Abstract
L-Selectin on neutrophils as well as inducible E- and P-selectin on endothelium are involved in the recruitment of neutrophils into inflamed tissue. Based on cell attachment assays, L-selectin was suggested to function as a carbohydrate presenting ligand for E- and P-selectin. However, previous affinity isolation experiments with an E-selectin–Ig fusion protein had failed to detect L-selectin among the isolated E-selectin ligands from mouse neutrophils. We show here that L-selectin from human neutrophils, in contrast to mouse neutrophils, can be affinity-isolated as a major ligand from total cell extracts using E-selectin–Ig as affinity probe. Binding of human L-selectin to E-selectin was direct, since purified L-selectin could be reprecipitated with E-selectin–Ig. Recognition of L-selectin was abolished by sialidase-treatment, required Ca2+, and was resistant to treatment with endoglycosidase F. Binding of L-selectin to a P-selectin–Ig fusion protein was not observed. In agreement with the biochemical data, the anti–Lselectin mAb DREG56 inhibited rolling of human neutrophils on immobilized E-selectin–Ig but not on P-selectin–Ig. No such inhibitory effect was seen with the anti–mouse L-selectin mAb MEL14 on mouse neutrophils. Rolling of E-selectin transfectants on purified and immobilized human L-selectin was inhibited by mAb DREG56. We conclude that L-selectin on human neutrophils is a major glycoprotein ligand among very few glycoproteins that can be isolated by an E-selectin affinity matrix. The clear difference between human and mouse L-selectin suggests that E-selectin–binding carbohydrate moieties are attached to different protein scaffolds in different species.
The selectins are a family of three Ca2+-dependent cell adhesion molecules that are involved in the initial attachment and rolling of leukocytes on the blood vessel wall (Lasky, 1995; McEver et al., 1995). This initiation of cell contact enables other adhesion molecules to stabilize the binding and allows the leukocytes to migrate across the barrier of the vessel wall (Springer, 1994). E- and P-selectin, which are both expressed on activated endothelium, mediate the entry of neutrophils and certain lymphocyte populations into inflamed tissue (Mayadas et al., 1993; Labow et al., 1994; Frenette et al., 1996). L-selectin on leukocytes is also involved in leukocyte adhesion to activated endothelium (Arbonés et al., 1994). In addition, L-selectin acts as a lymphocyte homing receptor that mediates the entry of lymphocytes into lymph node tissue (Gallatin et al., 1983).
Two glycoprotein ligands for P- and E-selectin were identified on myeloid cells by affinity isolation using soluble forms of P- and E-selectin as affinity probes. One of them, the P-selectin glycoprotein ligand-1 (PSGL-1)1, was originally cloned by expression cloning in COS cells (Sako et al., 1993) and was affinity isolated with human P-selectin (Moore et al., 1992) and mouse P-selectin–Ig (Lenter et al., 1994). Fucosylation of terminal saccharide structures on PSGL-1, which gives rise to epitopes reactive with antisLex antibodies, is necessary for the binding to P-selectin. In addition, sulfation on NH2-terminal tyrosine residues seems to be essential for the binding to P-selectin (Pouyani and Seed, 1995; Sako et al., 1995). PSGL-1 also binds to E-selectin; however, only fucosylation, but no sulfation on tyrosines is needed for this binding (Lenter et al., 1994; Asa et al., 1995; Li et al., 1996). The second ligand that was identified by direct affinity isolation was the E-selectin ligand ESL-1, a glycoprotein that requires sialic acid and fucose for binding (Levinovitz et al., 1993; Steegmaier et al., 1995). In contrast to PSGL-1, ESL-1 does not bind to P-selectin and is not a sialomucin. Instead, it requires some of its five potential N-linked carbohydrate side chains for ligand activity. PSGL-1 was shown to mediate rolling of human neutrophils on P- and E-selectin in vitro (Moore et al., 1995; Patel et al., 1995) and rolling of human neutrophils injected into rat mesenteric venules in vivo (Norman et al., 1995). Antibodies against mouse ESL-1 were found to inhibit adhesion of mouse neutrophils to endothelial E-selectin in a nonstatic (rotation) cell attachment assay (Steegmaier et al., 1995).
Three glycoprotein ligands for L-selectin, glycosylationdependent cell adhesion molecule-1 (GlyCAM-1) (Lasky et al., 1992), CD34 (Baumhueter et al., 1993), and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) (Briskin et al., 1993), were identified on the cuboidal endothelial cells of high endothelial venules. These L-selectin ligands are constitutively expressed and are thought to function as addressins or regulatory molecules in the trafficking of lymphocytes into lymphoid organs.
Which molecules on activated endothelium function as binding partners for L-selectin is less well understood. It has been shown in several in vitro adhesion studies that lymphocytes, neutrophils, and monocytes bind to cytokineactivated but not to nonactivated endothelial cells in an L-selectin-dependent manner (Spertini et al., 1991, 1992; Brady et al., 1992). Likewise, monoclonal antibodies against L-selectin can block rolling of neutrophils in cytokine-activated venules (Ley et al., 1991; von Andrian et al., 1993; Arbonés et al., 1994) and can partially block the entry of neutrophils into inflamed tissue such as inflamed peritoneum (Watson et al., 1991) or inflamed skin (Lewinsohn et al., 1987) or lung tissue (Mulligan et al., 1994). This interaction of leukocytes with activated endothelium could in part be due to the binding of L-selectin to not yet identified cytokine-inducible carbohydrate-presenting ligands (von Andrian et al., 1993).
Unexpectedly, indirect evidence suggested an alternative function of L-selectin as ligand for the endothelial selectins: Attachment of human neutrophils to E- and P-selectin–expressing cells in nonstatic (rotation) adhesion assays (Picker at al., 1991; Kishimoto et al., 1991) and in flow adhesion assays (Abbassi et al., 1993; Lawrence et al., 1994; Patel et al., 1995) was partially inhibited by antibodies against L-selectin. In addition, E-selectin transfectants bound to immobilized L-selectin. However, in sharp contrast to these results, L-selectin could not be affinity isolated from mouse neutrophils with mouse E-selectin–Ig and mouse P-selectin–Ig fusion proteins, although PSGL-1 and ESL-1 were readily detectable (Levinovitz et al., 1993; Lenter et al., 1994). Likewise, only PSGL-1, but no L-selectin, was detected by blotting assays with extracts of human neutrophil membranes, using purified, iodinated human P-selectin as affinity probe (Moore et al., 1992).
Here we present evidence that may explain these apparent discrepancies. We show that, in contrast to L-selectin on mouse neutrophils, L-selectin on human neutrophils can directly be isolated with an E-selectin–Ig affinity probe. Our results establish that human L-selectin not only supports rolling of E-selectin transfectants, but that indeed L-selectin is a preferred E-selectin ligand among all other cellular proteins. This is not the case for mouse L-selectin. Thus, as was predicted by Varki (1994), different repertoires of glycoprotein ligands for the selectins are generated by different species.
Materials and Methods
Cells
Mouse polymorphonuclear granulocytes (PMN) were isolated from bone marrow cells, flushed out of the femurs of 10-wk-old NMRI mice and filtered through nylon tissue, pelleted, resuspended in DME, layered onto 4 ml of Histopaque 1077 (Sigma Chemical Co., St. Louis, MO) and centrifuged for 30 min at 700 g. More than 90% of the pelleted cells were neutrophils as judged by staining with Diff-Quik (Baxter, Unterschleissheim, Germany). Neutrophils and mononuclear cells from human blood were isolated on density gradients of Histopaque 1077 and 1119 (Sigma Chemical Co.) according to manufacturer's instructions. Human bone marrow neutrophils (kindly provided by Dr. Roland Mertelsmann, Freiburg, Germany) were purified similarly. Mouse E-selectin–transfected CHO cells were obtained from Dr. Pierre Vassali (Geneva, Switzerland).
