Abstract
Calnexin is a membrane-bound lectin and a molecular chaperone that binds newly synthesized glycoproteins in the endoplasmic reticulum (ER). To analyze the oligomeric properties of calnexin and calnexin-substrate complexes, sucrose velocity gradient centrifugation and chemical cross-linking were used. After CHAPS solubilization of Chinese Hamster Ovary cells, the unoccupied calnexin behaved as a monomer sedimenting at 3.5 S20,W. For calnexin-substrate complexes the S-values ranged between 3.5–8 S20,W, the size increasing with the molecular weight of the substrate. Influenza hemagglutinin, a well-characterized substrate associated with calnexin in complexes that sedimented at 5–5.5 S20,W. The majority of stable complexes extracted from cells, appeared to contain a single calnexin and a single substrate molecule, with about one third of the calnexin in the cell being unoccupied or present in weak associations. However, when chemical cross-linking was performed in intact cells, the calnexin-substrate complexes and calnexin itself was found to be part of a much larger heterogeneous protein network that included other ER proteins. Pulse-chase analysis of influenza-infected cells combined with chemical cross-linking showed that HA was part of large, heterogeneous, cross-linked entities during the early phases of folding, but no longer after homotrimer assembly. The network of weakly associated resident ER chaperones which included BiP, GRP94, calreticulin, calnexin, and other proteins, may serve as a matrix that binds early folding and assembly intermediates and restricts their exit from the ER.
The ER maintains an efficient machinery for protein translation, folding, oligomeric assembly, and quality control (Gething and Sambrook, 1992; Helenius et al., 1992b ). The lumen provides an exclusive, highly specialized environment for the controlled folding and maturation of membrane proteins and soluble proteins most of which are destined for export to other organelles or for secretion. The redox environment and the ionic milieu are carefully maintained and concentration of molecular chaperones and folding enzymes such as protein disulfide isomerase (PDI),1 GRP 94, calnexin, calreticulin, and ERp72 is very high. At nearly millimolar concentrations, the most abundant of them greatly outnumber the substrate proteins.
The chaperone-assisted folding and the formation of disulfide bonds begin already while growing nascent chains enter the lumenal compartment (Bergman and Kuehl, 1979; Chen et al., 1995). It continues posttranslationally often with the formation of additional disulfide bonds and in many cases with the assembly of oligomers (Hurtley and Helenius, 1989; Braakman et al., 1991; Gething and Sambrook, 1992). Proteins that fail to fold or oligomerize properly are, as a rule, prevented from export, and get degraded (Hurtley and Helenius, 1989; Klausner, 1989; Hammond and Helenius, 1995).
During folding, polypeptides with N-linked oligosaccharides interact transiently and specifically with two homologous lectin-like chaperones that are unique to the ER, the membrane-bound calnexin and the soluble calreticulin. A transmembrane protein of 64 kD, calnexin interacts transiently with a large number of different glycoproteins during their folding and maturation (Ou et al., 1993). Calreticulin (46 kD), a soluble protein interacts with an overlapping but not identical group of proteins (Nauseef et al., 1995; Peterson et al., 1995; Wada et al., 1995). Both bind specifically to partially trimmed, monoglucosylated forms of the N-linked core glycans present on folding intermediates (Hammond et al., 1994; Hebert et al., 1995; Peterson et al., 1995; Ware et al., 1995; Spiro et al., 1996). They promote proper folding, prevent premature oligomerization, inhibit degradation, and mediate quality control for a variety of glycoproteins (David et al., 1993; Ou et al., 1993; Hammond and Helenius, 1994; Jackson et al., 1994; Kearse et al., 1994; Le et al., 1994; Loo and Clarke, 1994; Pind et al., 1994; Tector and Salter, 1995; Hebert et al., 1996; Vassilakos et al., 1996).
Despite the increasing understanding of the processes involved in protein maturation and quality control, it still remains unclear how the ER works as a functional entity and how calnexin and calreticulin carry out their functions. In this paper, we have focussed on calnexin. To characterize the properties of individual calnexin molecules and complexes between calnexin and its substrates, we analyzed detergent solubilized cell lysates by sucrose velocity gradient centrifugation and chemical cross-linking. To determine the nature of weaker contacts in situ, cross-linking in live cells was employed. Our results indicated that the unoccupied chaperones and chaperone complexes are small entities, but part of an extended network of molecules that form larger structures in the ER.
Materials and Methods
Cells, Viruses, and Chemicals
CHO15B cells and the Lec 23 cells used in this study are mutant cells that lack the medial-Golgi enzyme N-acetyl glucosamine transferase and glucosidase I, respectively (Gottlieb et al., 1975; Ray et al., 1991). They were grown as described (Balch et al., 1986; Ora and Helenius, 1995). The X31/ A/Aichi/1968 strain of Influenza virus was used to infect CHO15B cells (Braakman et al., 1991). Infected cells were used 4–6 h after infection for the pulse-chase experiments. Media and reagents for cell culture were obtained from Gibco BRL (Grand Island, NY). Brefeldin A (BFA) was purchased from Epicenter Technologies (Madison, WI). It was used at a final concentration of 5 μg/ml, from a stock of 1 mg/ml in ethanol. CHAPS and the chemical cross-linking reagents [Dithiobis(succinimidylpropionate)] (DSP), Disuccinimidyl Glutarate (DSG), and Disuccinimidyl Suberate (DSS) were purchased form Pierce (Rockford, IL). Castanospermine (CST), Cycloheximide (CHX), N-ethylmaleimide (NEM), and Triton X-100 were purchased from Sigma Chem. Co. (St. Louis, MO). [35S]-Promix containing cysteine and methionine was purchased from Amersham Corp. (Arlington Heights, IL).
Antibodies and Immunoprecipitations
The rabbit anti-influenza virus serum, the antiserum against the NH2-terminal 1-12 amino acid sequence of X31 influenza hemagglutinin (HA) referred to as NHA1, and the rabbit anti-calnexin COOH-terminal peptide antibodies have been described (Doms et al., 1985; Hammond et al., 1994; Peterson et al., 1995). The polyclonal rabbit anti–rat-calreticulin antisera were a gift from Dr. Hans Dieter Söling (Göttingen, Germany). The latter was affinity purified against rat calreticulin for use in immunoprecipitation (Peterson et al., 1995). The rabbit anti-calreticulin polyclonal antiserum and the rat anti-GRP94 monoclonal antiserum used in the cross-linking experiments in intact cells was purchased from Affinity Bioreagents (Neshanic Station, NJ). The mouse anti-BiP monoclonals were purchased from StressGen (Victoria, Canada).
Pulse-chase Analysis of HA Folding and Metabolic Labeling
The folding of HA was followed by the method described by Braakman et al. (1991). For studying calnexin binding the cells were lysed with 2% CHAPS in 0.2 M NaCl, 50 mM Hepes, pH 7.5 (HBS buffer, Hammond et al., 1994). For overnight labeling, the cells were starved for 15 min for methionine and cysteine and then 4 ml of complete α-MEM containing 0.3 mCi of radiolabeled methionine and cysteine (Amersham Promix) was added to each 60-mm dish.
Velocity Sedimentation on Sucrose Gradients
Monomeric and trimeric forms of HA were resolved on 5–25% continuous sucrose gradients in 20 mM MES, 100 mM NaCl, 30 mM Tris HCl, pH 8.6 (MNT buffer) with 0.1% Triton X-100 (Copeland et al., 1986). In experiments where the gradient fractions were to be used for cross-linking, the sucrose gradients were made in HBS buffer containing 0.1% Triton X-100. The cell lysates were layered on top of 5-ml gradients and centrifuged in an SW 55 rotor at 42,000 rpm for 15 h at 4°C (Beckman Instrs., Fullerton, CA). After centrifugation the gradients were fractionated manually from top to bottom. To characterize the size of the large cross-linked complex (Fig. 6), the cross-linked cell lysates were sedimented on a 5–25% sucrose gradient in TLS 55 rotor tubes. The tubes were sedimented at 160,000 g (50,000 rpm) for 3 h at 4°C in a Beckman table top ultracentrifuge. The gradients were fractionated into 12 fractions and precipitated with anti-HA antibodies.
Figure 6.
Effects of Brefeldin A. Ten confluent dishes of CHO15B cells were infected with Influenza virus. At 6 h postinfection five dishes were pretreated with Brefeldin A as described in Materials and Methods. All the dishes were pulse labeled and chased as indicated in the figure in the presence (lanes 6–10) or absence (lanes 1–5) of BFA and the chases were stopped with ice cold PBS with NEM. Cross-linking was performed before lysis as in Fig. 4 and the cell lysates were precipitated with anti-NHA1 antiserum. The immunoprecipitates were analyzed in the nonreduced form by 5% SDS-PAGE and fluorography. Top panels show uncross-linked samples and the bottom panels show cross-linked samples precipitated with anti-NHA1 antiserum.
