Abstract
Microtubules in the axon are uniformly oriented, while microtubules in the dendrite are nonuniformly oriented. We have proposed that these distinct microtubule polarity patterns may arise from a redistribution of molecular motor proteins previously used for mitosis of the developing neuroblast. To address this issue, we performed studies on neuroblastoma cells that undergo mitosis but also generate short processes during interphase. Some of these processes are similar to axons with regard to their morphology and microtubule polarity pattern, while others are similar to dendrites. Treatment with cAMP or retinoic acid inhibits cell division, with the former promoting the development of the axon-like processes and the latter promoting the development of the dendrite-like processes. During mitosis, the kinesin-related motor termed CHO1/MKLP1 is localized within the spindle midzone where it is thought to transport microtubules of opposite orientation relative to one another. During process formation, CHO1/ MKLP1 becomes concentrated within the dendrite-like processes but is excluded from the axon-like processes. The levels of CHO1/MKLP1 increase in the presence of retinoic acid but decrease in the presence of cAMP, consistent with a role for the protein in dendritic differentiation. Moreover, treatment of the cultures with antisense oligonucleotides to CHO1/MKLP1 compromises the formation of the dendrite-like processes. We speculate that a redistribution of CHO1/MKLP1 is required for the formation of dendrite-like processes, presumably by establishing their characteristic nonuniform microtubule polarity pattern.
Neurons are terminally postmitotic cells that use their microtubule arrays for process formation rather than cell division. In dividing cells, microtubules are organized into the mitotic spindle, which consists of two half-spindles that emanate from each spindle pole and partially overlap in the midzone region of the cell. Each half-spindle consists of uniformly oriented microtubules with their plus ends emanating from a centrosome at each pole (Euteneuer and McIntosh, 1982). The overlap of these microtubules in the midzone results in a region of nonuniformly oriented microtubules. Neurons also display characteristic uniform and nonuniform patterns of microtubule polarity orientation, specifically within the processes that they generate. Axons are long cylindrical processes that contain microtubules uniformly oriented with their plus-ends-distal to the cell body (Heidemann et al., 1981), while dendrites are short tapering processes that contain nonuniformly oriented microtubules (Baas et al., 1988). Developmental studies have established that the nonuniform microtubule polarity pattern of the dendrite, similar to the midzone region of the mitotic spindle, results from the superimposition of two populations of oppositely oriented microtubules (Baas et al., 1989; Sharp et al., 1995).
It is provocative to speculate that microtubules are organized in neuronal processes by modifications of the same mechanisms by which they are organized in the mitotic spindle. Recent studies have established that the critical factor in organizing spindle microtubules, even more fundamental than attachment to the centrosome, is the influence of motor proteins (Heald et al., 1996; for reviews see McIntosh, 1994; Hoyt, 1994; Barton and Goldstein, 1996; Moore and Endow, 1996). These studies indicate that motors generate forces among the microtubules to organize them into a bipolar spindle and to move them apart during mitotic progression. In recent studies, we demonstrated that expression of a portion of one of these mitotic motors in insect ovarian Sf9 cells causes these cells to extend processes with a thick tapering morphology and nonuniform microtubule polarity pattern highly reminiscent of neuronal dendrites (Kuriyama et al., 1994; Sharp et al., 1996). This kinesin-related motor protein, termed CHO1 or MKLP1, is present within the midzone region of the mitotic spindle where it is thought to transport oppositely oriented microtubules relative to one another (Sellitto and Kuriyama, 1988; Nislow et al., 1992). Specifically, it transports microtubules with minus-ends-leading toward the plus ends of other microtubules (Nislow et al., 1992), and hence would have the appropriate properties to intercalate minus-end-distal microtubules among plus-end-distal microtubules during dendritic development. Might CHO1/ MKLP1 redistribute during the conversion of a mitotic precursor cell into a postmitotic neuron to establish the nonuniform microtubule pattern of developing dendrites?
In the present study, we have used a system of cultured neuroblastoma cells that are ideally suited for testing whether such a redistribution occurs. These cells are mitotic, but also generate short processes during interphase. Some of the processes extended by these cells are axonlike with regard to their morphology and ultrastructure while others are dendrite-like (Ross et al., 1975). Our studies demonstrate that similar to bona fide axons and dendrites, the axon-like processes contain uniformly plus-end-distal microtubules, while the dendrite-like processes contain nonuniformly oriented microtubules. The cells can be rendered postmitotic by treatment with either cAMP, which promotes the development of the axon-like processes, or retinoic acid, which promotes the development of the dendrite-like processes (Prasad and Hsie, 1971; Shea et al., 1985; Fischer et al., 1986). Our studies reveal that CHO1/ MKLP1 is concentrated within the midzone region of the mitotic spindle during mitosis, but redistributes specifically to the dendrite-like processes both in cells undergoing normal interphase and in cells differentiated by retinoic acid. Also consistent with a role for the protein in dendritic differentiation, we show that the levels of CHO1/ MKLP1 increase in the presence of retinoic acid but decrease in the presence of cAMP. Finally, we show that treatment of the cells with antisense oligonucleotides that inhibit the expression of CHO1/MKLP1 compromises the formation of the dendrite-like processes.