Antibodies
The following monoclonal antibodies were used: DREG200 and DREG56 (anti–human L-selectin; Kishimoto et al., 1990), kindly provided by Dr. Kishimoto (Ridgefield, CT), MEL 14 (anti–mouse L-selectin; Gallatin et al., 1983), HECA452 (anti–sLex-like carbohydrate, also called CLA; Duijvestijn et al., 1988), M1/70.15.11.5.HL (anti–mouse Mac-1; American Type Culture Collection, Rockville, MD), and mAb 44 (anti–human Mac1; PharMingen, San Diego, CA). LAM1-110 and LAM1-116 (anti–human and murine L-selectin, adhesion blocking) and LAM1-14 (anti–human L-selectin, nonblocking; Kansas et al., 1991) were a generous gift from Dr. T.F. Tedder. The rabbit antisera 65 and 89060 against mouse ESL-1 have been described (Steegmaier et al., 1995). The anti–human PSGL-1 rabbit serum 84870 was raised against a fusion protein containing the first 298 amino acids of human PSGL-1 fused to the Fc part of human IgG1.
Selectin-Immunoglobin Chimeric Proteins
The construction of the mouse E-selectin–IgG and mouse P-selectin–IgG chimeric proteins has been described (Hahne et al., 1993). The pig, human, and rat E-selectin–IgG fusion proteins were kindly provided by Dr. Martyn K. Robinson (Celltech, Slough, UK) and contained the CH1, hinge, CH2, and CH3 domains of human IgG1, fused to pig lectin and EGF domains, the human lectin and EGF domains, or the rat lectin and human EGF domains, respectively.
Affinity Isolation of Metabolically Labeled E-Selectin Ligands
Metabolic labeling of neutrophils with [35S]methionine and [35S]cysteine and affinity isolation and reprecipitation experiments were essentially done as described (Lenter et al., 1994).
Sialidase and Endoglycosidase F Treatment of L-Selectin
L-Selectin was purified from metabolically labeled neutrophils using the CNBr-Sepharose–conjugated DREG200 affinity matrix, as described above, and was treated with neuraminidase of Arthrobacter ureafaciens or endoglycosidase F (both from Boehringer Mannheim, Manheim, Germany) as described (Lenter et al., 1994).
Purification of Human and Mouse L-Selectin
Human L-selectin was purified from 20 ml human serum with an E-selectin–Ig affinity matrix. Serum was precleared twice with 200 μl proteinA–Sepharose for 4 h and incubated overnight with 40 μg hIgG1 or E-selectin–IgG chimeric protein bound to 50 μl protein A–Sepharose. EDTAeluted material was either directly electrophoresed or subjected to reprecipitations with CNBr-Sepharose–conjugated DREG56.
For flow adhesion assays, L-selectin from 500 ml of heparinized human plasma was incubated with 200 μl CNBr-Sepharose beads conjugated with DREG56. Specifically bound proteins were eluted and reprecipitated with E-selectin–IgG as described above. Mouse L-selectin was purified from detergent extracts of freshly isolated neutrophils from 20 mice using a MEL14-conjugated CNBr-Sepharose affinity column.
Cell Attachment Assay under Flow
Glass coverslips were coated for 3 h at room temperature with 0.3 μg/ml human IgG, E-selectin–IgG, P-selectin–IgG, or human L-selectin or with 0.1 μg/ml mouse L-selectin in PBS and subsequently blocked with 5% BSA in PBS. Neutrophils (5 × 106/ml) were preincubated with 25 μg/ml human IgG1 in DME containing 10% FCS and 0.02% azide for 10 min at room temperature to block cell surface Fc-receptors. mAbs were subsequently added as 25 μg/ml purified antibody, as 1/50 diluted ascites, or as fivefold concentrated hybridoma supernatant and incubated with the cells for 30 min at room temperature. Cells were diluted to a final density of 1 × 106 cells/ml and perfused through a transparent rectangular perfusion chamber with a slit height of 0.3 mm and a width of 6 mm, as described by Kuijper et al. (1996). The flow rate was controlled with a syringe pump attached to the inlet. The whole experiment was videotaped and the number of rolling cells was quantified by counting cells on multiple images beginning 3 min after start of the flow during which tethering was allowed to occur. The wall shear stress in experiments performed with neutrophils was 2.1 and 1.6 dynes/cm2 with E-selectin–transfected CHO cells. The area of the field of view was 2.46 × 105 μm2.
Results
L-Selectin from Human, but Not from Mouse Neutrophils Can Be Affinity Isolated with E-Selectin–IgG
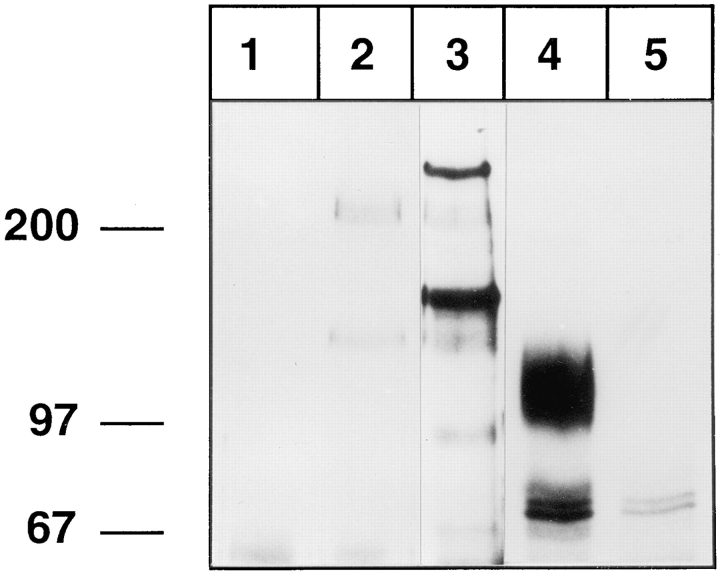
We performed affinity isolation experiments with metabolically labeled mouse neutrophils and antibody-like fusion proteins of mouse E- and P-selectin. Confirming earlier results (Levinovitz et al., 1993; Lenter et al., 1994), we detected the 150-kD ESL-1 and a 250-kD ligand as E-selectin–specific ligands, while a pair of 230/130-kD glycoproteins could be isolated with both selectin affinity probes (Fig. 1, lanes 2 and 3). The 230/130-kD pair was recognized by rabbit antisera against mouse PSGL-1 (Borges, E., and D. Vestweber, unpublished observation). No protein of the size of L-selectin could be precipitated with any of the two selectin affinity probes, although L-selectin was strongly detected in immunoprecipitations with the anti–L-selectin mAb MEL14, migrating as a broad band at 100–120 kD (Fig. 1, lane 4). Thus, in contrast to the high-affinity ligands ESL-1 and PSGL-1, L-selectin from mouse neutrophils does not bind to any of the two endothelial selectins with sufficient affinity that would allow affinity isolation.
Figure 1.
L-Selectin from mouse neutrophils cannot be affinity isolated with E- or P-selectin–IgG. Neutrophils from mouse bone marrow were metabolically labeled with [35S]methionine and [35S]cysteine and detergent extracts were incubated either with immobilized CD4-IgG (lane 1), P-selectin–IgG (lane 2), E-selectin–IgG (lane 3), mAb MEL14 (lane 4), or an isotype-matched control mAb (lane 5). Specifically bound proteins were eluted with EDTA (lanes 1–3) or with SDS (lanes 4 and 5) and electrophoresed on a 6% polyacrylamide gel under reducing conditions, and labeled proteins were visualized by fluorography. Molecular mass markers (in kD) are indicated on the left.