Cross-linking of Proteins in Cells, Cell Lysates, or in Gradient Fractions Using Homobifunctional Cross-linkers
Cross-linking was performed in three different ways: in intact cells, in cell lysates, and in isolated gradient fractions. In intact cells, cross-linking was performed as follows. After pulse and chase, the cells were washed and left in phosphate buffer saline (PBS) with 20 mM NEM for 10 min on ice and then washed once with cold PBS. Cells were scraped gently in 225 μl of PBS (for ∼106 cells) and 20 mM stock of DSP (freshly made for each experiment) was added to give a final concentration of 2 mM. The stock solution of DSP (20 mM) was prepared in dimethyl sulfoxide (DMSO). In control samples an equivalent amount of DMSO without DSP was added. The cell suspension was incubated on ice for 30 min (with intermittent shaking) after which the excess cross-linker was quenched with 50 mM glycine. Cells were lysed by adding an equal volume of 4% CHAPS in PBS. Lysates were spun at 1,500 g to obtain a postnuclear supernatant (PNS) which was used for immunoprecipitations. Cross-linking with DSG and DSS was performed in a similar way.
Cross-linking in cell lysates was performed by including 2 mM final concentration of DSP in the lysis buffer (HBS) containing 2% CHAPS. Cells were incubated in the presence of the lysis buffer for 30 min on ice and cross-linker was quenched with 20 mM Glycine. An equal amount of DMSO without DSP was added to uncross-linked controls.
For cross-linking of proteins in the fractions from the sucrose gradients, DSP at a concentration of 2 mM was added to individual fractions and the fractions were incubated on ice for 30 min. Excess DSP was quenched using 20 mM glycine and the fractions were processed further for immunoprecipitation.
Results
Substrate-free Calnexin
Substrate-free calnexin was generated in CHO cells in three different ways. First, prolonged incubation with cycloheximide was used to inhibit the synthesis of proteins thus depleting the calnexin of its substrates (Fig. 1 A). Previous pulse-chase studies have shown that most substrates bind to calnexin for not more than ∼60 min (Hammond et al., 1994; Ou et al., 1993). Castanospermine, an α-glucosidase inhibitor that prevents trimming of newly synthesized glycoproteins to the required monoglucosylated form (Elbein, 1991), was similarly used to prevent the formation of binding-competent substrates (Fig. 1 B) (Hammond et al., 1994).
Figure 1.
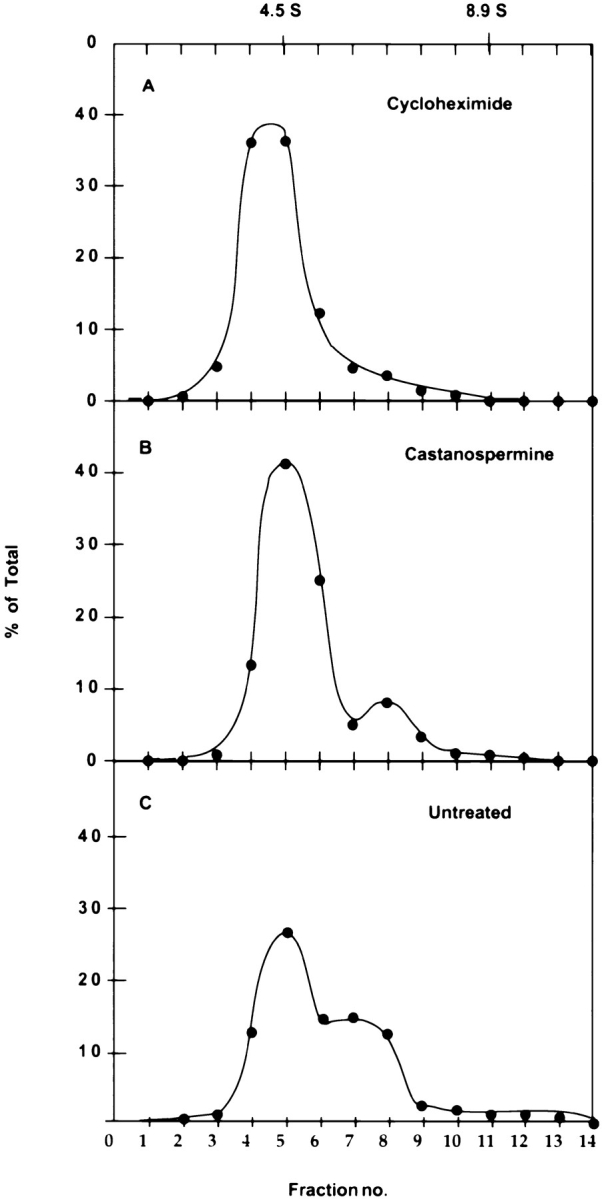
Sedimentation analysis of substrate-free calnexin and calnexin-substrate complexes. Three dishes of CHO15B were labeled for 16 h and chased for 5 h in the absence (C) or presence (A) of 500 μM cycloheximide. To one untreated dish of CHO15B cells, castanospermine (B) was added at a concentration of 1 mM in the last 1 h of chase. After the chase the cells were lysed in 2% CHAPS in HBS. The cell lysates were subjected to velocity sedimentation on sucrose gradients at 42,000 rpm for 15 h at 4°C. After centrifugation the gradients were fractionated from top to bottom and each fraction was precipitated with anti-calnexin antiserum. The precipitates were analyzed in Nonreduced form on 5% SDS-PAGE. The band corresponding to calnexin was quantified by scanning and plotted as the percentage of total for each gradient.
To incorporate radioactive label into calnexin, cells were labeled overnight with [35S]methionine and cysteine. They were then treated with inhibitors as described above for 5 h (cycloheximide) or 1 h (castanospermine), whereafter they were flooded with NEM, an alkylating agent that prevents further disulfide oxidation, and lysed with CHAPS. Postnuclear supernatants were subjected to sucrose gradient velocity centrifugation in the presence of the detergent. The gradient fractions were immunoprecipitated with the anti-calnexin antiserum, and analyzed by SDS-PAGE and fluorography. The bands were quantified by densitometry.
In each case, a major peak of calnexin was observed with an S-value of 3–4 S20,W. When samples from the main peak were cross-linked using a bifunctional chemical cross-linker DSP, no higher molecular weight bands were seen by SDS-PAGE suggesting that the peak corresponded to calnexin monomers (not shown). No evidence of additional labeled subunits was found. Notably, the proteins associated with the so-called SSR complex (SSR-α, SSR-β), previously reported to copurify with calnexin from MDCK cells (Wada et al., 1991), were absent. We concluded that in its unoccupied form, calnexin is solubilized from the ER mainly as a monomer.
Calnexin-Substrate Complexes
When the experiments in CHO cells described above were repeated without cycloheximide or castanospermine treatment, the endogenous calnexin-substrate complexes could be analyzed. The distribution of calnexin in the gradients was now clearly bimodal (Fig. 1 C). In addition to the peak at 3–4 S20,W which cosedimented with the unoccupied calnexin (A and B), a more prominent rapidly sedimenting population of calnexin molecules was apparent. Calnexin was distributed as heterogenously sized complexes over the 3–8 S20,W range.
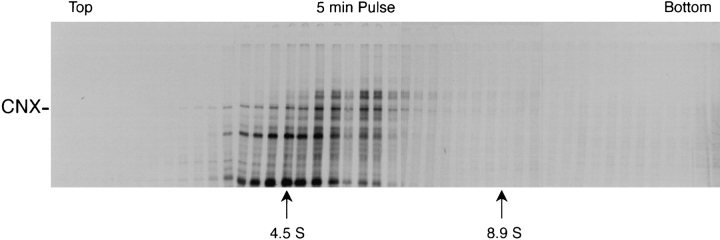
The proteins complexed to calnexin could be visualized when the cells were pulse-labeled for 5 min before solubilization (Fig. 2). As expected (Ou et al., 1993; Peterson et al., 1995), a large number of different cellular proteins were found coprecipitating with calnexin. These were present as a relatively discrete peak in the 4–8 S20,W range centered at ∼5.5 S20,W. The substrate proteins that had a higher apparent molecular weight were present in the faster sedimenting complexes indicating that their size determined the increment in sedimentation rate over that of calnexin alone. Given the relatively narrow size distribution and the low overall sedimentation rates, it seemed likely that most of the CHAPS-solubilized complexes contained a single calnexin and a single substrate molecule.
Figure 2.
Association of newly synthesized CHO-substrate proteins with calnexin. CHO15B cells were labeled for 5 min and lysed with 2% CHAPS containing buffer. The cell lysate was layered on a 5–25% sucrose gradient and centrifuged in SW 55 rotor at 42,000 rpm for 15 h. After centrifugation, the gradient was fractionated and each fraction was precipitated with anti-calnexin antiserum. The immunoprecipitates were analyzed in the nonreduced form using 7.5% SDS-PAGE and fluorography. The positions of the sedimentation markers are indicated; BSA, 4.5 S20,W, HA-trimers, 8.9 S20,W.
Analyzing Calnexin-Hemagglutinin Complexes by Cross-linking
To further analyze the properties of calnexin substrate complexes, we studied a single well-defined substrate molecule, influenza HA. This 84-kD glycoprotein associates with calnexin cotranslationally and remains associated for ∼4 min after chain completion until all the intrachain disulfides are formed (Hammond et al., 1994; Chen et al., 1995; Tatu et al., 1995). After dissociating from calnexin, HA monomers proceed to assemble into noncovalent homotrimers that are transported to the Golgi complex and eventually to the plasma membrane.
