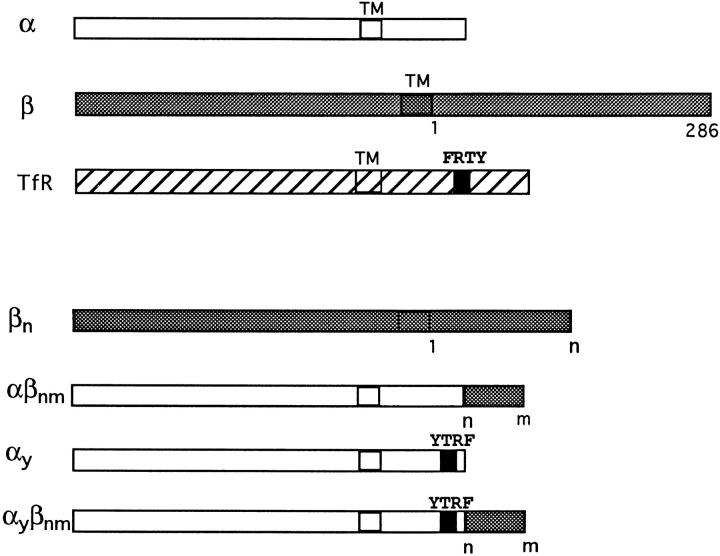
The IL2Rβ chain is a type I transmembrane protein, with 286 amino acids in its cytosolic part. We had previously shown that the β chain by itself, in the absence of the α and γ chains of the high-affinity IL2 receptor, is internalized and has a short half-life on the cell surface (22). To understand the molecular basis for the degradation of this protein, we constructed a truncated form of β, with only the 27 amino acids adjacent to the membrane of the cytosolic region (Fig. 2). This β27 construct was transfected in K562 cells, and its behavior was analyzed in several stably transfected clones.
Figure 2.
Schematic representation of the constructs used in this work. In all constructs, n and m designate amino acids at position n and m, respectively, after the transmembrane domain of β, assuming that Asp is the first cytosolic amino acid. The first 27 cytosolic amino acids of β starting from the membrane are the following: NCRNTGPWLKKVLKCNTPDPSKFFSQL. Amino acids 18–27 are underlined. TM, transmembrane domain; TfR, transferrin receptor.
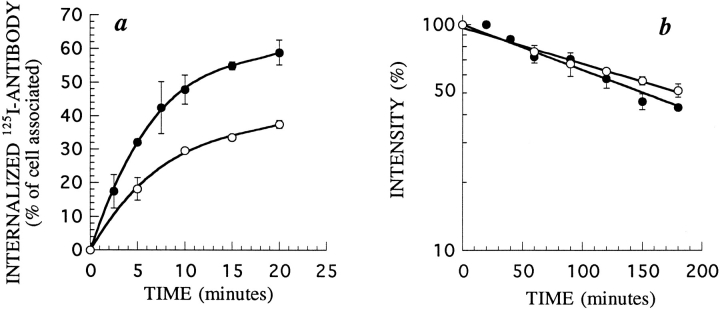
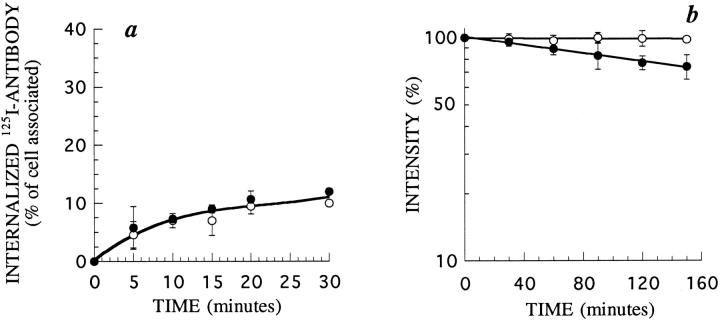
First, we measured the rate of endocytosis of β27 and βwt using radiolabeled anti-β mAb 561 (Fig. 3 a). We had previously shown that this antibody does not modify the kinetics of entry and degradation of the IL2 receptor (51). Both forms of the protein were internalized efficiently and β27 was even internalized faster than βwt. This difference may be due to the presence of negative signals for endocytosis in the βwt chain, as has already been described in other receptors (25). It is also possible that an internalization signal is better presented in the 27–amino acid tail than in the wild-type form of β. We concluded that the first 27 amino acids of β are sufficient to promote endocytosis.
Figure 3.
Endocytosis and cell surface half-life of β27 and βwt in K562 cells. (a) Kinetics of 125I-labeled mAb 561 internalization in cells stably transfected with βwt (○) or β27 (•). The antibody was added to the cells at 37°C. After further incubation for the indicated times, cells were cooled to 4°C and washed, and the amount of internalized antibody was measured. (b) Half-life of βwt (○) and β27 (•). Cell surface expression of β on cells treated for different times with 50 μM of cycloheximide was assessed by flow cytometry using mAb 341. Times indicated are after a 30-min preincubation in cycloheximide. In each case, one representative experiment out of three is shown. Error bars indicate standard deviations for averages of three or more experiments.
We also measured the half-life of β27 at the cell surface. Stably transfected β27 or βwt cells were incubated with the protein synthesis inhibitor cycloheximide for various times. The surface expression of the chain was then probed with anti-IL2Rβ mAb by flow cytometry, as described (23). The half-lives of both forms of β were the same, ∼150 min (Fig. 3 b). This represents a short half-life for a membrane receptor, and suggests that these proteins are degraded after endocytosis.
The 27th Amino Acid of β Is Necessary for its Short Half-Life on the Cell Surface, but Not for Its Endocytosis
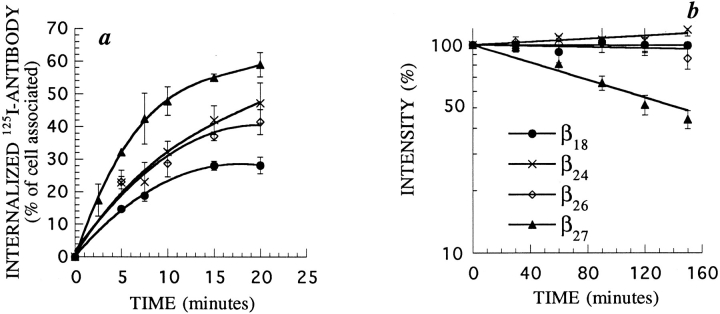
To further characterize the sequence responsible for β27 degradation, we constructed other truncated forms of β, with 18, 24, or 26 cytosolic amino acids (β18, β24, or β26, respectively). When protein synthesis was inhibited in stably transfected K562 cells, all these mutants were still expressed very stably at the cell surface (Fig. 4 b). This was not due to a defect in internalization, as all forms were internalized (Fig. 4 a). Internalization of β26 for example, is slower than that of β27, but is still very efficient, about as fast as that of βwt. Such a long half-life, associated with rapid constitutive internalization, indicates that the proteins are very efficiently recycled to the plasma membrane after internalization. Thus, all forms of β with a cytosolic tail shorter than 27 amino acids are recycled to the plasma membrane. The deletion of Leu27 had a dramatic effect on the half-life, while it had only a small effect on endocytosis of the protein. Larger truncations, to Pro18, also led to a progressive loss of endocytosis (Fig. 4 a). This region of β therefore seems to be important for its endocytosis and intracellular sorting, and we decided to analyze its potential structure.