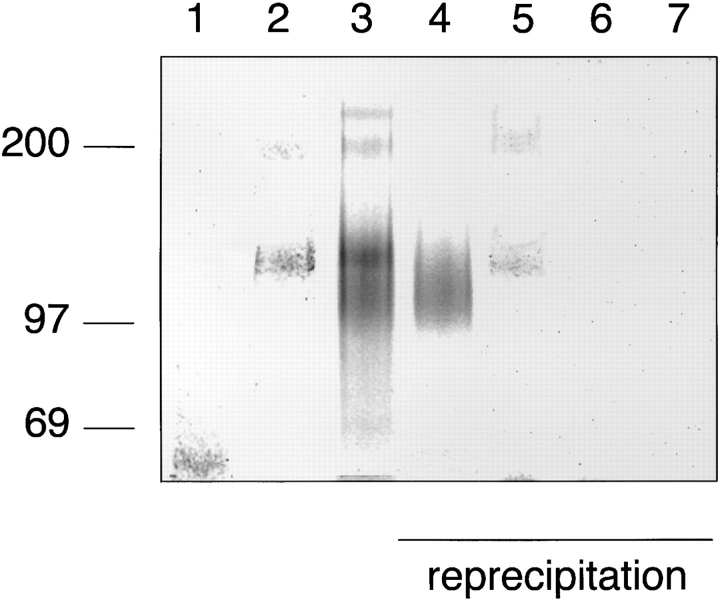
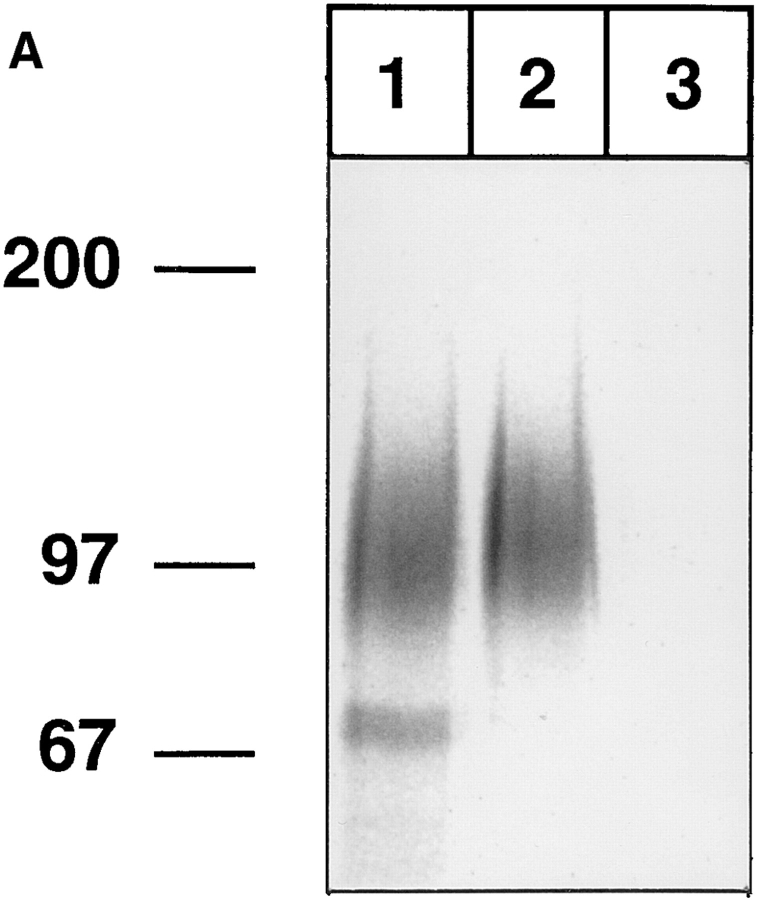
Human neutrophils isolated from peripheral blood were analyzed in the same way as mouse neutrophils. P-selectin– IgG precipitated exclusively a similar pair of proteins, as in the case of mouse neutrophils, migrating at a slightly lower apparent molecular mass of 110/220 kD (Fig. 2, lane 2). With E-selectin–IgG, protein bands of the same size were detected in addition to a broad band between 90 and 120 kD and another protein at 250 kD (Fig. 2, lane 3). Aliquots of the material that was eluted with EDTA from the E-selectin– IgG affinity matrix were further analyzed in immunoprecipitations with the mAb DREG200 against human L-selectin and polyclonal antibodies against human PSGL-1. These reprecipitation experiments identified the protein migrating at 90–120 kD apparent molecular mass as L-selectin and the pair of proteins at 110/220 kD as PSGL-1 (Fig. 2, lanes 4 and 5). The 250-kD protein may be the human homologue of the 250-kD mouse protein, which can be precipitated with E-selectin–IgG from mouse neutrophils. ESL-1 was only weakly detectable on human neutrophils, as tested in reprecipitation experiments with affinity-purified polyclonal antibodies against mouse ESL-1, which crossreacted with a human glycoprotein of similar molecular mass (not shown).
Figure 2.
L-selectin from human peripheral blood neutrophils can be affinity isolated with E-selectin–IgG, but not with P-selectin– IgG. Neutrophils from human peripheral blood were isolated by density gradient centrifugation and labeled as described in Fig. 1, and detergent extracts were incubated either with immobilized CD4-IgG (lane 1), P-selectin–IgG (lane 2), or E-selectin–IgG (lane 3). One aliquot of the EDTA-eluted proteins from the E-selectin–IgG matrix was directly electrophoresed (lane 3), while four aliquots were subjected to reprecipitations with immobilized mAb DREG200 (lane 4), IgG from rabbit antiserum 84870 against human PSGL-1 (lane 5), control rat mAb (lane 6), and nonimmune rabbit IgG (lane 7). Specifically bound proteins were eluted with EDTA (lanes 1–3) or SDS (lanes 4–7), electrophoresed on a 6% polyacrylamide gel under reducing conditions, and visualized by phosphorimaging. The material in lanes 1–3 corresponds to 10% of the total cell extract and in lanes 4–7 to 17.5%. Molecular mass markers (in kD) are indicated on the left.
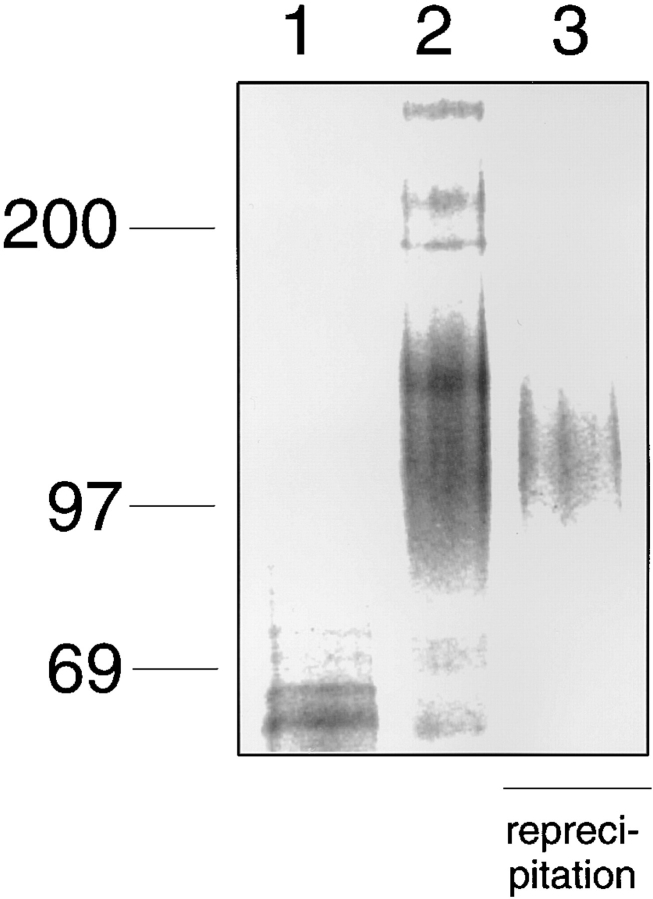
After neutrophils had been removed by density gradient centrifugation, the residual human peripheral blood leukocytes were analyzed as described above by affinity isolation with E-selectin–IgG. As shown in Fig. 3, several proteins were eluted with EDTA from the E-selectin affinity matrix (lane 2). In reprecipitation experiments with mAb DREG200, a polyclonal antiserum against human PSGL-1, and two different antisera against mouse ESL-1, four of the protein bands could be identified as L-selectin, PSGL-1, and ESL-1 (Fig. 3). In addition to these, an unknown protein migrating at 170–180 kD was reproducibly found. Depending on the sample of human blood cells, the ratio of the amount of this protein and of ESL-1 varied. A second unknown protein, which was detected at slightly higher molecular mass than L-selectin, was not reproducibly found.