Using sucrose gradients, we have previously shown that calnexin-HA complexes sediment at 5–5.5 S20,W, calnexinfree HA subunits at 4.5–5 S20,W, and mature HA trimers at 8.9 S20,W (Tatu et al., 1995). Fig. 3 A shows the sedimentation of the folding intermediates (IT1, IT2), the fully oxidized NT, and the trimers of HA on sucrose gradients. As shown in Fig. 3 B, anti-calnexin antiserum brought down IT1, IT2, and some of the fully oxidized HA (NT) from the 5–5.5 S20,W region of the gradient. Calnexin itself was seen as a weakly labeled band just above IT1 (Fig. 3 B).
Figure 3.
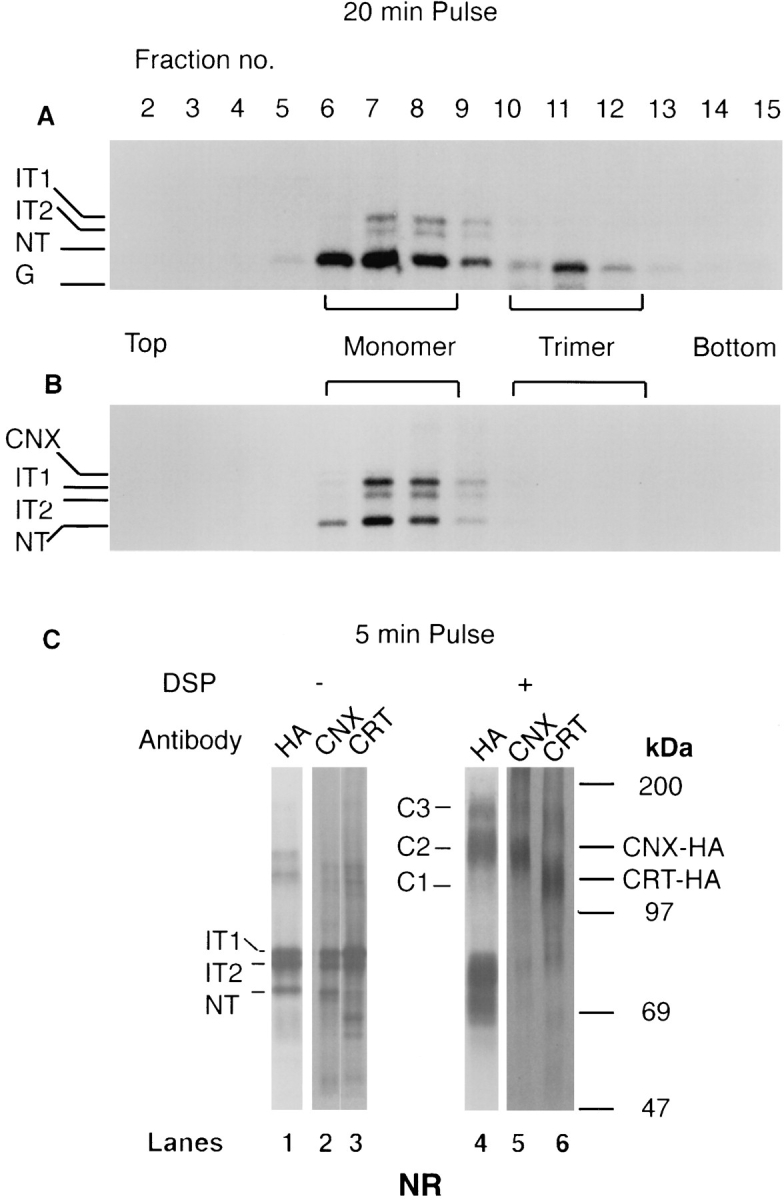
Calnexin and calreticulin form cross-linkable complexes with folding intermediates of HA. Influenza-infected CHO15B cells were labeled for 20 or 5 min and lysed as described above. The lysates were analyzed by velocity centrifugation on 5–25% sucrose gradients. After centrifugation the gradient was fractionated from top into 15 fractions. For the sample pulsed for 20 min, the fractions were immunoprecipitated with anti-HA antiserum (A) and with anticalnexin antiserum (B), and analyzed in nonreduced form by 7.5% SDS-PAGE. For the 5-min pulsed samples, each fraction was divided into two parts. Crosslinker DSP was added to one aliquot and cross-linking was performed as described in Materials and Methods. To the other, an equal amount of DMSO was added. The cross-linked (+ DSP, lanes 4, 5, and 6) and the uncross-linked (− DSP, lanes 1, 2, and 3) samples were further divided into two parts. These were immunoprecipitated with anti-HA (lanes 1 and 4), with anti-calnexin (CNX) antisera (lanes 2 and 5), or with anti-calreticulin (CRT) antiserum (lanes 3 and 6). The immunoprecipitates were analyzed in the nonreduced form by 7.5% SDS-PAGE and fluorography.
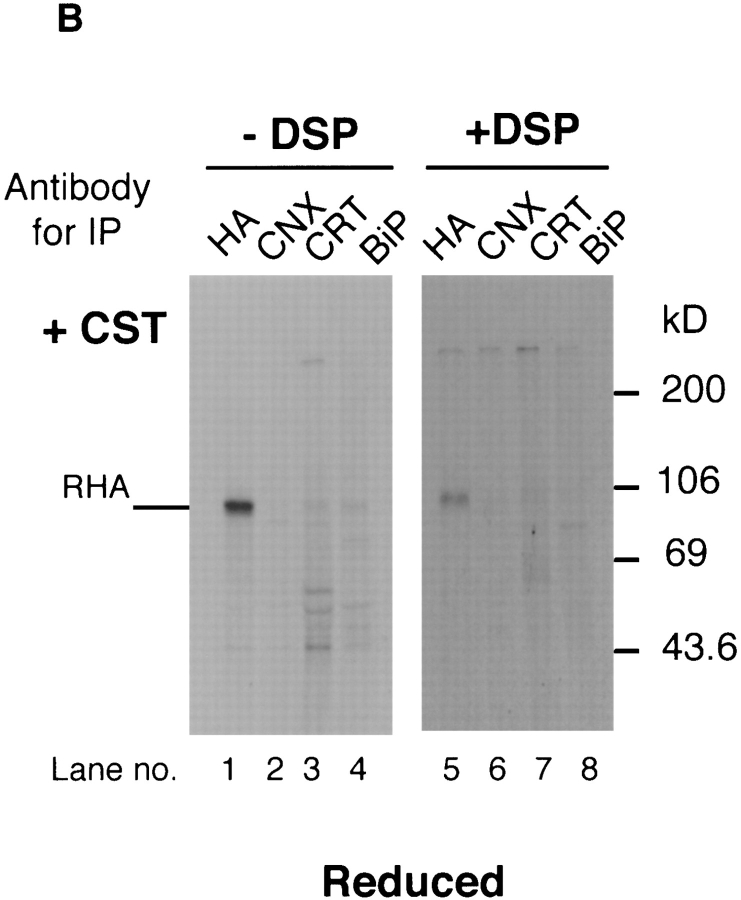
To characterize the oligomeric structure of the detergent solubilized complexes, influenza-infected cells were pulsed for 5 min, lysed, and fractionated on similar sucrose gradients. Fractions 5–7, which contained the calnexin and calreticulin complexes with HA, were pooled and aliquots were subjected to chemical cross-linking using the cleavable cross-linker DSP. Together with uncross-linked controls, the samples were then immunoprecipitated with antiHA, anti-calnexin, and anti-calreticulin, respectively, and analyzed in nonreduced form by SDS-PAGE and fluorography.
As shown in Fig. 3 C, anti-HA precipitates of uncrosslinked samples contained IT1, IT2, and NT (lane 1). The cross-linked sample showed the presence of three crosslinked species, C1, C2, and C3 (lane 4). The most prominent was C2 which had an approximate molecular mass of 140–160 kD. Since this band was also precipitated by anticalnexin (lane 5) it corresponded to the 1:1 HA-calnexin complex. Less intense cross-linked bands were seen at 120 kD (C1) and just below 200 kD (C3). Of these, C1 could be precipitated with anti-calreticulin (lane 6) suggesting that it corresponded to 1:1 HA-calreticulin complexes. The composition of C3 remains unclear. It was, however, precipitable both with anti-HA and anti-calnexin suggesting either a higher order complexes of these two polypeptides or a ternary complex containing an additional component. In some experiments, the C3 band was also precipitated with anti-calreticulin raising the possibility that it corresponded to ternary HA-calnexin-calreticulin complexes. Presence of such ternary complexes has also been observed when HA is expressed in microsomes (Hebert, D., and A. Helenius, manuscript in preparation). Occasionally we also saw higher molecular mass cross-links (>200 kD) in anti-calnexin and anti-calreticulin immunoprecipitates. The composition of these complexes is unclear.
Taken together, the results indicated that when cells were solubilized with nondenaturing detergent and subjected to centrifugation on sucrose gradients, the majority of calnexin- and calreticulin-bound HA was contained in discrete calnexin and calreticulin complexes. Some higher molecular weight complexes were also present.