Figure 4.
Endocytosis and cell surface half-life of truncated forms of β in K562 cells. (a) Kinetics of 125I-labeled mAb 561 internalization in cells stably transfected with different truncated forms of β, as indicated. (b) Half-life of these proteins. Experiments were performed as described in Fig 3. In each case, one representative experiment out of three is shown. Error bars indicate standard deviations for averages of three or more experiments.
The Sorting Signal of β27 Adopts a Turn-Helix Structure
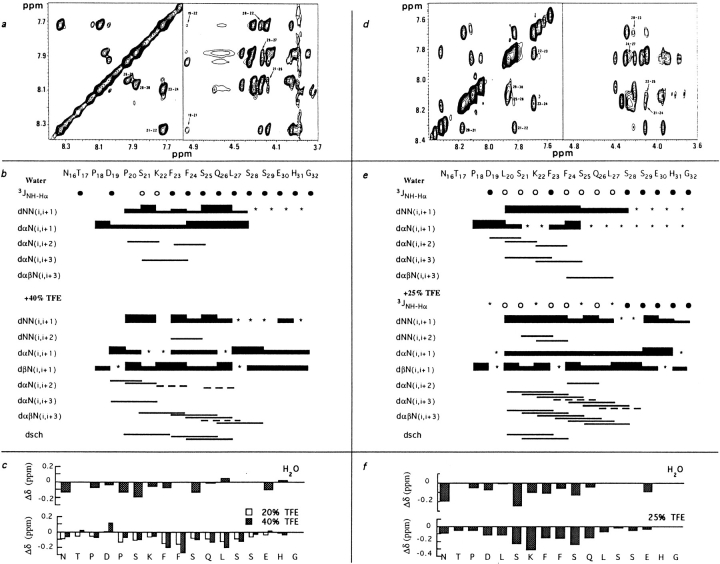
A peptide encompassing amino acids 16 to 32 of β was synthesized and will be called BETApep. NMR analysis of this peptide in solution in water at pH 5.3 and 3.4 was performed. At the two pH values, the presence of NHiNHi+1, αCHi-NHi+2, and αCHi-NHi+3 dipolar connectivities indicates turn-like structures in the 20–27 fragment (Fig. 5). The Pro20Hα-Lys22NH and the Pro20Hδ-Ser21NH NOE connectivities as well as the fact that the trans conformation was the major one for both prolines, suggest that the Asp19-Lys22 fragment forms a type I or type I′ β turn (13). The small value observed for the Ser21 3JNH-Hα would be in favor of a type I′ β turn rather than a type I β turn (Fig. 5 b). The possibility that a secondary structure might arise through aggregation was ruled out by running a 10fold diluted sample: identical chemical shifts were observed.
Figure 5.
NMR analysis of BETApep and P20Lpep. (Top) Sections of a two-dimensional NOESY spectrum at 25°C of BETApep in 40% TFE (a) and of P20Lpep in 25% TFE (d). Sequential amide NH-NH NOE are indicated by residue number (left part of a and d). Cross-peaks arising from medium range NOE NH-Hα (i, i + 2) and (i, i + 3) are indicated by an arrow and residue numbers (right part of a and d). (Middle) Summary of 1H-1H NOE connectivities for BETApep (b) and P20Lpep (e) in 40% and 25% TFE, respectively. The intensity of NOE cross-peaks is indicated by the thickness of the lines, and correlations close to the diagonal and ambiguous crosspeaks resulting from overlapping are indicated by an asterisk or a dashed line (c). Coupling constants are labeled as follows: • 3JNH-Hα > 6Hz, ○ 3JNH-Hα ⩽ 6Hz. (Bottom) Changes in the chemical shifts (Δδ) of the CHα protons of BETApep (c) or P20Lpep (f) in water or upon addition of TFE. Hα induced chemical shifts are given in water as compared to random coil values, and in TFE as compared to values observed in water.
The presence of molecules in an ordered conformation is confirmed by other NMR parameters, such as chemical shift and temperature coefficients. Alpha protons displayed slight upfield shifts throughout the sequence Pro18 to Ser25 with the exception of the Phe24 Hα proton, probably due to a Phe23 ring current effect (Fig. 5 c). The chemical shifts of all amide protons varied linearly with the temperature between 0°C and 40°C, implying no conformational changes. The temperature coefficients (Δδ/δT) obtained in aqueous solution vary between 4 and 8 × 10−3 ppm/°C. The lowest value was observed for Glu30 (4 × 10−3 ppm/°C), while intermediate values (4.8 to 6 × 10−3 ppm/°C) were obtained for the amide protons of fragment Ser21 to Gln26, indicative of a partial shielding of these protons from the solvent, probably through the formation of a hydrogen bond. Finally, these NMR studies in water strongly suggest that, at 25°C, BETApep adopts a turn between residues Asp19 and Lys22 and a nascent helix from Phe23 to Leu27.
To better define the structure, spectra were obtained in a TFE/water mixture, a mixed solvent known to increase the population of existing folded conformations (39). Indeed, the CD spectrum of BETApep in water displayed weak negative ellipticities at 220 nm and 199 nm, together with a stronger positive ellipticity at 193 nm, suggesting that a small population of BETApep molecules are in a helical conformation, in agreement with the NMR measurements. As the percentage of TFE increased, these spectra exhibited characteristic features of an α-helical structure.
NMR spectra were obtained in 20% and 40% TFE/water solutions. Addition of TFE induced substantial shifts of the proton resonances. In fragment 20 to 28, the peptide Hα resonances shifted upfield as compared to their position in water (Fig. 5 c). The induced shifts of Hα protons show that, in TFE/water solution, residues 20 to 28 form an α helix (60).