Figure 3.
Affinity isolation of E-selectin ligands from human peripheral blood mononuclear leukocytes. After removal of neutrophils by density gradient centrifugation, residual human peripheral blood mononuclear leukocytes were metabolically labeled as described in Fig. 1, and detergent extracts were incubated either with CD4-IgG (lane 1) or E-selectin–IgG (lane 2). One aliquot of the EDTA-eluted proteins from the E-selectin–IgG matrix was directly electrophoresed (lane 2), while five aliquots were subjected to reprecipitations with immobilized mAb DREG200 (lane 3), IgG from rabbit antiserum 84870 against human PSGL-1 (lane 4), affinity-purified IgG from the two rabbit antisera 65 (lane 5), and 89060 against ESL-1 (lane 6) or nonimmune rabbit IgG (lane 7). Specifically bound proteins were eluted with SDS (lanes 3–7), electrophoresed on a 6% polyacrylamide gel under reducing conditions, and visualized by phosphorimaging. The material in each of lanes 1 and 2 corresponds to 10% of the total cell extract and in each of lanes 3–7 to 16% of the total extract. Molecular mass markers (in kD) are indicated on the left.
L-Selectin from Human Bone Marrow Neutrophils Binds to E-Selectin
Since the analyzed human and mouse neutrophils had been isolated from human peripheral blood and from mouse bone marrow, respectively, we needed to test whether the strikingly different results were due to different tissue sources or to species differences. Therefore, we analyzed neutrophils from human bone marrow by affinity isolation with E-selectin–IgG. As shown in Fig. 4, a broad protein band ranging from 90 to 120 kD was isolated with E-selectin– IgG, which could be reprecipitated with DREG200 (lane 3). Thus, independent from the tissue source of the neutrophils, L-selectin of human neutrophils can be recognized by E-selectin.
Figure 4.
L-selectin from human bone marrow neutrophils binds to E-selectin–IgG. Neutrophils were isolated from human bone marrow by density gradient centrifugation and labeled as described in Fig. 1, and detergent extracts were incubated with immobilized human IgG (lane 1) or E-selectin–IgG (lane 2). 40% of the EDTAeluted material from the E-selectin–IgG matrix was directly electrophoresed (lane 2), and 60% were subjected to reprecipitation with mAb DREG200 (lane 3). Labeled proteins were electrophoresed on a 6% polyacrylamide gel under reducing conditions and visualized by phosphorimaging. Molecular mass markers (in kD) are indicated on the left.
Human L-Selectin Is Directly Recognized by E-Selectin
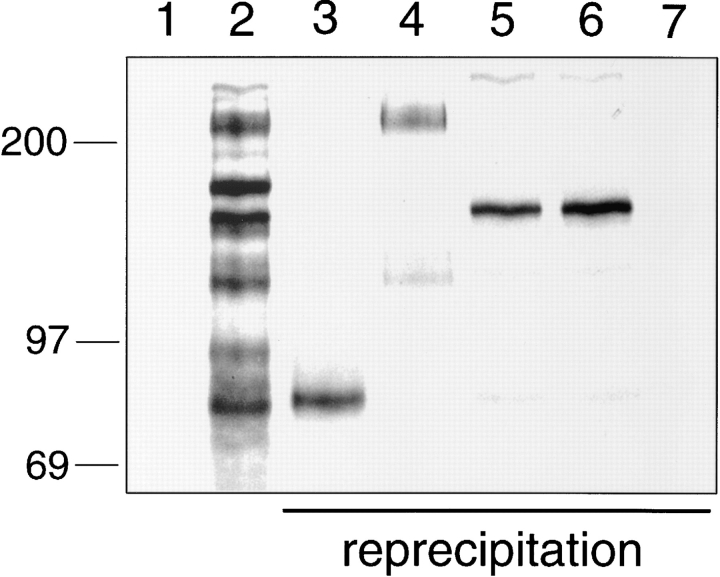
We wanted to test whether L-selectin was directly recognized by E-selectin or whether it was indirectly coprecipitated because of its possible association with other proteins. To this end, we used the anti–human L-selectin mAb DREG200 coupled to CNBr-Sepharose as an affinity matrix to first purify L-selectin from human blood neutrophils and then to test whether the purified L-selectin could be bound by E-selectin–IgG in a second reprecipitation. The neutrophils were labeled as before and detergent extracts were incubated with the DREG200 affinity matrix. The precipitated L-selectin was eluted by pH-shift, and after neutralization, equal aliquots of the eluted material were subjected to reprecipitation either with E-selectin IgG or P-selectin IgG coupled to protein A–Sepharose. The reprecipitated material was specifically eluted with EDTA. Purified L-selectin (Fig. 5 A, lane 1) could be reprecipitated with E-selectin–IgG (lane 2) but not with P-selectin–IgG (lane 3). These results indicate that L-selectin derived from human neutrophils binds directly to E-selectin and not to P-selectin.
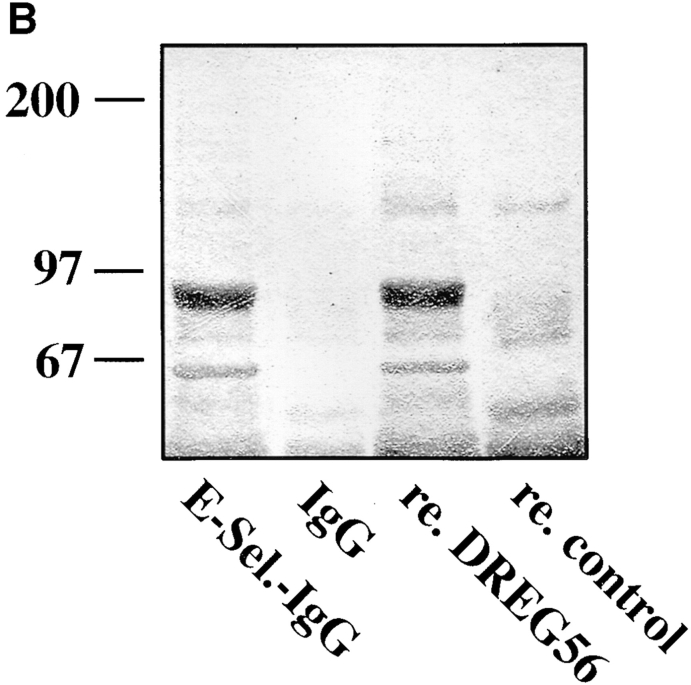
Figure 5.
Purified human L-selectin directly binds to E-selectin–IgG. (A) Neutrophils from human peripheral blood were metabolically labeled as described in Fig. 1 and subjected to immunoprecipitation with mAb DREG200. L-selectin was eluted from the affinity matrix by pH-shift and either directly electrophoresed (lane 1) or reprecipitated with E-selectin–IgG (lane 2) or P-selectin– IgG (lane 3). Isolated proteins were electrophoresed on a 6% polyacrylamide gel under reducing conditions and visualized by phosphorimaging. Molecular mass markers (in kD) are indicated on the left. (B) Shedded human L-selectin is the major glycoprotein precipitated from human serum using E-selectin–IgG as a probe. 20 ml human serum were incubated with affinity matrices carrying E-selectin–IgG (E-Sel.-IgG) or, as control, human IgG. One third of the EDTA-eluted material was directly electrophoresed (E-Sel.-IgG and IgG) or reprecipitated with immobilized DREG56 (re. DREG56) or immobilized control antibody (re. control). Purified proteins were electrophoresed on a 6% polyacrylamide gel and visualized by Coomassie staining.
The specificity and efficiency of the interaction between E- and L-selectin was further highlighted by the fact that shedded L-selectin could be affinity isolated from human serum with an E-selectin–Ig matrix. As shown in Fig. 5 B, shedded L-selectin was purified in a single step purification with E-selectin Ig (E-Sel.-IgG). The identity of the purified serum protein was confirmed by reprecipitating the EDTAeluted material with the mAb DREG56 (re.DREG56).