Transient Association of HA with Larger Complexes
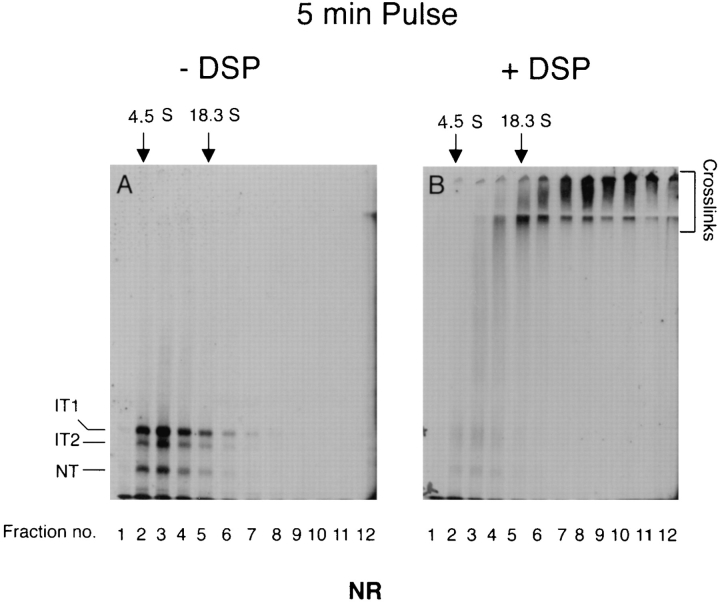
Next, we used cross-linking in situ to determine whether additional associations existed between the newly synthesized HA and resident ER proteins. We pulse-labeled influenza-infected cells for 3 min and chased for various times. The cells were then incubated with the cross-linker DSP (which is membrane permeable), lysed with CHAPS, immunoprecipitated with different antibodies, and analyzed by SDS-PAGE and fluorography. In the 5% gels used in this experiment, the separation of IT1, IT2, NT, and G (G is the Golgi form of HA) was improved, and large cross-linked complexes were more completely resolved than above.
The results shown in Fig. 4 A, demonstrated that the newly synthesized HA could be cross-linked inside the ER of the cell. The products turned out to be quite different than those seen after solubilization. When DSP was added within the first 2 min of chase, cross-linking was very efficient as shown by the virtually complete disappearance of the monomeric HA species (lanes 6 and 7, Nonreduced). That the HA was present in the material on top of the stacking gel was shown by boiling the samples in the presence of DTT, thus breaking the DSP links (lanes 6 and 7, Reduced).
Figure 4.
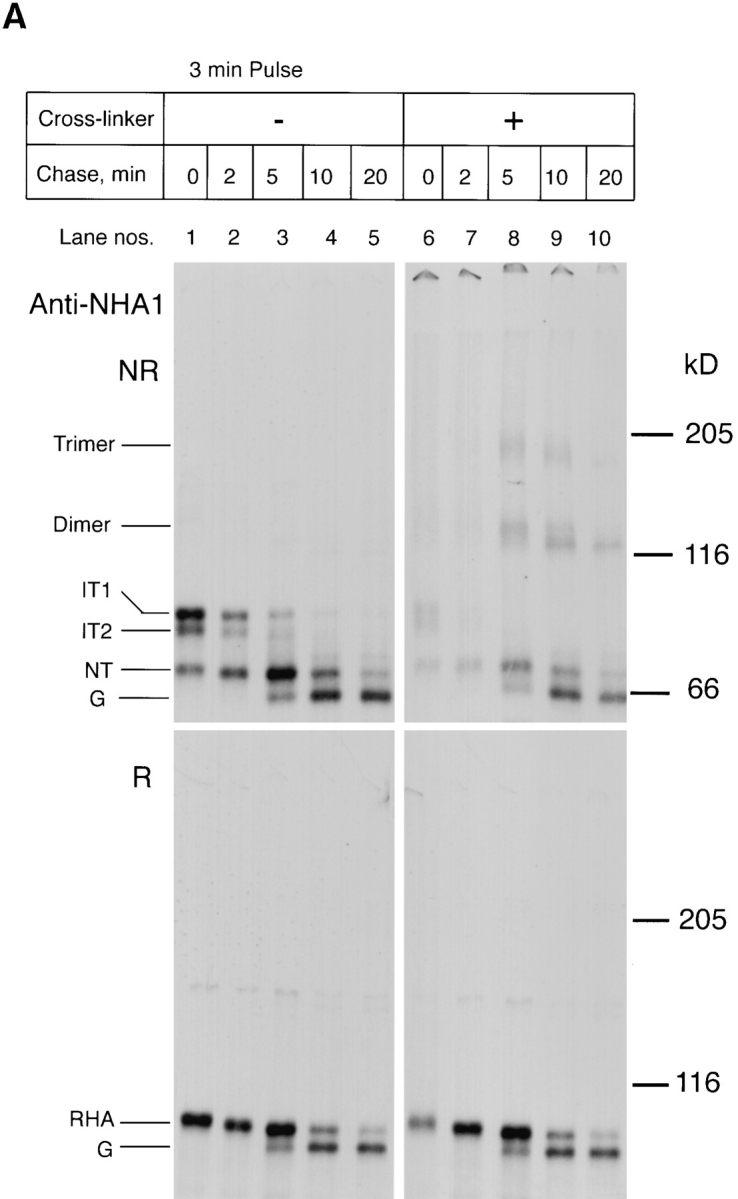
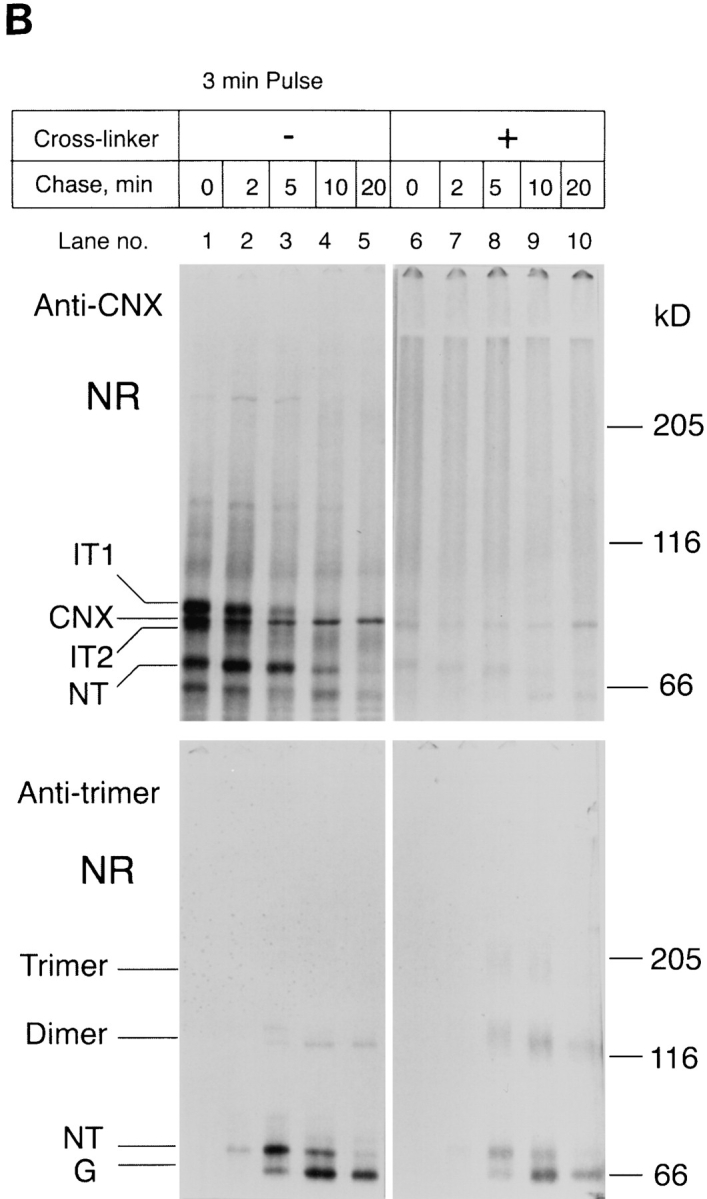
Newly synthesized HA is a part of large complexes until it assembles into trimers. Influenza-infected CHO15B cells were labeled for 3 min and chased for different time intervals as indicated. The chase was stopped by ice cold PBS containing alkylating agent NEM. The cells in each dish were scraped and divided into two aliquots. One aliquot was cross-linked with DSP as described in Materials and Methods and the other was used as an uncross-linked control. After cross-linking the cells were lysed. The lysates from the crosslinked (lanes 6–10) and the control samples (lanes 1–5) were immunoprecipitated with anti-NHA1 (Fig. 4 A), anti-calnexin (CNX, Fig. 4 B, top panel), and the trimer specific monoclonal antibody (Fig. 4 B, bottom panel) as described in Materials and Methods. The anti-calnexin and anti-trimer immunoprecipitates were analyzed only in their nonreduced form (Fig. 4 B). The anti-NHA1 immunoprecipitates were analyzed both in the nonreduced (Fig. 4 A, top panel) and the reduced forms (Fig. 4 A, bottom panel). The immunoprecipitates were analyzed by 5% SDS-PAGE and fluorography.
The result indicated that early folding forms of HA were efficiently cross-linked to covalent complexes most of which were so large that they did not enter the resolving gel. The same large HA-containing complexes could also be precipitated by anti-calnexin (Fig. 4 B, lanes 6 and 7), suggesting that the 1:1 calnexin-HA complexes observed by velocity centrifugation in CHAPS lysates (Fig. 3 C) were part of much larger cross-linkable complexes in situ.