The various connectivities observed in the NOESY spectra for the peptide in water and in the TFE/water mixture are presented in Fig. 5. The NHi-NHi+1 interactions and the large number of medium range NOEs seen in segment Pro20 to Ser29 show that these residues are part of a stable α-helical structure. In conclusion, in the presence of TFE, residues 19 to 29 of BETApep form an α helix.
Disruption in the Turn at the Beginning of the Sorting Signal Does Not Affect Receptor Trafficking
In aqueous solution at pH 5.4 two structural elements, a type I′ β turn for fragment Asp19 to Lys22 and a nascent helix for fragment Phe23 to Leu27 were observed for the peptide. To elucidate the relative importance of these two structural elements in the biological function, a peptide called P20Lpep was synthetized, in which Pro20 was replaced by a leucine to remove the turn. The structural analysis was done in the same conditions with P20Lpep as with BETApep, and similar results were obtained, except that the turn characterized in BETApep was absent from P20Lpep. In water, a nascent helix was observed throughout fragment Leu20 to Ser28. This helix was stabilized in the presence of TFE (Fig. 5, d, e, and f).
A mutation replacing Pro20 by Leu was introduced in the β27 construct and the behavior of this mutant was analyzed after transfection in K562 cells. The kinetics of endocytosis and the half-life on the cell surface of this mutant were the same as those of β27 (not shown). Therefore, disruption of the β turn does not modify the sorting of β27.
Amino Acids 18–27 of β Divert from Recycling a Receptor Internalized Via Clathrin-coated Pits
If this nascent helix is responsible for the sorting of β27, and if it can act more or less independently of its location in the protein, then it might act as a sorting signal that could be transferred into another internalized membrane protein and remain active. The best characterized mechanism for gaining entry into the cell is the clathrin-coated vesicle one (reviewed in reference 45). Membrane receptors which carry an internalization signal in their cytosolic domain are concentrated in the clathrin-coated regions of the membrane. Eventually, clathrin-coated pits invaginate until a closed clathrin-coated vesicle is formed. This mechanism of internalization is very efficient and is used by many membrane proteins, such as the transferrin receptor. The internalization signal of the transferrin receptor has been well characterized. It consists of four amino acids, YTRF, which promote the internalization of the receptor independently of their location in the cytosolic tail, provided that at least 7 residues separate the tetrapeptide from the transmembrane region (8, 27).
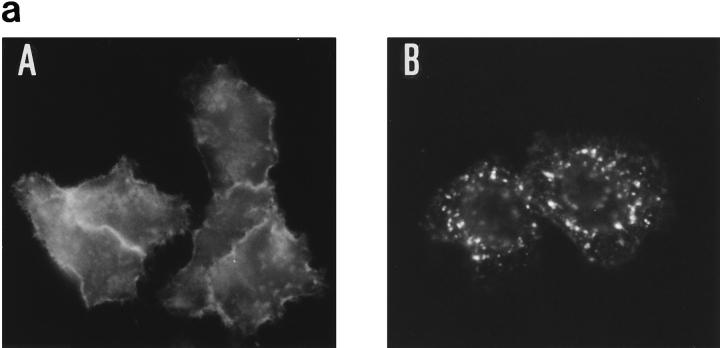
We prepared chimeric receptors using the α chain of the IL2 receptor. This α chain recycles to the plasma membrane when internalized as part of high-affinity IL2 receptors (23). It has been used previously to prepare other chimeric membrane proteins, because good antibodies against its extracellular domain are available (29). When the α chain is expressed alone, without β and γ, it is internalized inefficiently. We therefore designed a construct, αY, in which the transferrin receptor coated-pit internalization signal was inserted in the cytosolic part of α. In αY, the EPLSYTRF sequence from the transferrin receptor was inserted at the COOH terminus of α (Fig. 2). HeLa cells were transfected with α or αY constructs, and 2 d after transfection, the cells were processed for immunofluorescence using anti–α mAb. Staining of α-transfected cells showed mostly a strong surface labeling, while in αY-transfected cells, αY was found in intracellular compartments (Fig. 6 a). We measured the internalization of αY using radiolabeled anti–α mAb 7G7B6. We had previously shown that 7G7B6 mAb does not modify the kinetics of entry and degradation of the IL2 receptor (51). The endocytosis of αY in stably transfected K562 cells was very efficient (Fig. 6 b). We next inserted in this construct the putative sorting signal by adding amino acids 17 to 27 or 18 to 27 of β to the COOH-terminal extremity of αY. The chimeras were named αYβ17–27 and αYβ18–27, respectively (Fig. 2). These chimeras were also rapidly internalized with the same kinetics as αY (Fig. 7 a). When protein synthesis was inhibited, αY expression was stable, indicating that after internalization, αY is efficiently recycled to the plasma membrane, as is the case for the transferrin receptor. Conversely, the chimeras containing the putative sorting signal, αYβ17–27 and αYβ18–27, had a half-life of ∼200 min (Fig. 7 b). This indicates that these chimeras were degraded after internalization via the clathrin-coated pits.
Figure 6.
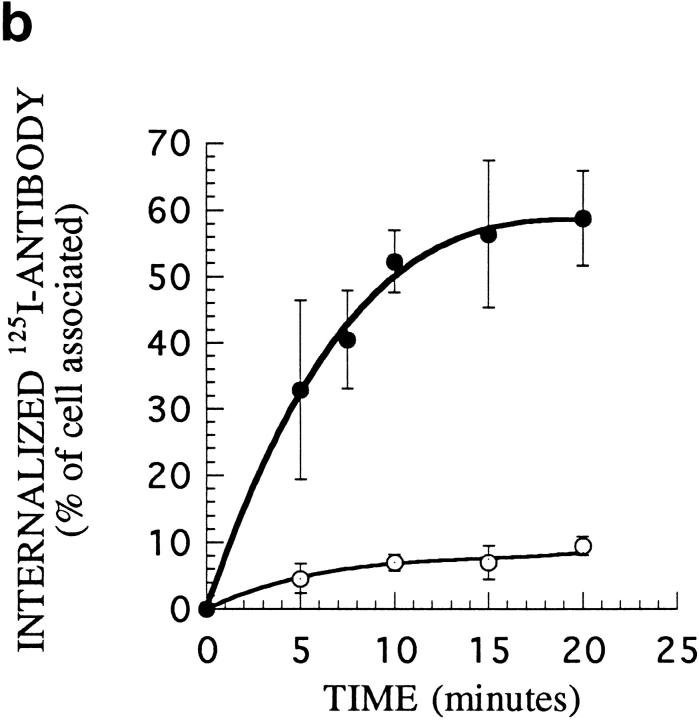
Internalization of 7G7B6 antibody in cells transfected with α or αY. (a) HeLa cells transiently transfected with α (A) or αY (B) were incubated for 30 min with 7G7B6 mAb. After fixation and permeabilization, the antibody was revealed using Texas red–labeled antimurine IgG2a antibody. Magnification: 630. (b) Kinetics of 125I-labeled 7G7B6 mAb internalization in K562 cells stably transfected with α (○) or αY (•). The antibody was added to the cells at 37°C. After further incubation for the indicated times, cells were cooled to 4°C, and the amount of internalized antibody was measured. One representative experiment out of three is shown. Error bars indicate standard deviations for averages of three or more experiments.