Human L-Selectin Binds to E-Selectin from Four Different Species
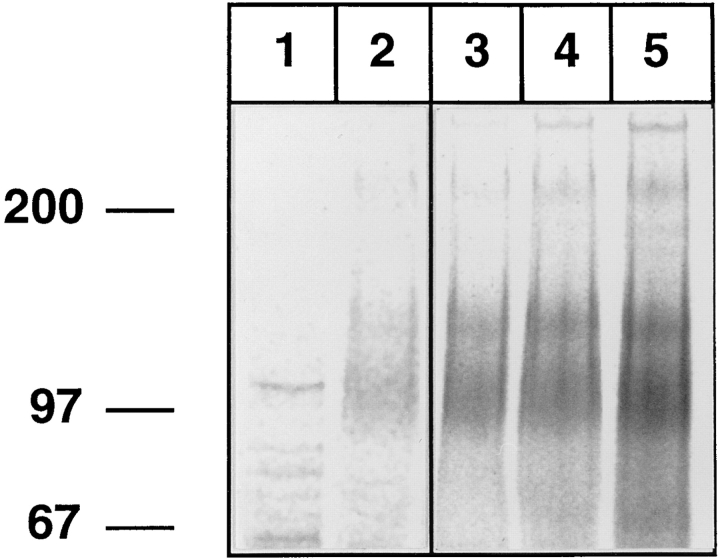
Since our analysis of E-selectin ligands on human and mouse neutrophils had been based on the use of a mouse E-selectin–IgG fusion protein, we also tested recombinant forms of E-selectin from other species, namely from human, rat, and pig. The same pattern of ligands representing L-selectin and the 110/220-kD forms of PSGL-1 was isolated with each of the four E-selectin fusion proteins (Fig. 6, lanes 2–5). Ligand recovery increased from human to pig, rat, and mouse E-selectin, respectively. This result reveals that human neutrophilic L-selectin is recognized by E-selectin, independently of the species origin of E-selectin.
Figure 6.
Recombinant forms of E-selectin from four different species can bind to human L-selectin. Neutrophils from human peripheral blood were metabolically labeled as described in Fig. 1 and detergent extracts were incubated with immobilized human IgG (lane 1), or various E-selectin–IgG fusion proteins generated from human (lane 2), pig (lane 3), rat (lane 4), or mouse (lane 5) E-selectin. EDTA-eluted proteins were electrophoresed on a 6% polyacrylamide gel under reducing conditions and visualized by phosphorimaging. Lanes 1 and 2 were exposed five times longer than lanes 3–5. Molecular mass markers (in kD) are indicated on the left.
Binding Requires the Presence of Sialic Acid on L-Selectin and Is Resistant to Treatment with Endoglycosidase F
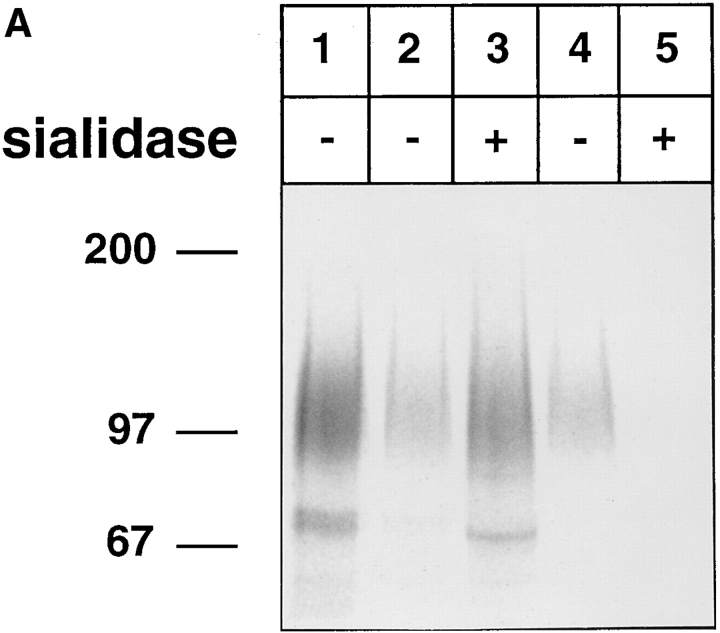
We wanted to test whether the high-affinity binding of L-selectin to E-selectin is dependent on the presence of carbohydrates on L-selectin. L-selectin was isolated from [35S]methionine/[35S]cysteine-labeled human neutrophils using the DREG200-affinity matrix and was subsequently treated with sialidase from Arthrobacter ureafaciens at 37°C overnight. This treatment reduced the apparent molecular mass of L-selectin only slightly (Fig. 7 A, lane 3). When aliquots of the sialidase and of the mock-treated samples were subjected to reprecipitations with E-selectin–IgG, no binding of the sialidase-treated L-selectin was observed (Fig. 7 A, lane 5) while the mock-treated material bound well to E-selectin–IgG (Fig. 7 A, lane 4). Thus, sialic acid on L-selectin is necessary for the binding to E-selectin.
Figure 7.
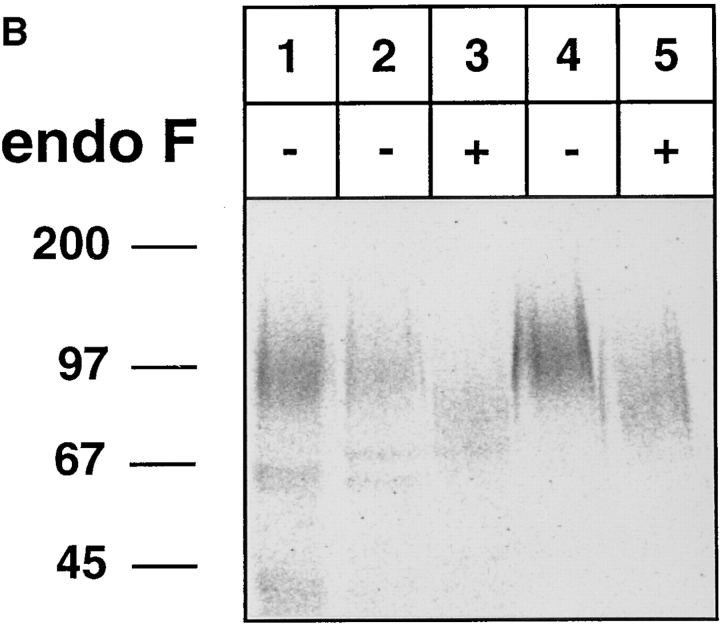
Binding of human L-selectin to E-selectin is sensitive to sialidase treatment and resistant to endo F treatment of L-selectin. L-selectin was immunoprecipitated from metabolically labeled human neutrophils with mAb DREG200. (A) L-selectin was either directly electrophoresed (lane 1) or treated with (lanes 3 and 5) or without (lanes 2 and 4) sialidase from Arthrobacter ureafaciens. 40% of the treated samples were directly electrophoresed (lanes 2 and 3) and 60% reprecipitated with E-selectin–IgG (lanes 4 and 5). (B) Similar to A, except that endo F instead of sialidase was used. Proteins were electrophoresed on a 6% polyacrylamide gel under reducing conditions and visualized by phosphorimaging. Molecular mass markers (in kD) are indicated on the left.
This result prompted us to test whether N-linked carbohydrates on L-selectin are relevant for the binding process. Treatment of L-selectin labeled and isolated as described above with endoglycosidase F caused a significant reduction of its apparent molecular mass (Fig. 7 B, lane 3). However, endo F–digested L-selectin still bound efficiently to the E-selectin–IgG fusion protein (Fig. 7 B, lane 5). This indicates, that endo F–sensitive N-linked carbohydrate side chains on L-selectin are not necessary for binding to E-selectin.