As HA maturation proceeded during the chase, the pattern of cross-linked products began to change. After 5–20 min of chase, the HA appeared in the form of three discrete bands (Fig. 4 A, lanes 8–10). Unlike the previous large complexes, these were not coprecipitated with anticalnexin. One of them corresponded to the noncrosslinked monomeric species, the two others to HA homotrimers and homodimers familiar from previous studies (Doms and Helenius, 1986). All three bands were precipitable with trimer-specific monoclonal antibodies to HA (Copeland et al., 1986) (Fig. 4 B, anti-trimer). Moreover, within each band, a slight shift occurred with time from a slower to a somewhat faster migrating band. This shift is caused by mannose trimming of N-linked glycans in the Golgi complex (Balch et al., 1986). The shift revealed that the trimeric HA had moved from pre-Golgi compartments to the Golgi complex.
Similar large cross-linked complexes were obtained using another cross-linker DSG, indicating that the results were not cross-linker dependent (not shown). Moreover, when DSP was included in the lysis buffer, similar large cross-linked complexes were observed (not shown). Thus, the interactions between the HA and its cross-linking partners were apparently stable for some time after solubilization and dilution. Together with experiments described below (Fig. 7 B), this result indicated that the extensive cross-linking of early folding forms of HA was not just due to a high local protein concentration in the ER.
Figure 7.
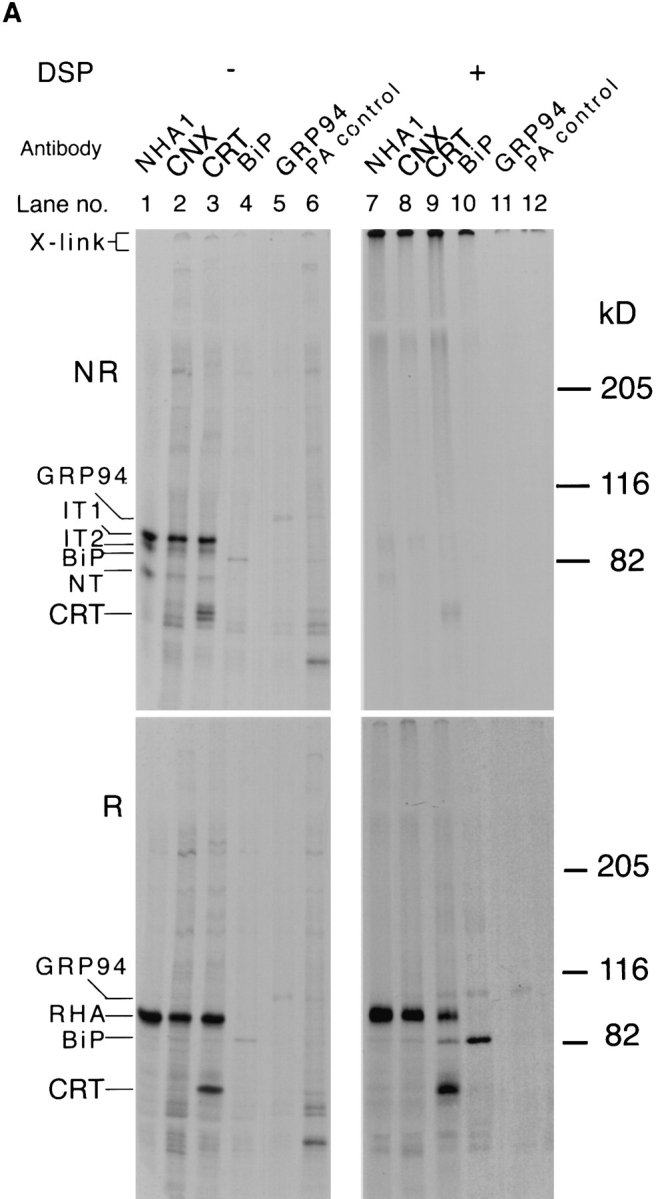
Composition of the folding complex. (A) CHO15B cells were prelabeled for 36 h and then infected with the Influenza virus. 6 h after infection the cells were pulse labeled for 5 min and the pulse was stopped with ice cold PBS containing NEM. The cells were scraped from the dish and divided into two aliquots. One aliquot was cross-linked with DSP as in Fig. 4. The cells were lysed with 2% CHAPS in HBS after cross-linking, and the lysates from the cross-linked and the control samples were divided into aliquots. These were precipitated with antibodies against HA (lanes 1 and 7), calnexin (CNX, lanes 2 and 8), calreticulin (CRT, lanes 3 and 9), BiP (lanes 4 and 10), GRP94 (lanes 5 and 11) and with Protein A beads without any antibody (lanes 6 and 12). The precipitates were analyzed in nonreduced (top panels) and the reduced form (bottom panels) by 5% SDS-PAGE and fluorography. (B) Two confluent dishes of CHO cells were infected with Influenza virus. 4 h after infection one dish was preincubated with 1 mM castanospermine for 1 h and then pulse labeled for 5 min in the presence of 1 mM castanospermine. The other untreated dish was pulse labeled without castanospermine for 5 min. Cross-linking was performed as above and cells were lysed with 2% CHAPS in PBS. Lysates were divided into four aliquots and immunoprecipitated as above. The immunoprecipitates were analyzed in the reduced form by 7.5% SDS-PAGE and fluorography.
To arrive at a size estimate for the cross-linked complexes containing HA, velocity sedimentation on sucrose gradients was carried out following cross-linking. After precipitating with anti-HA antibodies, the fractions were analyzed on SDS-PAGE in Nonreduced form (Fig. 5). Sedimentation standards (urease, 18.9 S20,W and BSA, 4.5 S20,W) were analyzed on identical gradients. While the uncrosslinked HA was found in fractions 2, 3, 4, and 5, the crosslinked complexes containing HA were present throughout the gradient indicating heterogeneous size. The cross-linked complexes ranged in size from ∼8 to more than 40 S20,W.
Figure 5.
Velocity gradient centrifugation to determine the size of the cross-linked complex containing HA. CHO cells were prelabeled for 15 h with [35S]methionine and cysteine and then infected with Influenza virus. 6 h after infection cells were pulse labeled for 5 min and the pulse was stopped with ice cold PBS containing 20 mM NEM. Cross-linking and cell lysis was carried out as in Fig. 5 and as described in Materials and Methods. The cross-linked and the control samples were layered on a 5–25% sucrose gradient in a 2-ml tube and sedimented in TLS 55 rotor in a table top ultracentrifuge as described under Materials and Methods. The gradients were fractionated and the fractions were precipitated with anti-HA antibodies. The immunoprecipitates were analyzed in the nonreduced form by 5% SDS-PAGE and fluorography.
Taken together, the results showed that early folding forms of HA (IT1, IT2, and NT) are cross-linked to large, heterogeneous calnexin-containing complexes. Evidently, the HA-calnexin and HA-calreticulin complexes present at this stage of maturation were part of extensive structures that could be stabilized by cross-linking in situ and in lysates immediately after solubilization. As folding proceeded, HA reached its trimeric form and was no longer directly associated with this structure. Instead, cross-linking now occurred between the subunits of the mature HA homotrimer.
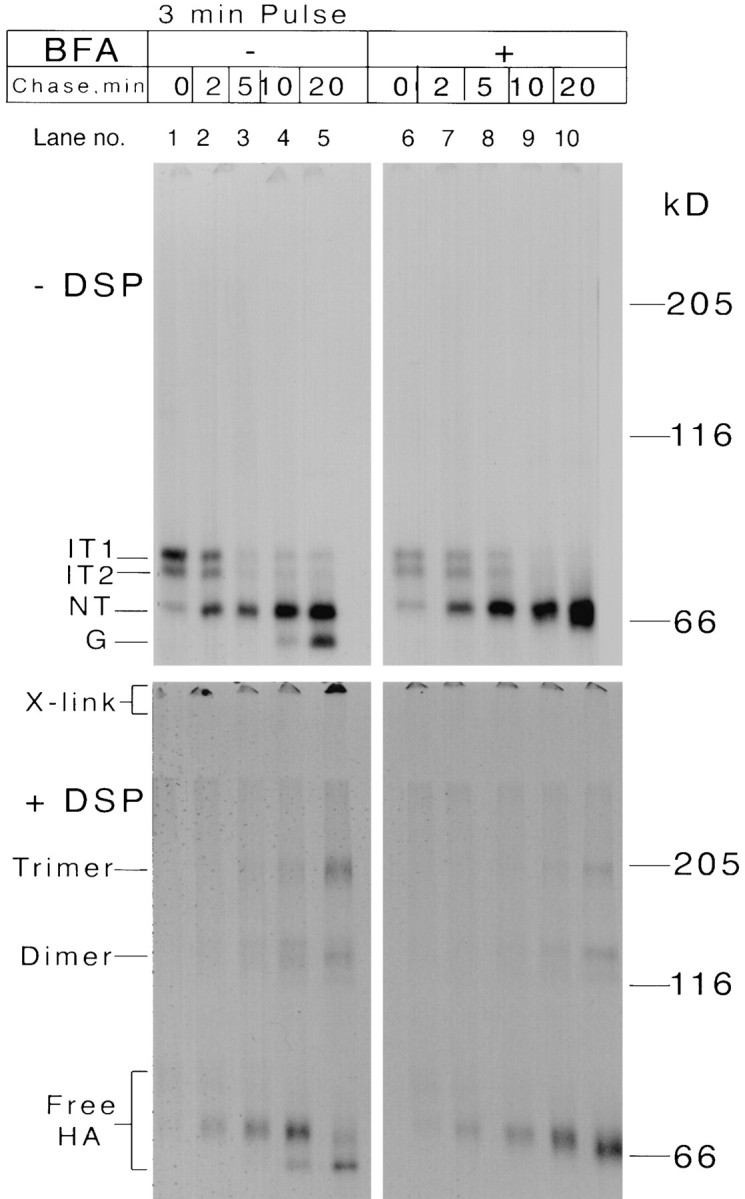
The Effects of Brefeldin A
That the HA trimers escaped cross-linking after trimer formation could mean that they were still in the ER but dissociated from the chaperone complexes, or it could be simply explained by their export out of the ER. To test whether HA trimers retained in the ER would cross-link to the large complexes, we employed BFA, a drug that prevents transport of HA and other proteins from the ER to the Golgi complex (Klausner et al., 1992). As shown in Fig. 6 (lanes 6–10, +DSP), BFA had no effect on the crosslinking of HA either early or late in the chase. That the inhibitor was working, was demonstrated by the lack of conversion of HA to the mannose-trimmed form marked G in the figure.