Figure 7.
Endocytosis and cell surface half-life of α, αYβ17–27 and αYβ18–27 in K562 cells. (a) Kinetics of 125I-labeled 7G7B6 mAb internalization in cells stably transfected with αY, αYβ17–27 or αYβ18–27. Experiments were performed as described in Fig. 6 b. (b) Half-life of these chimeras. Cell surface expression of α on cells treated for different times with 50 μM of cycloheximide was assessed by flow cytometry using 7G7B6 mAb. Times indicated are after a 30min preincubation in cycloheximide. In each case, one representative experiment out of three is shown. Error bars indicate standard deviations for averages of three or more experiments.
The αYβ18–27 Chimera Is Found in Transferrin Negative Compartments
Transferrin and its receptor recycle very efficiently to the plasma membrane after endocytosis and are widely used as markers of early/recycling endosomes. If αY is in fact internalized and recycles as the transferrin receptor, both receptors should be localized in the same intracellular compartments; whereas if the αYβnm chimeras are sorted to a degradation pathway, they should not colocalize entirely with the transferrin receptor.
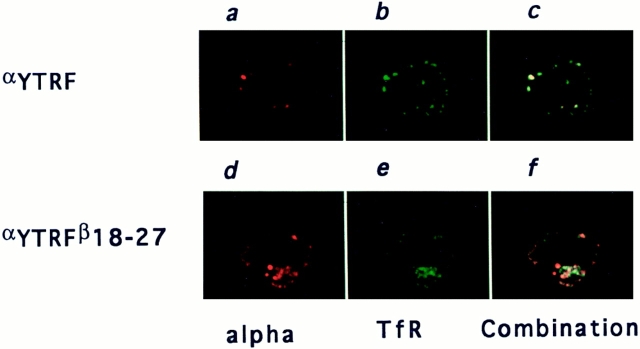
We had previously shown that 7G7B6 mAb was a suitable marker for following the endocytosis of α, as it accompanies α along its recycling pathway (23). To study the intracellular distribution of αY or αYβ18–27 after endocytosis, cells stably transfected with these chimeras were incubated for 120 min at 37°C with 7G7B6 mAb before fixation and permeabilization. Early/recycling endosomal compartments were then labeled using anti-transferrin receptor mAb OKT9. Subclass-specific antibodies were chosen to reveal each marker. In αY and αYβ18–27 transfected cells, there was strong intracellular labeling with both antibodies. Most of the compartments were double-labeled, as expected because the transferrin receptor and the αY or αYβ18–27 constructs have a clathrin-coated pit internalization signal and are found in the same endosomal compartments. In αY-transfected cells, almost every compartment labeled with 7G7B6 was also labeled with anti-transferrin receptor mAb (Fig. 8 c). On the other hand, in αYβ18–27 transfected cells, we could detect a significant number of 7G7B6 positive compartments that were negative for transferrin receptor labeling (Fig. 8 f). Therefore, when the 18–27 sequence is added to the cytosolic tail of a recycling receptor, the resulting chimera can exit from early/ recycling endosomes.
Figure 8.
Localization after endocytosis of αY or αYβ18–27 relative to transferrin receptor. K562 cells stably transfected with αY (top) or αYβ18–27 (bottom) were incubated at 37°C for 2 h with anti-α mAb 7G7B6. The cells were then washed at 4°C to stop endocytosis, fixed, permeabilized and incubated with anti-transferrin receptor mAb OKT9. Anti-α and antitransferrin receptor mAb were then revealed with Texas red– labeled anti–mouse IgG2a and FITC labeled anti–mouse IgG1, respectively. One representative medial optical section is represented. (a and d) α staining; (b and e) transferrin receptor staining; and (c and f) combinations of the two stainings.
The Sorting Signal of the β Chain Does Not Function as a Transferable Internalization Signal
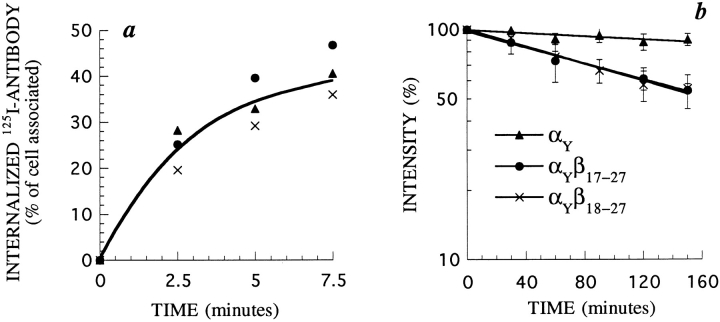
In most of the cases previously described, sequences necessary to orient proteins towards lysosomes are very similar to internalization signals and function as such. We therefore determined if this is also the case for the 18–27 β chain sequence. The α chain of the IL2 receptor, without β and γ, is internalized very inefficiently when transfected in K562 cells. However, this internalization is measurable (Fig. 9 a). We constructed a chimera between the IL2Rα chain and amino acids 18–27 from the cytosolic part of the β chain, named αβ18–27 (Fig. 2). We measured the internalization of the chimera using radiolabeled anti–α mAb 7G7B6. Internalization of the chimera αβ18–27 was slow and inefficient, identical to that of α, with only 10% of the molecules being internalized at steady state (Fig. 9 a). This result was surprising because amino acids 18–27 contain part of the information for the endocytosis of β27, as β27 is internalized faster than β18 (Fig. 3 a). One could therefore expect that amino acids 18–27 of β may contribute a positive signal for the internalization of α in the αβ18–27 chimera. As this does not appear to be the case, it seems that the internalization of β27 requires other parts of the protein.
Figure 9.