Attachment of Human, but Not of Mouse Neutrophils to E-Selectin under Flow Is Partially Blocked by Anti–L-Selectin mAb
To study neutrophil adhesion to E- and P-selectin under flow conditions, mouse and human neutrophils were infused into parallel-plate laminar flow chambers with coverslips coated either with E-selectin–IgG, P-selectin–IgG, or human IgG1. Attached cells that rolled on the proteincoated glass surface were quantified at a defined wall shear stress of 2.1 dynes/cm2, which is in the range found in postcapillary venules. No attachment or rolling on human IgG was observed.
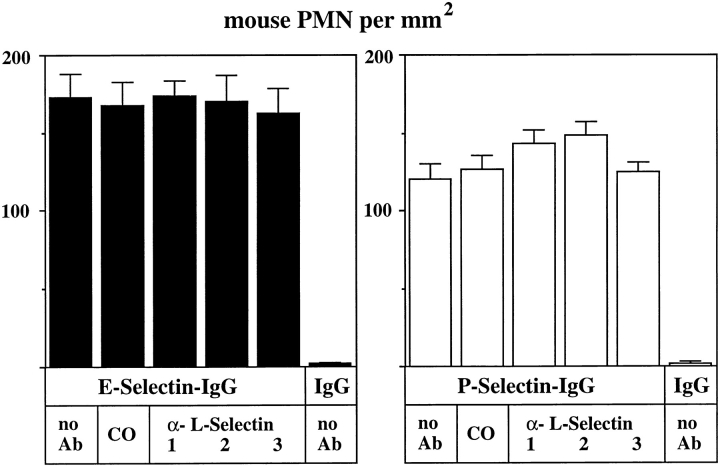
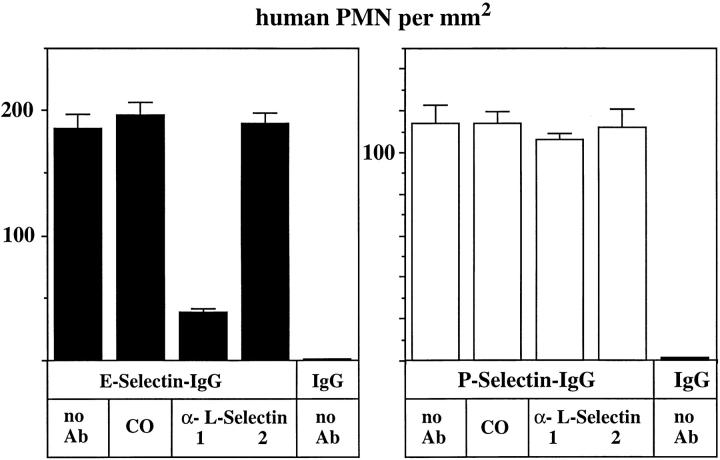
The number of rolling mouse neutrophils on E- and P-selectin–IgG could neither be inhibited by the mAb MEL14 nor by the mAbs LAM1-110 and LAM1-116 (Fig. 8). All three of these antibodies can block the lectin function of L-selectin. Shedding of L-selectin from the surface of the analyzed mouse neutrophils, due to a possible activation of the cells during preparation, was excluded by flow cytometry with mAb MEL14 (data not shown). Our results indicate that L-selectin is not involved in tethering of mouse neutrophils to E- or P-selectin under flow conditions. In contrast to this, tethering of human neutrophils to E-selectin–IgG was clearly reduced by the anti–human L-selectin mAb DREG56 (Fig. 9, left). Neutrophil binding to P-selectin–IgG was not affected by the antibody (Fig. 9, right). Again, no cells bound to immobilized human IgG and control incubations with the nonblocking mAb LAM114 against human L-selectin and an mAb against Mac-1 did not affect neutrophil tethering to either E-selectin– or P-selectin–IgG. Interactions between rolling human neutrophils were only rarely observed in our flow adhesion assays. Only cells that rolled on the coated glass surface without prior interactions with other neutrophils were counted. Increasing the shear stress or cell density caused neutrophils to accumulate in areas, possibly because of neutrophil– neutrophil interactions (not shown). However, such accumulations were only rarely observed under the conditions used in the experiments depicted in Figs. 8 and 9. Thus, the inhibitory effect of DREG56 as depicted in Fig. 8 is most likely due to an inhibitory effect on interactions between neutrophils and E-selectin. Our results indicate that L-selectin on human neutrophils mediates tethering to E-selectin, but not to P-selectin, under flow conditions.
Figure 8.
Attachment of flowing mouse neutrophils to E-selectin is not inhibited by three different anti–L-selectin mAbs. Flow adhesion assays were performed with mouse neutrophils (PMN) in a planar laminar flow chamber containing cover slips coated with E-selectin–IgG, P-selectin– IgG, or (as control) human IgG1 (as indicated). Before the assay, cells were preincubated either with DME/10% FCS containing no antibody (no Ab), 25 μg/ml of an mAb against mouse Mac-1 (CO), 25 μg/ml of MEL14 (1), LAM1-110 (2), or LAM1-116 (3). All three mAbs are reactive against the lectin domain of L-selectin. The number of rolling cells per area were quantitated by visual examination of videotaped images. Data presented are mean ± SEM of ten different areas evaluated. The depicted experiment represents one of three similar experiments.
Figure 9.
Attachment of flowing human neutrophils to E-selectin is inhibited by an mAb against L-selectin. Flow adhesion assays with human neutrophils (PMN) were performed and evaluated as described in Fig. 8. Cells were preincubated either with DME/10% FCS containing no antibody (no Ab), 25 μg/ml of an mAb against human Mac-1 (CO), 25 μg/ml of DREG56 (1), or 25 μg/ml of LAM1-14 (2). DREG56 recognizes the lectin domain of L-selectin while LAM1-14 recognizes an short consensus repeats domain.
Rolling of E-Selectin–transfected CHO Cells on Immobilized Human L-Selectin Is Blocked by DREG56
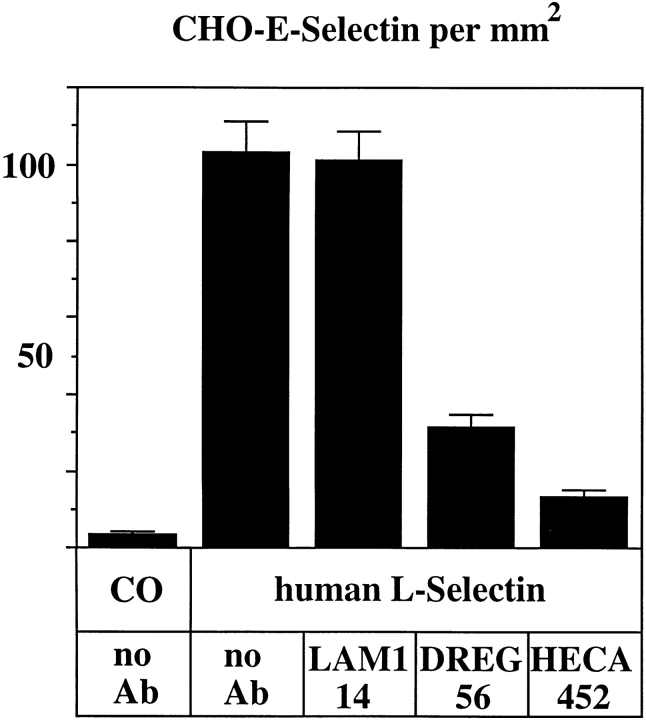
To study the interaction between E-selectin and human L-selectin in the absence of other adhesive mechanisms, we analyzed the rolling of E-selectin–transfected CHO cells on purified human L-selectin. To this end, human L-selectin was purified in a two-step procedure: first with a DREG56 affinity column, and second with an E-selectin– IgG affinity column from human serum and coated onto coverslips. As shown in Fig. 10, E-selectin transfectants rolled on human L-selectin, and this interaction was largely inhibited by mAb DREG56, while mAb LAM1-14 did not inhibit. Rolling was dependent on L-selectin–associated carbohydrates since it could be blocked by the anticarbohydrate antibody HECA452. These data demonstrate that DREG56 can interfere with the recognition of L-selectin– based carbohydrates by E-selectin.