The result indicated that HA trimers, even though present in the ER, did not get cross-linked to large complexes of ER proteins. The interaction of early folding forms with constituent ER proteins was thus qualitatively different than that of assembled trimers. It was concluded that dissociation of HA from the chaperone machinery proceeded or coincided with trimer assembly, and that this led to a situation where cross-linking no longer occurred. We have previously shown that HA dissociates from calnexin just before trimer formation takes place (Tatu et al., 1995).
Composition of In Situ Cross-linked Complexes
To determine which lumenal ER proteins were part of the HA-containing, cross-linked complexes formed in situ, long term (36 h) labeling of CHO cells with [35S]methionine and cysteine was performed so that the resident proteins would be labeled. The cells were then infected with influenza virus, and 5 h postinfection the HA synthesized was labeled using a brief 5-min pulse. Half of the cells were cross-linked with DSP, and the other half used as an uncross-linked control. The lysates were immunoprecipitated with antibodies against five different antigens; HA, calnexin, calreticulin, BiP, and GRP94. A background control received protein A beads without antibody (“PA control”). The immunoprecipitates were analyzed in nonreduced form to determine whether cross-linking had occurred (NR), and in reduced form (R) to determine the identity of the cross-linked components.
Judging by the amount of cross-linked HA precipitated with anti-calnexin and anti-calreticulin compared to antiHA, almost all the HA was covalently cross-linked to either one or both of these chaperones (Fig. 7, Reduced, lanes 7–9). A small amount of BiP was also present in the complexes precipitated with anti-HA (bottom right panel, lane 7). We have previously shown that ∼5–10% of HA misfolds and binds to BiP (Hurtley et al., 1989). No detectable HA-precipitation occurred with anti-GRP94, but precipitations with this antibody were not very efficient (bottom right panel, lane 11).
The converse experiment using anti-HA did not bring down detectable amounts of labeled cellular proteins except a small amount of BiP (bottom right panel, lane 7). Considering the excess of chaperones present in the ER over HA, this was not unexpected since only a small fraction of each would bind at any given time to the newly synthesized HA molecules. From these results, we tentatively concluded that the large cross-linkable complexes containing HA also contained calnexin and calreticulin and possibly some BiP. Whether other ER proteins such as PDI were present, remains to be determined.
To rule out the possibility that the cross-linking of newly synthesized HA into large complexes containing ER chaperones in situ was merely a result of the high protein concentration, we carried out a cross-linking experiment in castanospermine-treated cells. Castanospermine inhibits HA association with calnexin and calreticulin by preventing trimming of glucose residues from the N-linked core oligosaccharides (Elbein, 1991; Hammond et al., 1994; Peterson et al., 1995). If cross-linking would simply occur as a result of high protein concentration, the lack of specific binding of HA to these chaperones should not prevent formation of large calnexin and calreticulin complexes containing HA. In castanospermine-treated cells, the newly synthesized HA failed, however, to precipitate with anticalnexin, anti-calreticulin, or anti-BiP antibodies regardless of whether the cells had been treated with DSP or not (Fig. 7 B, lanes 2 and 3 without DSP, lanes 6 and 7 after DSP cross-linking and reduction). The result indicated that to be cross-linked with a complex containing calnexin and calreticulin, HA had to be specifically associated with these chaperones. It was not enough that they were present in the same compartment.
Constitutive Association of Chaperone Proteins in the ER
Interestingly, the precipitations shown in Fig. 7 revealed extensive cross-linking of calreticulin with BiP and GRP94, and of GRP94 with BiP (see lanes 3 and 4). To examine whether these associations were dependent on the presence of newly synthesized substrate proteins, the cells were prelabeled as described. Then cycloheximide was added for 5 h before cross-linking in order to clear the ER of substrate proteins.
It was evident from the results (Fig. 8, A and B) that, under conditions where no newly synthesized proteins were present, the chaperones were associated with each other in a variety of cross-linkable combinations. The anticalnexin immunoprecipitates after reduction of the large cross-linked complexes formed (Fig. 8, lane 2) showed, for example, bands corresponding to calnexin, calreticulin, BiP, and GRP94. In addition to BiP, anti-BiP precipitates showed GRP94 and some calreticulin. Also, in Lec 23 cells where calnexin and calreticulin do not bind to substrates (Ora and Helenius, 1995) calreticulin could be cross-linked to calnexin, BiP, and GRP94 (Fig. 8 A, lane 3).
Figure 8.
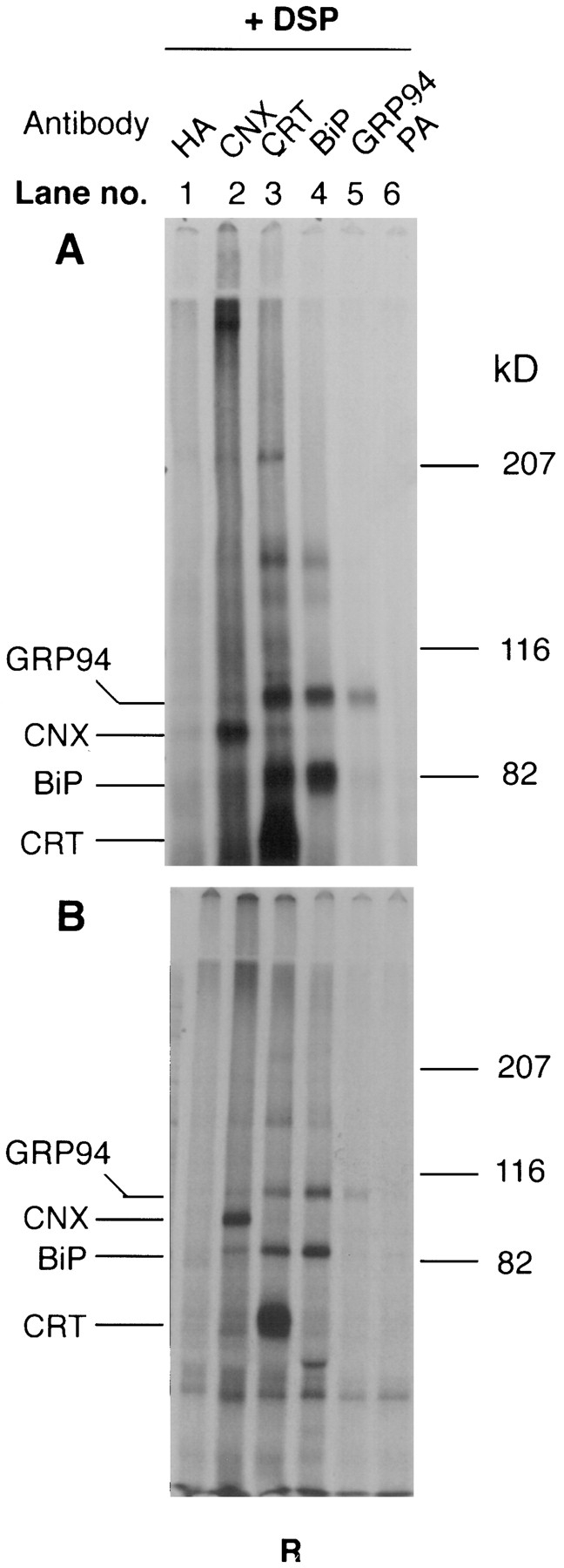
Constitutive association of the chaperone proteins of the ER. Lec 23 (A) and CHO (B) cells were prelabeled for 36 h as described in Materials and Methods. CHO's were treated with 500 μM cycloheximide for 5 h and cross-linking was performed, in both, as in Fig. 4. The cell lysates were divided into six different aliquots and immunoprecipitated with five antibodies. Lane 1, anti-NHA1; lane 2, anti-calnexin (CNX); lane 3, anti-calreticulin (CRT), lane 4, anti-BiP; lane 5, anti-GRP94. One aliquot was used for the protein A control without any antibody. The immunoprecipitates were analyzed in the reduced form by 5% SDSPAGE and fluorography. Only the cross-linked samples are shown.