Endocytosis and cell surface half-life of α and αβ18–27 in K562 cells. (a) Kinetics of 125I-labeled 7G7B6 mAb internalization in cells stably transfected with α (○) or αβ18–27 (•). (b) Half-life of these chimeras. Experiments were performed as described in Fig 7. In each case, one representative experiment out of three is shown. Error bars indicate standard deviations for averages of three or more experiments.
We measured the half-lives of α and αβ18–27 at the cell surface, as described above, in stably transfected K562 cells. Their surface expression was probed with anti–α mAb 7G7B6. As seen in Fig. 9 b, the α chain has a very long half-life, while the half-life of αβ18–27 was ∼300 min.
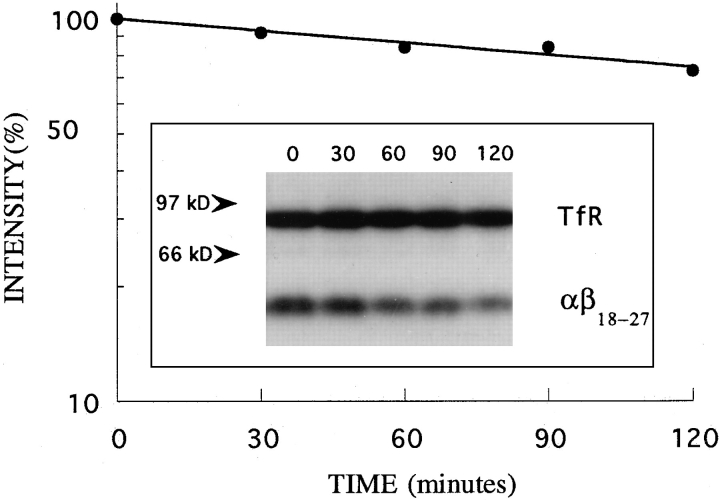
To show that this half-life of αβ18–27 was due to degradation, the rate of turn over of αβ18–27 was measured in these cells by a pulse-chase experiment. Cells were iodinated and incubated in chase medium at 37°C for different times as described in the Materials and Methods section. Proteins were quantitatively immunoprecipitated from cell lysates using antibodies against the IL2Rα chain and the transferrin receptor, the precipitates resolved on SDSpolyacrylamide gels, and the radioactivity in the band corresponding to the αβ18–27 chimera or to the transferrin receptor was quantitated by phosphorimager analysis (Fig. 10). The transferrin receptor was stable over the 2 h of chase, while the intensity of the band corresponding to the αβ18–27 chimera decreased with a half-life of ∼300 min. This value is the same as that measured for the half-life of αβ18–27 on the cell surface (Fig. 9) and represents a 3–5fold increase in the rate of turnover of αβ18–27 compared to α. In conclusion, the transfer of amino acids 18–27 from β to the α chain induces degradation of this membrane protein but does not promote its endocytosis.
Figure 10.
Turnover of αβ18–27 in K562 cells. The cells were iodinated, washed, and incubated in chase medium. At the indicated times, cells were harvested and lysed. αβ18–27 chimera (mol wt ∼55 kD) and transferrin receptor (mol wt ∼90 kD) were immunoprecipitated from the lysates with 7G7B6 anti-IL2Rα and OKT9 anti-transferrin receptor mAb, and separated by SDSPAGE (inset). Radioactivity in the bands was quantitated by phosphorimager analysis. The ratio of αβ18–27 chimera to transferrin receptor was calculated at each time point. The values in the graph represent the percentage of this ratio at different times relative to that at time zero.
Discussion
The high-affinity IL2 receptor is continuously internalized, and after receptor endocytosis, the β chain is found in late endocytic compartments (23). Here we show by a pulse chase experiment that it is degraded. Most growth factor and cytokine receptors are degraded after internalization, which is important for the regulation of their expression and function, but the mechanism for their sorting towards degradation is not understood. Based on the hypothesis that short sequences act as tags for intracellular routing of receptors, we looked for a signal in the cytosolic tail of the β chain that would direct it towards degradation compartments. We observed that the 27 amino acids adjacent to the membrane of the cytosolic tail were sufficient for this function. We prepared a modified IL2 receptor α chain by adding to its cytosolic tail an efficient coated-pit internalization signal, the transferrin receptor signal YTRF. This construct αY was rapidly internalized and recycled. By confocal microscopy, it was colocalized with the transferrin receptor which labels early/recycling endocytic compartments. Conversly, αYβ18–27, a chimera containing, in addition to YTRF, amino acids 18–27 of the β chain, was internalized with the same kinetics as αY, but its half-life was shortened to ∼3 h. This represents a 4–6-fold increase in the rate of turnover of αYβ18–27 compared to αY. By confocal microscopy, αYβ18–27 was not always colocalized with the transferrin receptor, indicating that it was sorted from the recycling pathway. Therefore the P18DPSKFFSQL27 sequence of the cytosolic tail of β is sufficient to provide a determinant to divert a normally rapidly internalized recycling receptor from recycling compartments. Since we have shown that the β chain as well as the αβ18–27 construct are degraded, it is most likely that this signal targets proteins towards late endocytic compartments where they are degraded.
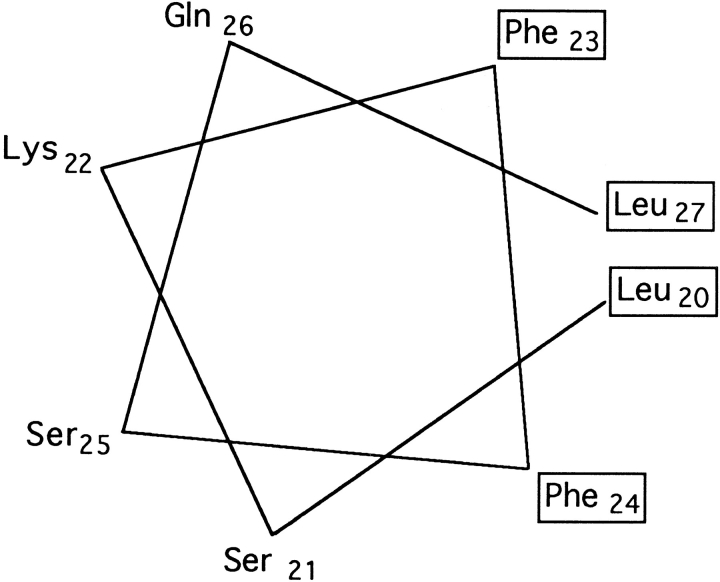
A peptide, named BETApep, including the sorting signal and corresponding to amino acids 16–32 of the β chain, was synthesized and its structure was studied by CD and NMR. This peptide formed a nascent helix involving Phe23 to Leu27, preceded by a type I′ β turn formed by amino acids Asp19 to Lys22. It is noteworthy that this α helix is amphipathic with hydrophobic residues Phe23, Phe24, and Leu27 on one side, and hydrophilic residues Lys22, Ser25, and Gln26 on the other side. Strikingly, the fragment that is highly structured in the peptide containing amino acids 16–32 of β corresponds to the sorting signal defined in this work by its biological properties, i.e., amino acids 18–27. However, it cannot be ruled out that NMR data might not reflect the structural requirements in vivo. Additional experiments are needed to illustrate the structure-function relationship in this signal.