Figure 10.
Rolling of E-selectin–transfected CHO cells on immobilized human L-selectin can be blocked by DREG56 and the anticarbohydrate antibody HECA452. Coverslips coated either with hIgG (Co) or human L-selectin as indicated were treated with DME/25% FCS containing no antibody (no Ab), 25 μg/ml of the functionally silent anti–L-selectin mAb LAM1-14, 25 μg/ml of the blocking anti–L-selectin mAb DREG56, or with fivefold concentrated hybridoma supernatant of the anticarbohydrate mAb HECA452 (as indicated). Prepared coverslips were exposed to a flowing cell suspension of E-selectin–transfected CHO cells. The assay was evaluated as described for Fig. 8.
Similar studies were tried with mouse L-selectin. However, the amount of L-selectin that could be prepared from mouse neutrophils using a Mel14 affinity column was very low. From the neutrophils of 20 mice, not more than 40 ng of L-selectin could be purified, as was roughly estimated by silver staining. A complete preparation coated in 400 μl on a coverslip (100 ng/ml) did not give rise to any rolling of E-selectin–transfected cells (not shown).
Discussion
Since the first reports about the binding of endothelial selectins to L-selectin (Picker et al., 1991; Kishimoto et al., 1991; Abbassi et al., 1993; Lawrence et al., 1994; Patel et al., 1995), it has been a matter of debate whether L-selectin indeed functions as a ligand for the endothelial selectins. The proposed ligand-function was mainly based on two lines of evidence: First, monoclonal antibodies against L-selectin blocked the interaction of human neutrophils with E-selectin. Second, purified L-selectin from human neutrophils was able to support binding of E-selectin transfectants in nonstatic (rotation) adhesion assays.
The two major points of criticism against this argumentation were: First, the inhibitory effect of the anti–L-selectin antibody could be due to indirect effects, which are possibly due to steric hindrance of other, closely associated adhesion molecules. Indeed, L-selectin and PSGL-1 were both shown to be enriched on the microvillous surface of neutrophils (Picker et al., 1991; Moore et al., 1995; von Andrian et al., 1995). Second, L-selectin on human neutrophils carries sLex-like carbohydrate epitopes. Since even neoglycoproteins, such as BSA, conjugated to sLex can support interactions with E-selectin transfectants, binding assays with coated L-selectin only demonstrate that L-selectin carries E-selectin–binding carbohydrate epitopes. The purpose of our work presented here was to examine whether human L-selectin not only supports rolling of E-selectin transfectants, but whether L-selectin would indeed be a preferred ligand among all other cellular proteins. This could only be tested by affinity isolation experiments and was all the more important since previous affinity isolation experiments with mouse neutrophils had failed to detect L-selectin among the isolated E-selectin ligands (Levinovitz et al., 1993; Lenter et al., 1994). Our results reported here clarify the apparent discrepancy between the published indirect evidence for a ligand function of human L-selectin and our previous work: human L-selectin indeed binds to E-selectin, while mouse L-selectin does not. Furthermore, the selective affinity isolation of human L-selectin with E-selectin–Ig demonstrates that human L-selectin is a preferred ligand for E-selectin, which binds better than almost all other glycoproteins of human neutrophils, besides PSGL-1 and ESl-1. Shedded L-selectin could even be isolated from human serum in a single-step purification procedure with an E-selectin affinity column. No other serum protein was isolated with similar efficiency in such experiments. We believe that the selective binding of L-selectin, PSGL-1, and ESL-1 to E-selectin is based on the selective posttranslational modification of these ligands with E-selectin–binding carbohydrate epitopes. Indeed, ESL-1 was recently shown to be the only carrier of the HECA452 carbohydrate epitope and to be the exclusive E-selectin ligand of CHO cells upon transfection of the fucosyltransferases VII or IV (Zöllner and Vestweber, 1996).
While indirect evidence for the binding of E-selectin to L-selectin was reported by numerous groups, P-selectin binding to L-selectin was only reported once (Picker et al., 1991). In contrast to these data, we could not block attachment of flowing human neutrophils on P-selectin with the anti–L-selectin mAb DREG56 (in agreement with Patel et al., 1995), and we could not affinity isolate human L-selectin with P-selectin–IgG (in agreement with Moore et al., 1992). Thus, we could not provide evidence for the binding of P-selectin to human L-selectin.
It has been shown that human neutrophils can roll on each other in an L-selectin–dependent fashion (Bargatze et al., 1994). PSGL-1 was recently shown to be a ligand for L-selectin (Spertini et al., 1996) and to be involved in L-selectin–dependent rolling of neutrophils on neutrophils (Walcheck et al., 1996). Based on these observations, we need to consider that the inhibitory effect of DREG56 on the rolling of neutrophils on E-selectin–coated surfaces could be due in part to the inhibition of L-selectin–mediated interactions between neutrophils. With this possibility in mind, we have carefully analyzed our experiments. Indeed, areas of accumulating neutrophils were occasionally observed where rolling neutrophils had served as obstacles for flowing neutrophils, causing the latter to slow down and facilitating their attachment to the glass surface. However, such areas were ignored for the quantitative analysis. Instead, only single, isolated cells that rolled on the E-selectin–coated surface without prior engagement with other rolling neutrophils were counted. Therefore, we conclude that the quantitated inhibitory effect of DREG56 in our experiments was due to interference with the interactions of single neutrophils with the E-selectin–coated support. Evidence that DREG56 not only blocks the lectin function of L-selectin but also the carbohydrate presentation to E-selectin was based on the inhibitory effect of DREG56 on the rolling of E-selectin transfectants on coated purified human L-selectin, an interaction that was also blocked by the anticarbohydrate antibody HECA452.
We have to consider that the biochemical evidence for selective binding of a glycoprotein to a selectin does not guarantee that it is indeed relevant as a ligand for cell adhesion or even physiologically relevant during leukocyte extravasation. Especially for the possible role of L-selectin as an E-selectin ligand, this is difficult to decide. L-selectin is certainly present on the surface of neutrophils at sites that are privileged for rolling interactions. In addition, purified L-selectin supports rolling of E-selectin transfectants, and DREG56 inhibits rolling of human neutrophils on E-selectin. However, L-selectin as well as E-selectin also bind to additional partner molecules, which are possibly involved in leukocyte extravasation. Besides additional E-selectin ligands such as ESL-1 and PSGL-1, in vitro adhesion studies have demonstrated that L-selectin on human neutrophils binds to cytokine-inducible, not yet characterized ligands on human umbilical vein endothelial cells (Smith et al., 1991; Spertini et al., 1991, 1992; Brady et al., 1992) whose expression kinetics were distinct from that of E-selectin. In vivo studies in mice indeed suggest that such additional cytokine-inducible ligands for L-selectin exist (Arbonés et al., 1994; Tedder et al., 1995). In addition, the function of L-selectin as capturing molecule in the process of neutrophil rolling on rolling neutrophils on the blood vessel wall is yet another level on which L-selectin could mediate leukocyte extravasation. Thus, the results presented here do not yet allow us to decide whether or to what extent the ability of E-selectin to recognize L-selectin on human neutophils is of physiological relevance for neutrophil extravasation.
It has been suggested that because of the posttranslational nature of the recognition motifs of selectins, there might be significant differences in functionally active glycoprotein ligands among different animal species (Varki, 1994). L-selectin appears to be the first example for such a species variation. The analysis of the carbohydrate structures on human L-selectin should prove useful to determine the structure of high-affinity carbohydrate ligands for E-selectin in humans. It would be interesting to compare these structures with those that were recently reported for human PSGL-1 (Wilkins et al., 1996).
Acknowledgments
We thank Dr. Kei Kishimoto for the generous gift of DREG56 and DREG200 antibodies, Dr. Thomas F. Tedder for kindly providing the LAM-1 antibodies, Dr. Martyn K. Robinson for generously providing human, pig, and rat E-selectin–IgG fusion proteins, Dr. Roland Mertelsmann for kindly providing samples of human bone marrow, Dr. Pierre Vassalli for E-selectin–transfected CHO cells, and Dr. Jürgen Brosius for critically reading the manuscript.