Discussion
The results indicated that calnexin and possibly calreticulin formed stable, discrete 1:1 complexes with substrate glycoproteins in the ER. These complexes were resistant to detergent solubilization, gradient centrifugation, and immunoprecipitation. In the ER of intact cells, calnexin and calreticulin were, however, part of a larger network of interacting proteins which included other ER chaperones. While this network was stabilized by weaker contacts that dissociated upon detergent solubilization it could be analyzed using chemical cross-linkers in situ.
In CHAPS solubilized samples, substrate-free calnexin molecules were found to sediment at 3–4 S20,W. No evidence for the presence of additional, tightly bound subunits was observed although a shoulder of faster sedimenting forms were occasionally seen. This indicated that calnexin is a monomer when it is not associated with a substrate molecule. Judging by the sedimentation, we estimated that no more than about a third of the calnexin in CHO cells was normally in an unoccupied state. The rest was associated with a variety of different substrate proteins (see also Ou et al., 1993; Peterson et al., 1995).
The sedimentation rate (4–8 S20,W) in sucrose gradients after solubilization with CHAPS, indicated that complexes between calnexin and its substrate glycoproteins were only somewhat larger than calnexin alone. The sedimentation profiles suggested that most of them contained no more than a single substrate molecule. The molecular weight of the substrate proteins determined how much faster the complex sedimented than calnexin itself. Very few of the complexes were in the 12 S20,W range previously reported by Ou et al. (1993) to be the average size of calnexin-substrate complexes after cholate solubilization (Ou et al., 1993). The majority of complexes were in the size range observed for calnexin/α1-antitrypsin complexes (Le et al., 1994) i.e., smaller than originally assumed.
The 1:1 stoichiometry was found to apply to the majority of stable calnexin-HA and calreticulin-HA complexes extracted from virus-infected cells. While monomeric HA has an S-value of ∼4.5 S20,W (Doms and Helenius, 1986), the calnexin-HA complexes sedimented at ∼5–5.5 S20,W. When the gradient fractions were treated with the chemical cross-linker, the main cross-linked, HA-containing species had a MW of 140–160 kD, consistent with a 1:1 complex. Calreticulin-HA dimers somewhat smaller than the calnexin-HA dimers could also be demonstrated. Only a small amount of larger cross-linked species were observed, possibly representing ternary complexes containing both cross-linked calnexin, calreticulin, and HA. Previously we have shown that on sucrose gradients calnexin associated folding intermediates IT1 and IT2 sediment somewhat faster than calnexin associated NT (Tatu et al., 1995). Together with the observation that calreticulin preferentially binds to IT1 and some IT2 (Hebert et al., 1996), it seems probable that the folding intermediates form ternary complexes of HA, calnexin, and calreticulin. That ternary complexes can occur, has been recently shown for HIV gp160, a protein which has 24 N-linked glycans and therefore a large number of potential attachment sites for calnexin and calreticulin (Otteken and Moss, 1996). Others have shown that calnexin can bind to oligomeric assembly intermediates such as assemblies of α, β, and invariant chains of MHC class II antigens (Anderson and Cresswell, 1994). If present in lysates from CHO cells, such large oligomeric, calnexin-containing complexes were too few to be detectable in our gradients.
The observation that calnexin and calreticulin interact with their substrates as monomers renders them somewhat unusual because most lectins are homooligomeric (Weis and Drickamer, 1996). Even if lectins have extended binding sites that recognize more than just a single terminal residue (as calnexin and calreticulin do [Ware et al., 1995; Spiro et al., 1996]), the affinity for individual glycans generally reaches only the millimolar range which is not sufficient for stable association (Weis and Drickamer, 1996). Most lectins have, therefore, either multiple lectin domains in the same polypeptide chain or they are multimeric. For example, other membrane-bound lectins in the secretory pathway, the two mannose-6-P receptors and ERGIC 53, are oligomeric (Schweizer et al., 1988; Zhongmin et al., 1992). The members of the calnexin family of lectins have, however, conserved sequence repeats (Wada et al., 1995) which could provide as many as 3–4 glycan-binding domains per polypeptide.
While calnexin and calreticulin seem to bind to their substrates as monomers, the in situ cross-linking experiments indicated that they share weaker interactions with larger chaperone entities. Particularly obvious were crosslinks between calreticulin and BiP, calreticulin and GRP94, calreticulin and calnexin, as well as BiP and GRP94. Similar contacts have been previously reported in pancreatic acinar microsomes (Baksh et al., 1995) and in chondrocytes (Nakai et al., 1992). Since inhibition of protein synthesis had no effect on the cross-linking patterns, associations are present whether substrate proteins are synthesized or not. They probably represent authentic interactions in a dynamic network throughout the rough ER. Inhibition of substrate binding to calnexin and calreticulin by castanospermine inhibited cross-linking of HA to them further supporting that the cross-links represent highly specific interactions and ruling out the possibility of nonspecific crosslinks simply due to high protein concentrations in the ER.
The existence of a network of resident ER proteins has been postulated for some time (Kreibich et al., 1978; Hortsch et al., 1987; Booth and Koch, 1990; Sambrook, 1990; Helenius et al., 1992a ; Hammond and Helenius, 1995). At a concentration exceeding 100 mg/ml, the lumenal resident proteins, most of which are chaperones and folding enzymes, easily outnumber the newly synthesized substrate proteins present in the ER (Koch, 1987; Marquardt et al., 1993). Electronmicrographs of the ER reveal a lace-like material spanning the lumenal space. The majority of resident proteins have COOH-terminal KDEL sequences that provide a signal for retrieval from the Golgi complex (Pelham, 1991), and most of them have long negatively charged sequences that constitute low affinity, high capacity calcium-binding sites. Both of these sequence elements contribute to efficient localization of calreticulin in the ER lumen (Sönnichsen et al., 1994). While providing structure to the lumen and the membrane of the rough ER, the matrix is probably very dynamic and highly responsive to the fluctuations in free calcium and ATP concentrations (Sambrook, 1990; Nigam et al., 1994).
Pulse-chase experiments combined with in situ crosslinking showed that early full-length folding intermediates of HA were connected to the network. Once the HA reached its mature trimeric conformation, it was no longer crosslinked. While BiP and GRP94 were part of larger crosslinked structures, there was no evidence that they interacted directly with HA (Hurtley et al., 1989).
The ER matrix is likely to provide a structural framework for the resident chaperones and associated substrates, and functional structure necessary for efficient quality control. Thus, it may serve as a complex, mixedbed, “affinity chromatography matrix” that transiently adsorbs incompletely folded and assembled proteins, and prevents their nonspecific aggregation with each other. The substrate proteins undergo controlled on- and -off cycles with selected chaperone components of the matrix. Glycoproteins will, for example, associate with calnexin and calreticulin, with glucosidase II and UDP-glucose:glycoprotein glucosyl transferase driving the on- and -off cycle (Hammond and Helenius, 1993). These interactions may promote folding, suppress irreversible aggregation, and at the same time limit the mobility of the newly synthesized folding and assembly intermediates, preventing their premature exit from the ER.
Acknowledgments
The authors wish to thank Dr. Jani Simons for critically reading the manuscript and all the members of the Helenius-Mellman labs for helpful discussions and comments.
This work was supported by National Institutes of Health grants GM 38346 and CA 46128 to A. Helenius. U. Tatu was supported by a fellowship from the American Heart Association, Connecticut Affiliate, and a Human Frontier Science Program grant to A. Helenius.
Abbreviations used in this paper
- BFA
Brefeldin A
- DSP
dithiobis(succinimidylpropionate)
- DSG
disuccinimidyl glutarate
- HA
hemagglutinin
- NEM
N-ethylmaleimide
- PDI
protein disulfide isomerase
Footnotes
Please address all correspondence to A. Helenius, Department of Cell Biology, Yale University School of Medicine, 333 Cedar Street, P.O. Box 3333, New Haven, CT 06520-8002. Tel.: (203) 785-4301. Fax: (203) 7857226.