To assess the potential role of the helix distortion at the NH2-terminal extremity due to Pro20, we also studied the structure of a synthetic peptide identical to the previous one, except that Pro20 had been replaced by a leucine, a helix favoring residue (42). In aqueous solution at pH 5.4, this peptide, P20Lpep, exhibited NOE characteristics of a nascent helix from Leu20 to Ser28, stabilized in the presence of TFE. A schematic view of this amphipathic α helix is presented in Fig. 11. In parallel with this NMR analysis, we have studied the routing after endocytosis of a mutated β27 receptor in which Pro20 had been replaced by a leucine. This receptor was internalized with the same kinetics as β27 and its half-life was identical to that of β27. Therefore when Pro20 is replaced by a leucine, receptor routing is not affected. The corresponding structure is α-helical, having lost the NH2-terminal kink due to the proline in the wildtype sequence. These results indicate that the kink is not important for the signal. It is interesting to compare this signal with the internalization signal of the invariant chain: both have the same kind of structure, but in the latter case, the kink of the invariant chain seems to be required for internalization (38). Other regular structures have already been reported for signals involved in the endocytic pathway, for coated pit localization of a few receptors (4, 14, 38, 58), targeting to synaptic vesicles of VAMP (17), and ER retention of CD3-ε (31). The known structures fall into two categories, those which form a tight β-turn (4, 14) and those which form an α helix (17, 38, 58). This α helix is often preceded or followed by a turn (17, 31, 38, 58). Here we show that the turn at the beginning of the α helix can be removed without altering the signal function.
Figure 11.
Helical wheel representation of amino acids 20 to 27 of P20Lpep. Hydrophobic amino acids are boxed.
Most of the signals which sort membrane glycoproteins at different steps of their traffic along the endocytic pathway fall into two categories, the tyrosine-containing and dileucine–containing signals. The targeting sequences found in lysosomal glycoproteins belong to these two categories (20, 24, 33, 43, 59). It is also the case for proteins which are targeted to late endocytic compartments such as the CD3γ and CD3δ chains of the T cell surface antigen receptor complex (29), the invariant chain (41), CD4 in Nef expressing cells (2), a CD8/gp75 chimera in fibroblasts (55), and the β chain of HLA-DM (32). The sorting signal that we found in the IL2Rβ chain is not related to the tyrosine or di-leucine based motives. It also shares no clear sequence homology with other receptors that are degraded after endocytosis.
Tyrosine and di-leucine based motifs have first been described as internalization signals. Since then, they have been found to function at other sorting steps, in addition to their capacity to promote endocytosis (2, 26, 29, 41). The signal we describe here specifically functions as a signal of sorting towards degradation compartments: although it induced the degradation of the α chain when added to its cytosolic tail, it did not enhance its low internalization rate.
In this study, we have found that the half-life of the wildtype β chain at the cell surface of K562 cells is 150 min. This value is similar to that of P-selectin, between 75 and 150 min, and faster than that of lysosomal acid phosphatase, ∼3–4 h, or of the β chain of HLA-DM in HeLa cells, ∼3 h (7, 15, 32). The half-life of β27 is the same as that of the whole β chain, while its internalization is about three times faster. This observation is in agreement with a recent study showing that there was no simple correlation between the internalization and degradation rates (61). Also, there may be another sequence, further downstream in the β chain, that is also involved in degradation.
Under physiological conditions, β is not expressed by itself: in lymphocytes or natural killer cells, the γ chain is also present. When the ligand is present, these two chains associate to bind the ligand and the trimolecular complex thus formed is internalized. The half-life of β, in the absence of IL2, is about twice as long as when the ligand is present (22 and our unpublished results). This cannot be attributed simply to differences in internalization rates, since these rates are similar (Subtil, A., unpublished results). Thus, in the absence of ligand, the β chain is sorted to degradation compartments less efficiently than when the ligand is present. In the latter case, β and γ associate and the receptors become phosphorylated. The sorting signal described here, or other potential signals in the rest of the chain, might function more efficiently in the presence of ligand when β and γ are associated and phosphorylated. Alternatively, the γ chain may also have a degradation signal which might account for the more efficient sorting. The β and γ chains, in combination with other chains of the cytokine receptor family, can constitute different cytokine receptors which can be expressed simultaneously on the same cells. The expression of each of the chains on the cell surface may be rapidly modulated by the efficiency of its degradation after endocytosis, which allows for subtle control of receptor expression and function.
Over the years, the term “receptor-mediated endocytosis” has become synonymous with internalization via clathrin-coated pits. However, other receptor-mediated endocytosis pathways have also been reported (reviewed in reference 28). When clathrin-coated pit endocytosis is inhibited, IL2 receptors are still internalized (51). When this classical pathway functions, the proportion of IL2 receptors entering via either pathway is not known. Even if a significant proportion of IL2 receptors entered cells by a clathrin-independent mechanism, since both pathways seem to rejoin in early endosomes (19), where sorting probably occurs (34), one would expect the signal to function regardless of the entry pathway used. The sorting signal of the β chain was functional in a chimera having the transferrin receptor coated-pit localization signal, αYβ18–27. Therefore this signal directs receptors towards degradation compartments when they are internalized via coated-pits.
The way sorting towards degradation compartments occurs is not known. In some cases, aggregation of recycling receptors has been shown to drive them out of the recycling pathway and towards lysosomes. We do not know if the sorting signal we describe in this paper can cause aggregation. However this seems unlikely, since point mutations in the signal disrupt its function and the resulting receptors have an increased half-life (manuscript in preparation). Alternatively, the degradation signal might be recognized by a component of a cytoplasmic coat, such as COP proteins, which seem to be involved in endosome function (3, 57).
It is striking that sorting motifs which function at different sites in cells and which target membrane glycoproteins to various organelles share common features in their sequence or in their structure. Although the nature of the specific molecules that are able to recognize these motifs is not known, the similarity of the signals suggest that the machineries that decipher them at different cell sites may also be related. Further studies will be needed to understand how these various signals function to specifically target receptors to different organelles.