This work was supported by grants of the Deutsche Forschungsgemeinschaft to D. Vestweber.
Abbreviations used in this paper
- ESL
E-selectin ligand
- PMN
polymorphonuclear granulocytes
- PSGL
P-selectin glycoprotein ligand
Footnotes
Address all correspondence to Dietmar Vestweber, Institute of Cell Biology, ZMBE, Technologiehof, Mendelstr. 11, D-48149 Münster, Germany. Tel.: 49 251 83 86 17. Fax: 49 251 83 86 16.
References
- Abbassi O, Kishimoto TK, McIntire LV, Anderson DC, Smith CW. E-Selectin supports neutrophil rolling in vitro under conditions of flow. J Clin Invest. 1993;92:2719–2730. doi: 10.1172/JCI116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbonés ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Asa D, Raycroft L, Ma L, Aeed PA, Kaytes PS, Elhammer AP, Geng J-G. The P-selectin glycoprotein ligand functions as a common human leukocyte ligand for P- and E-selectins. J Biol Chem. 1995;270:11662–11670. doi: 10.1074/jbc.270.19.11662. [DOI] [PubMed] [Google Scholar]
- Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994;180:1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumhueter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA. Binding of L-selectin to the vascular sialomucin CD34. Science (Wash DC) 1993;262:436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- Brady HR, Spertini O, Jimenez W, Brenner BM, Marsden PA, Tedder TF. Neutrophils, monocytes, and lymphocytes bind to cytokine- activated kidney glomerular endothelial cells through L-selectin (LAM-1) in vitro. J Immunol. 1992;149:2437–2444. [PubMed] [Google Scholar]
- Briskin MJ, McEvoy LM, Butcher EC. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature (Lond) 1993;363:461–464. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- Duijvestijn AM, Horst E, Pals ST, Rouse BT, Steere AC, Picker LJ, Meijer CJLM, Butcher EC. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988;130:147–155. [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature (Lond) 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Hahne M, Jäger U, Isenmann S, Hallmann R, Vestweber D. Five TNF-inducible cell adhesion mechanisms on the surface of mouse endothelioma cells mediate the binding of leukocytes. J Cell Biol. 1993;121:655–664. doi: 10.1083/jcb.121.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas GS, Spertini O, Stoolman LM, Tedder TF. Molecular mapping of functional domains of the leucocyte receptor for endothelium, LAM-1. J Cell Biol. 1991;114:351–358. doi: 10.1083/jcb.114.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: a rapidly down regulated adhesion molecule. Proc Natl Acad Sci USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto TK, Warnock RA, Jutila MA, Butcher EC, Lane C, Anderson DC, Smith CW. Antibodies against human neutrophil LECAM-1 (LAM-1/Leu-8/DREG-56 antigen) and endothelial cell ELAM-1 inhibit a common CD18-independent adhesion pathway in vitro. Blood. 1991;78:805–811. [PubMed] [Google Scholar]
- Kuijper PH, Gallardo HI, Torres, van der Linden JA, Lammers JW, Sixma JJ, Koenderman L, Zwaginga JJ. Platelet-dependent primary hemostasis promotes selectin- and integrin-mediated neutrophil adhesion to damaged endothelium under flow conditions. Blood. 1996;87:3271–3281. [PubMed] [Google Scholar]
- Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML, et al. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Lasky LA. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem. 1995;64:113–139. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- Lasky LA, Singer MS, Dowbenko D, Imai Y, Henzel WJ, Grimley C, Fennie C, Gillett N, Watson SR, Rosen SD. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992;69:927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Bainton DF, Springer TA. Neutrophil tethering to and rolling on E-selectin are separable by requirement for L-selectin. Immunity. 1994;1:137–145. doi: 10.1016/1074-7613(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Lenter M, Levinovitz A, Isenmann S, Vestweber D. Monospecific and common glycoprotein ligands for E- and P-selectin on myeloid cells. J Cell Biol. 1994;125:471–481. doi: 10.1083/jcb.125.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinovitz A, Mühlhoff J, Isenmann S, Vestweber D. Identification of a glycoprotein ligand for E-selectin on mouse myeloid cells. J Cell Biol. 1993;121:449–459. doi: 10.1083/jcb.121.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn DM, Bargatze RF, Butcher EC. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987;138:4313–4321. [PubMed] [Google Scholar]
- Ley K, Gaethgens P, Fennie C, Singer MS, Lasky LA, Rosen SR. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991;77:2553–2555. [PubMed] [Google Scholar]
- Li F, Wilkens PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem. 1996;271:3255–3264. [PubMed] [Google Scholar]
- Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- McEver R, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992;118:445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KL, Patel KD, Bruehl RE, Fugang L, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MS, Miyasaka M, Tamatani T, Jones ML, Ward PA. Requirement for L-selectin in neutrophil-mediated lung injury in rats. J Immunol. 1994;152:832–840. [PubMed] [Google Scholar]
- Norman KE, Moore KL, McEver RP, Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood. 1995;86:4417–4421. [PubMed] [Google Scholar]
- Patel KD, Moore KL, Nollet MU, McEver RP. Neutrophils use both shared and distinct mechanisms to adhere to selectins under static and flow conditions. J Clin Invest. 1995;96:1887–1896. doi: 10.1172/JCI118234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butcher EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Pouyani T, Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995;83:333–343. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- Sako D, Chang X-J, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin-binding. Cell. 1995;83:323–331. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Smith CW, Kishimoto TK, Abbassi O, Hughes B, Rothlein R, McIntire LV, Butcher E, Anderson DC, Abbassi O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J Clin Invest. 1991;87:609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini O, Luscinskas FW, Kansas GS, Munro JM, Griffin JD, Gimbrone MA, Jr, Tedder TF. Leukocyte adhesion molecule-1 (Lam-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion. J Immunol. 1991;147:2565–2573. [PubMed] [Google Scholar]
- Spertini O, Luscinskas FW, Gimbrone MA, Jr, Tedder TF. Monocyte attachment to activated human vascular endothelium in vitro is mediated by leukocyte adhesion molecule-1 (L-Selectin) under nonstatic conditions. J Exp Med. 1992;175:1789–1792. doi: 10.1084/jem.175.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini O, Cordey A-S, Monai N, Giuffrè L, Schapira M. P-selectin glycoprotein ligand 1 is a ligand for L-selectin on neutrophils, monocytes, and CD34+hematopoietic progenitor cells. J Cell Biol. 1996;135:523–531. doi: 10.1083/jcb.135.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Steegmaier M, Levinovitz A, Isenmann S, Borges E, Lenter M, Kocher HP, Kleuser B, Vestweber D. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature (Lond) 1995;373:615–620. doi: 10.1038/373615a0. [DOI] [PubMed] [Google Scholar]
- Tedder TF, Steeber DA, Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Chambers JD, Berg EL, Michie SA, Brown DA, Karolak D, Ramezani L, Berger EM, Arfors K-E, Butcher EC. L-selectin mediates neutrophil rolling in inflamed venules through sialyl Lewisx-dependent and -indepentent recognition pathways. Blood. 1993;82:182–191. [PubMed] [Google Scholar]
- von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- Walcheck B, Moore KL, McEver RP, Kishimoto TK. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. J Clin Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SR, Fennie C, Lasky LA. Neutrophil influx into an inflammatory site inhibited by a soluble homing receptor-IgG chimaera. Nature (Lond) 1991;349:164–167. doi: 10.1038/349164a0. [DOI] [PubMed] [Google Scholar]
- Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoproteinligand-1 from HL60 cells. J Biol Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- Zöllner, O., and D. Vestweber. 1996. The E-selectin ligand-1 is selectively activated in Chinese hamster ovary cells by the α(1,3)-fucosyltransferases IV and VII. J. Biol. Chem. In press. [DOI] [PubMed]