References
- Anderson KS, Cresswell P. A role for Calnexin (IP90) in the assembly of class II molecules. EMBO (Eur Mol Biol Organ) J. 1994;13(3):675–682. doi: 10.1002/j.1460-2075.1994.tb06306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksh S, Burns K, Andrin C, Michalak M. Interaction of calreticulin with protein disulfide isomerase. J Biol Chem. 1995;270:31338–31344. doi: 10.1074/jbc.270.52.31338. [DOI] [PubMed] [Google Scholar]
- Balch WE, Elliott MM, Keller DS. ATP-coupled transport of vesicular stomatitis virus G protein between the endoplasmic reticulum and the Golgi. J Cell Biol. 1986;261:14681–14689. [PubMed] [Google Scholar]
- Bergman LW, Kuehl WM. Formation of intermolecular disulfide bonds on nascent immunoglobulin polypeptides. J Biol Chem. 1979;254:5690–5694. [PubMed] [Google Scholar]
- Booth C, Koch LE. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1990;59:729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Braakman I, Hoover-Litty H, Wagner KR, Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991;114:401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Helenius J, Braakman I, Helenius A. Cotranslational folding and calnexin binding of influenza hemagglutinin in the endoplasmic reticulum. Proc Natl Acad Sci USA. 1995;92:6229–6233. doi: 10.1073/pnas.92.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland CS, Doms RW, Bolzau EM, Webster RG, Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986;103:1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David V, Hochstenbach F, Rajagopalan S, Brenner MB. Interaction with newly synthesized and retained proteins in the endoplasmic reticulum suggests a chaperone function for human integral membrane protein IP90(calnexin) J Biol Chem. 1993;268:9585–9592. [PubMed] [Google Scholar]
- Doms RW, Helenius A. Quaternary structure of influenza hemagglutinin after acid treatment. J Virol. 1986;60:833–839. doi: 10.1128/jvi.60.3.833-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Helenius A, White J. Membrane fusion activity of the influenza virus hemagglutinin: the low pH -induced comformational change. J Biol Chem. 1985;260:2973–2981. [PubMed] [Google Scholar]
- Elbein AD. Glucosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB (Fed Am Soc Exp Biol) J. 1991;5:3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- Gething M-J, Sambrook J. Protein folding in the cell. Nature (Lond) 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb C, Baenziger J, Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine: glycoprotein N-acetylglucosaminetransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975;250:3303–3309. [PubMed] [Google Scholar]
- Hammond C, Helenius A. A chaperone with a sweet tooth. Curr Biol. 1993;3:884–885. doi: 10.1016/0960-9822(93)90226-e. [DOI] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science (Wash DC) 1994;266:456–458. doi: 10.1126/science.7939687. [DOI] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway. Curr Biol. 1995;7:523–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharides, glucose trimming and calnexin during glycoprotein folding in the endoplasmic reticulum. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert D, Foellmer G, Helenius A. Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO (Eur Mol Biol Organ) J. 1996;15:2961–2968. [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Glucose trimming and reglucosylation determines glycoprotein association with calnexin. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Helenius A, Marquardt T, Braakman I. The endoplasmic reticulum as a protein folding compartment. TICB. 1992a;2:227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- Helenius, A., U. Tatu, T. Marquardt, and I. Braakman. 1992b. Protein folding in the endoplasmic reticulum. In Cell Biology and Biotechnology. R.G. Rupp and M.S. Oka, editors. Springer Verlag, Berlin/Heidelberg.
- Hortsch, M., C. Crimaudo, and D.I. Meyer. 1987. Structure and function of the endoplasmic reticulum. In Integration and Control of Metabolic Processes: Pure and Applied Aspects. O.L. Kon, editor. ICSU Miami, Miami. pp. 3.
- Hurtley SM, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Hurtley SM, Bole DG, Hoover-Litty H, Helenius A, Copeland CS. Interactions of misfolded Influenza hemagglutinin with binding protein (BiP) J Cell Biol. 1989;108:2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MR, Cohen-Doyle MF, Peterson PA, Williams DB. Regulation of MHC Class I transport by the molecular chaperone, calnexin(p88,IP90) Science (Wash DC) 1994;263:384–387. doi: 10.1126/science.8278813. [DOI] [PubMed] [Google Scholar]
- Kearse KP, Williams DB, Singer A. Persistence of glucose residues on core oligosaccharides prevents association of TCRα and TCRβ with calnexin and results specifically in accelerated degradation of nascent TCRα proteins within the endoplasmic reticulum. EMBO (Eur Mol Biol Organ) J. 1994;13:3678–3686. doi: 10.1002/j.1460-2075.1994.tb06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD. Architectural editing: determining the fate of newly synthesized membrane proteins. The New Biologist. 1989;1:3–8. [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch GLE. Reticuloplasmins: a novel group of proteins in the endoplasmic reticulum. J Cell Sci. 1987;87:491–492. doi: 10.1242/jcs.87.4.491. [DOI] [PubMed] [Google Scholar]
- Kreibich G, Ulrich BL, Sabatini DD. Proteins of rough microsomal membranes related to ribosome binding. J Cell Biol. 1978;77:464–487. doi: 10.1083/jcb.77.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Steiner JL, Ferrell GA, Shaker JC, Sifers RN. Association between calnexin and a secretion-incompetent variant of human α1- antitrypsin. J Biol Chem. 1994;269:7514–7519. [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Prolonged association of temperature-sensitive mutants of human P-glycoprotein with calnexin during biogenesis. J Biol Chem. 1994;269:28683–28689. [PubMed] [Google Scholar]
- Marquardt T, Hebert DN, Helenius A. Post-translational folding of influenza hemagglutinin in isolated endoplasmic reticulum-derived microsomes. J Biol Chem. 1993;268:19618–19625. [PubMed] [Google Scholar]
- Nakai A, Satoh M, Hirayoshi K, Nagata K. Involvement of the stress protein HSP47 in procollagen processing in the endoplasmic reticulum. J Cell Biol. 1992;117:903–914. doi: 10.1083/jcb.117.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef WM, McCormick SJ, Clark RA. Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase. J Biol Chem. 1995;270:4741–4747. doi: 10.1074/jbc.270.9.4741. [DOI] [PubMed] [Google Scholar]
- Nigam SK, Goldberg AL, Ho S, Rohde MF, Bush KT, Sherman MY. A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca(2+)-binding proteins and members of the thioredoxin superfamily. J Biol Chem. 1994;269:1744–1749. [PubMed] [Google Scholar]
- Ora A, Helenius A. Calnexin fails to associate with substrate proteins in glucosidase-deficient cell lines. J Biol Chem. 1995;270:26060–26062. doi: 10.1074/jbc.270.44.26060. [DOI] [PubMed] [Google Scholar]
- Otteken A, Moss B. Calreticulin interacts with newly synthesized human immunodeficiency virus type I envelope glycoprotein, suggesting a chaperone function similar to that of calnexin. J Biol Chem. 1996;271:97–103. doi: 10.1074/jbc.271.1.97. [DOI] [PubMed] [Google Scholar]
- Ou W-J, Cameron PH, Thomas DY, Bergeron JJM. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature (Lond) 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Pelham HR. Recycling of proteins between the endoplasmic reticulum and the Golgi complex. Curr Opin Cell Biol. 1991;3:585–591. doi: 10.1016/0955-0674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Ora A, Nguyen P, Van, Helenius A. Calreticulin is a lectin-like molecular chaperone for glycoproteins in the endoplasmic reticulum. Mol Biol Cell. 1995;6:1173–1184. doi: 10.1091/mbc.6.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pind S, Riordian JR, Williams DB. Participation of the endoplasmic reticulum chaperone calnexin(p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1994;269:12784–12788. [PubMed] [Google Scholar]
- Ray MK, Yang J, Sundaram S, Stanley P. A novel glycosylation phenotype expressed by Lec23, chinese hamster ovary mutant deficient in α-glucosidase I. J Biol Chem. 1991;266:22818–22825. [PubMed] [Google Scholar]
- Sambrook JF. The involvement of calcium in the transport of secretory proteins from the endoplasmic reticulum. Cell. 1990;61:197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Fransen JAM, Bächi T, Ginsel L, Hauri H-P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B, Füllefrug J, Nguyen P, Van, Diekmann W, Robinson DG, Meiskes G. Retention and retrieval: both mechanisms cooperate to maintain calreticulin in the endoplasmic reticulum. J Cell Sci. 1994;107:2705–2717. doi: 10.1242/jcs.107.10.2705. [DOI] [PubMed] [Google Scholar]
- Spiro RG, Zhu Q, Bhoyroo V, Söling H-D. Definition of the lectin-like properties of the molecular chaperone, calreticulin, and demonstration of its copurification with endomannosidase from rat liver Golgi. J Biol Chem. 1996;271:11588–11594. doi: 10.1074/jbc.271.19.11588. [DOI] [PubMed] [Google Scholar]
- Tatu U, Hammond C, Helenius A. Folding and oligomerization of influenza hemagglutinin in the ER and the intermediate compartment. EMBO (Eur Mol Biol Organ) J. 1995;14:1340–1348. doi: 10.1002/j.1460-2075.1995.tb07120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tector M, Salter RD. Calnexin influences folding of human class I histocompatibility proteins but not their assembly with B2-microglobulin. J Biol Chem. 1995;270:19638–19642. doi: 10.1074/jbc.270.33.19638. [DOI] [PubMed] [Google Scholar]
- Vassilakos A, Cohen-Doyle MF, Peterson PA, Jackson MR, Williams DB. The molecular chaperone calnexin facilitates folding and assembly of class I histocompatibility molecules. EMBO (Eur Mol Biol Organ) J. 1996;15:1495–1506. [PMC free article] [PubMed] [Google Scholar]
- Wada I, Imai S-i, Kai M, Sakane F, Kanoh H. Chaperone function of calreticulin when expressed in the endoplasmic reticulum as the membrane-anchored and soluble forms. J Biol Chem. 1995;270:20298–20304. doi: 10.1074/jbc.270.35.20298. [DOI] [PubMed] [Google Scholar]
- Wada I, Rindress D, Cameron PH, Ou W-J, Doherty JJ, II, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJM. SSRα and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]
- Ware FE, Vassilakos A, Peterson PA, Jackson MR, Lehrman MA, Williams DB. The molecular chaperone calnexin binds Glc1Man9 GlcNAc2oligosaccharides as an initial step in recognizing unfolded glycoproteins. J Biol Chem. 1995;270:4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- Weis WI, Drickamer K. Structural basis of lectin carbohydrate recognition. Annu Rev Biochem. 1996;65:441–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- Zhongmin M, Grubb JH, Sly WS. Divalent cation dependent stimulation of ligand binding to the 46 kDa mannose 6-phosphate receptor correlates with divalent cation-dependent tetramerization. J Biol Chem. 1992;267:19017–19022. [PubMed] [Google Scholar]