Acknowledgments
This work was supported by the Ligue nationale contre le Cancer, comité de Paris, and by a grant BIO2-CT92-0164 from the European Commission.
Abbreviations used in this paper
- CD
circular dichroism
- IL2
interleukin 2
- NOE
nuclear Overhauser effect
- NOESY
two-dimensional NOE spectroscopy
- NMR
nuclear magnetic resonance
- ROESY
rotating frame NOE
- TFE
trifluoroethanol
- TOCSY
total correlation spectroscopy
Footnotes
We greatly appreciate the help of Dr. Agnès Hémar at the beginning of this work. We are grateful to Dr. H. Gahery for helping with initial β constructs, to E. Gilard for helping with NMR analyses, to Drs. C. Bonnerot, P. Cosson, and T. Kono for providing plasmids NT, T-XO and pdKCRβ, respectively, and to Dr. R. Robb for the gift of antibodies 341 and 561. We are grateful to Drs. Anne Lecroisey, Sebastian Amigorena, Emmanuel Morelon, and David Ojcius for critical reading of the manuscript.
Please address all correspondence to A. Dautry-Varsat, Unite de Biologie des Interactions Cellulaires, Institut Pasteur, 25 Rue du Dr Roux, 75724 Paris Cedex 15, France. Tel.: 33 1 45 68 8574. Fax: 33 1 40 61 3238. E-mail: adautry@pasteur.fr
References
- 1.Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1993. Current Protocols in Molecular Biology. K. Janssen, editor. Wiley, J. & Sons, Inc.
- 2.Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 3.Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal βCOP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal A, Gierasch LM. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1991;67:1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- 5.Bazan JF. Haemopoietic receptors and helical cytokines. Immunology Today. 1990;11:350–354. doi: 10.1016/0167-5699(90)90139-z. [DOI] [PubMed] [Google Scholar]
- 6.Bothner-By A, Stephens RL, Lee J-M. Structure determination of a tetrasaccharide: transient nuclear overhauser effects in the rotating frame. J Am Chem Soc. 1984;106:811–813. [Google Scholar]
- 7.Braun M, Waheed A, von Figura K. Lysosomal acid phosphatase is transported to lysosomes via the cell surface. EMBO (Eur Mol Biol Organ) J. 1989;8:3633–3640. doi: 10.1002/j.1460-2075.1989.tb08537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collawn JF, Stangel M, Kuhn LA, Esekogwu V, Jing S, Trowbridge IS, Tainer JA. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63:1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- 9.DiSanto JP, Dautry-Varsat A, Certain S, Fischer A, de Saint G, Basile IL-2Rγ chain mutations in SCIDX1 result in the loss of high-affinity IL-2 receptor binding. Eur J Immunol. 1994;24:475–479. doi: 10.1002/eji.1830240232. [DOI] [PubMed] [Google Scholar]
- 10.DiSanto JP, Rieux-Laucat F, Dautry-Varsat A, Fischer A, de Saint G, Basile Defective human interleukin 2 receptor γ chain in an atypical X chromosome-linked severe combined immunodeficiency with peripheral T cells. Proc Natl Acad Sci USA. 1994;91:9466–9470. doi: 10.1073/pnas.91.20.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duprez V, Dautry-Varsat A. Receptor mediated endocytosis of interleukin 2 in a human tumor T cell line: degradation of interleukin 2 and evidence for the absence of recycling of interleukin 2 receptors. J Biol Chem. 1986;261:15450–15454. [PubMed] [Google Scholar]
- 12.Duprez V, Cornet V, Dautry-Varsat A. Down regulation of high affinity interleukin 2 receptors in a human tumor T cell line: IL2 increases the rate of surface receptor decay. J Biol Chem. 1988;263:12860–12865. [PubMed] [Google Scholar]
- 13.Dyson HJ, Rance M, Houghten RA, Lerner RA, Wright PE. Folding of immunogenic peptide fragments of proteins in water solution. J Mol Biol. 1988;201:161–200. doi: 10.1016/0022-2836(88)90446-9. [DOI] [PubMed] [Google Scholar]
- 14.Eberle W, Sander C, Klaus W, Schmidt B, von Figura K, Peters C. The essential tyrosine of the internalization signal in lysosomal acid phosphatase is part of a β turn. Cell. 1991;67:1203–1209. doi: 10.1016/0092-8674(91)90296-b. [DOI] [PubMed] [Google Scholar]
- 15.Green SA, Setiadi H, McEver RP, Kelly RB. The cytoplasmic domain of P-selectin contains a sorting determinant that mediates rapid degradation in lysosomes. J Cell Biol. 1994;124:435–448. doi: 10.1083/jcb.124.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griesinger C, Otting G, Wüthrich K, Ernst RR. Clean TOCSY for 1H spin system identification in macromolecules. J Am Chem Soc. 1988;110:7870–7872. [Google Scholar]
- 17.Grote E, Hao JC, Bennett MK, Kelly RB. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 18.Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- 19.Hansen SH, Sandvig K, van Deurs B. Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors. J Cell Biol. 1993;123:89–97. doi: 10.1083/jcb.123.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harter C, Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol. 1992;117:311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hémar A, Dautry-Varsat A. Cyclosporin A inhibits the interleukin 2 receptor α chain gene transcription but not its cell surface expression: the α chain stability can explain this discrepancy. Eur J Immunol. 1990;20:2629–2635. doi: 10.1002/eji.1830201216. [DOI] [PubMed] [Google Scholar]
- 22.Hémar A, Lieb M, Subtil A, DiSanto JP, Dautry-Varsat A. Endocytosis of the β chain of interleukin 2 receptor requires neither interleukin 2 nor the γ chain. Eur J Immunol. 1994;24:1951–1955. doi: 10.1002/eji.1830240902. [DOI] [PubMed] [Google Scholar]
- 23.Hémar A, Subtil A, Lieb M, Morelon E, Hellio R, Dautry-Varsat A. Endocytosis of interleukin 2 receptors in human T lymphocytes: distinct intracellular localization and fate of the receptor α, β, and γ chains. J Cell Biol. 1995;129:55–64. doi: 10.1083/jcb.129.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Höning S, Hunziker W. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-1) in MDCK cells. J Cell Biol. 1995;128:321–332. doi: 10.1083/jcb.128.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Z, Chen Y, Nissenson RA. The cytoplasmic tail of the G-protein-coupled receptor for parathyroid hormone and parathyroid hormone-related protein contains positive and negative signals for endocytosis. J Biol Chem. 1995;270:151–156. doi: 10.1074/jbc.270.1.151. [DOI] [PubMed] [Google Scholar]
- 26.Hunziker W, Geuze HJ. Intracellular trafficking of lysosomal membrane proteins. BioEssays. 1996;18:379–389. doi: 10.1002/bies.950180508. [DOI] [PubMed] [Google Scholar]
- 27.Jing S, Spencer T, Miller K, Hopkins C, Trowbridge IS. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990;110:283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamaze C, Schmid SL. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 29.Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 30.Macura S, Ernst RR. Elucidation of cross relaxation in liquids by two-dimensional N.M.R. spectroscopy. Mol Phys. 1980;41:95–117. [Google Scholar]
- 31.Mallabiabarrena A, Jiménez MA, Rico M, Alarcon B. A tyrosine-containing motif mediates ER retention of CD3-ε and adopts a helix-turn structure. EMBO (Eur Mol Biol Organ) J. 1995;14:2257–2268. doi: 10.1002/j.1460-2075.1995.tb07220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks MS, Roche PA, van Donselaar E, Woodruff L, Peters PJ, Bonifacino JS. A lysosomal targeting signal in the cytoplasmic tail of the β chain directs HLA-DM to MHC class II compartments. J Cell Biol. 1995;131:351–369. doi: 10.1083/jcb.131.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathews PM, Martinie JB, Fambrough DM. The pathway and targeting signal for delivery of the integral membrane glycoprotein LEP100 to lysosomes. J Cell Biol. 1992;118:1027–1040. doi: 10.1083/jcb.118.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrifield RB. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J Am Chem Soc. 1963;85:2149–2154. [Google Scholar]
- 36.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–267. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 37.Minamoto S, Mori H, Hatakeyama M, Kono T, Doi T, Ide T, Uede T, Taniguchi T. Characterization of the heterodimeric complex of human IL2 receptor α β chains reconstituted in a mouse fibroblast cell line, L929. J Immunol. 1990;145:2177–2182. [PubMed] [Google Scholar]
- 38.Motta A, Bremnes B, Castiglione MA, Morelli, Frank RW, Saviano G, Bakke O. Structure-activity relationship of the leucine-based sorting motifs in the cytosolic tail of the major histocompatibility complex-associated invariant chain. J Biol Chem. 1995;270:27165–27171. doi: 10.1074/jbc.270.45.27165. [DOI] [PubMed] [Google Scholar]
- 39.Nelson JW, Kallenbach NR. Stabilization of the ribonuclease S-peptide α-helix by trifluoroethanol. Proteins. 1986;1:211–217. doi: 10.1002/prot.340010303. [DOI] [PubMed] [Google Scholar]
- 40.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 41.Odorizzi CG, Trowbridge IS, Xue L, Hopkins C, Davis CD, Collawn JF. Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment. JCell Biol. 1994;126:317–330. doi: 10.1083/jcb.126.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padmanabhan S, Marqusee S, Ridgeway T, Laue TM, Baldwin RL. Relative helix-forming tendencies of nonpolar amino acids. Nature (Lond) 1990;344:268–270. doi: 10.1038/344268a0. [DOI] [PubMed] [Google Scholar]
- 43.Peters C, Braun M, Weber B, Wendland M, Schmidt B, Pohlmann R, Waheed A, von Figura K. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO (Eur Mol Biol Organ) J. 1990;9:3497–3506. doi: 10.1002/j.1460-2075.1990.tb07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rance M, Sorensen OW, Bodenhausen G, Wagner G. Improved spectral resolution in COSYlH NMR spectra of proteins via double quantum filtering. Biochem Biophys Res Commun. 1983;117:479–485. doi: 10.1016/0006-291x(83)91225-1. [DOI] [PubMed] [Google Scholar]
- 45.Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 46.Robinson MS, Watts C, Zerial M. Membrane dynamics in endocytosis. Cell. 1996;84:13–21. doi: 10.1016/s0092-8674(00)80988-5. [DOI] [PubMed] [Google Scholar]
- 47.Rohrer J, Schweizer A, Russell D, Kornfeld S. The targeting of Lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J Cell Biol. 1996;132:565–576. doi: 10.1083/jcb.132.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubin LA, Kutman CC, Fritz ME, Biddison WE, Boutin B, Yarchoan R, Nelson DL. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. . J Immunol. 1985;135:3172–3177. [PubMed] [Google Scholar]
- 49.Sandoval IV, Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 1994;4:292–297. doi: 10.1016/0962-8924(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 50.States DJ, Haberkorn RA, Ruben DJ. A two-dimensional nuclear overhauser experiment with pure absorption phase in four quadrants. J Magn Reson. 1982;48:286–292. [Google Scholar]
- 51.Subtil A, Hémar A, Dautry-Varsat A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J Cell Sci. 1994;107:3461–3468. doi: 10.1242/jcs.107.12.3461. [DOI] [PubMed] [Google Scholar]
- 52.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin 2 receptor γ chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 53.Sutherland R, Delia D, Schneider C, Newman R, Kemshead J, Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Immunology. 1981;78:4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trowbridge IS, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 55.Vijayasaradhi S, Xu Y, Bouchard B, Houghton AN. Intracellular sorting and targeting of melanosomal membrane proteins: identification of signals for sorting of the human brown locus protein, GP75. J Cell Biol. 1995;130:807–820. doi: 10.1083/jcb.130.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voss SD, Leary TP, Sondel PM, Robb RJ. Identification of a direct interaction between interleukin 2 and the p64 interleukin 2 receptor γ chain. Proc Natl Acad Sci USA. 1993;90:2428–2432. doi: 10.1073/pnas.90.6.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 58.Wilde A, Dempsey C, Banting G. The tyrosine-containing internalization motif in the cytoplasmic domain of TGN38/41 lies within a nascent helix. J Biol Chem. 1994;269:7131–7136. [PubMed] [Google Scholar]
- 59.Williams MA, Fukuda M. Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol. 1990;111:955–966. doi: 10.1083/jcb.111.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wishart DS, Sykes BD, Richards FM. Relationship between nuclear magnetic resonance chemical shift and protein secondary structure. J Mol Biol. 1991;222:311–333. doi: 10.1016/0022-2836(91)90214-q. [DOI] [PubMed] [Google Scholar]
- 61.Zwart DE, Brewer CB, Lazarovits J, Henis YI, Roth MG. Degradation of mutant influenza virus hemagglutinins is influenced by cytoplasmic sequences independent of internalization signals. J Biol Chem. 1996;271:907–917. doi: 10.1074/jbc.271.2.907. [DOI] [PubMed] [Google Scholar]