Materials and Methods
Cell Culture
The neuroblastoma cell line Neuro-2a was obtained from Amer. Type Cell Collection (Rockville, MD). The cells were routinely cultured in DMEM (Gibco/BRL Laboratories, Grand Island, NY) containing 10% FBS (Hyclone Laboratories, Logan, UT) and antibiotics. For immunofluorescence analyses, the cells were plated onto polylysine-coated glass coverslips adhered to the bottom of a 35-mm tissue culture dish into which a 1-cm hole had been drilled. For morphometric, biochemical, and ultrastructural analyses, the cells were plated directly onto the plastic tissue culture dishes. For all of these analyses, the concentration of serum was reduced to 2%, and the cells were plated at a density of 200 cells/mm2. In some experiments, either retinoic acid or dibutyryl cyclic AMP (db cAMP)1 was added to the culture 6 h after plating. These reagents were obtained from Sigma Chem. Co. (St. Louis, MO), and introduced into cultures at final concentrations of 20 μM for the retinoic acid or 1 mM for the db cAMP. Antisense or sense oligonucleotides were also added to some of the cultures at this time.
Antisense and Sense Oligonucleotides
Before antisense experiments, Northern blot analyses were performed on purified messenger RNA as previously described (Nislow et al., 1992) to confirm the presence of the CHO1/MKLP1 message in the mitotic neuroblastoma cells and in cells rendered postmitotic with retinoic acid or db cAMP. Having confirmed this, translation of CHO1/MKLP1 was blocked by treatment of cultures with phosphorothioate substituted DNA oligonucleotides (Research Genetics, Huntsville, AL). An antisense oligonucleotide consisting of the sequence 5′-CAGGTTTCCTGGGCATCTT-3,′ which is the inverse complement of the coding sequence +19+37 of hamster CHO1/MKLP1 transcript (Kuriyama et al., 1994), was used in all experimental conditions. We termed this sequence AS1. Dose/response experiments were performed to determine the lowest concentration of oligonucleotide which induced the maximal inhibition of CHO1/MKLP1 expression. Oligonucleotides were stored in serum-free medium, aliquoted, and frozen at −80°C. 6 h after cells were plated, the medium was removed and new medium containing 2% serum and a concentration of 1 μM, 5 μM, 10 μM, or 20 μM antisense oligonucleotides was added. This medium was changed every 6 h and dishes were fixed at 24, 48, or 72 h and prepared for quantitative immunofluorescence microscopy. On the basis of the results of the dose-response analyses, a concentration of 10 μM was used for morphometric and microtubule polarity analyses. For a positive control, the antisense oligonucleotide consisting of the sequence 5′-AGCTTTCGCTGGTTTCATG-3,′ which is the inverse complement of the sequence −1+18 of hamster CHO1/MKLP1 transcript, was added to cultures at a concentration of 10 μM. We termed this sequence AS2. For negative controls, two oligonucleotides consisting of the sequences 5′-AAGATGCCCAGGAAACCTG-3′ and 5′-CATGAAACCAGCGAAAGCT-3,′ which are the inverse compliment of the two antisense oligonucleotides mentioned above, respectively, were used at 10 μM. These sequences were termed S1 and S2. BLAST searches indicated no other matches for the antisense or sense sequences.
Immunofluorescence Microscopy
For immunofluorescence analyses, the cultures were fixed for 6 min in cold methanol (−20°C), rinsed three times for five min each in PBS, and incubated for 1 h in a blocking solution containing 5% normal goat serum in PBS. The cells were then exposed overnight at 4°C to mouse monoclonal antibodies that specifically recognize either CHO1/MKLP1 (used at 1:5,000; Sellito and Kuriyama, 1988), β-tubulin (used at 1:500; Amersham Corp., Arlington Heights, IL), microtubule-associated protein-2 (MAP2) (used at 1:100; provided as a kind gift from Dr. I. Fischer, Philadelphia, PA), or the cytoplasmic dynein intermediate chain (used at 1: 1,000; provided as a kind gift from Dr. K.K. Pfister; see Pfister et al., 1996). The cells were then rinsed extensively in PBS, treated again for 1 h in blocking solution, and exposed for 1 h at 37°C to an appropriate fluorescent secondary antibody used at 1:100 (Jackson Immunoresearch Laboratories, West Grove, PA). Images were captured with the LSM 410 Laser Confocal Microscope (Carl Zeiss Incorporated, Thornwood, NY). For quantitative analyses on the levels of CHO1/MKLP1 within individual cells, the pinhole on the confocal system was opened maximally to allow the complete visualization of fluorescently labeled material in a single image. Fluorescence intensities were quantified using NIH Image software (provided free of charge from the National Institutes of Health, Bethesda, MD). Fluorescence intensities were calculated for cells treated with or without antisense oligonucleotides, sense oligonucleotides, retinoic acid, or db cAMP, and for cells treated with various combinations of these reagents. Intensities were expressed in arbitrary fluorescence units (AFU). One hundred cells were analyzed for each condition.
Microtubule Polarity Analyses
The polarity orientation of microtubules within processes extended under the various experimental conditions was determined using our recent modification (Sharp et al., 1996) of a previously described method (Heidemann and McIntosh, 1980). This method involves the decoration of existing microtubules with exogenous brain tubulin using a buffer that promotes the formation of lateral protofilament sheets. These sheets appear as curved appendages termed “hooks” on the microtubules when viewed in cross-section under the electron microscope. A clockwise hook indicates that the plus-end of the microtubule is directed toward the observer, while a counterclockwise hook indicates that the minus-end is directed toward the observer. Cultures were rinsed briefly in PBS and then incubated at 37°C for 20 min in a solution containing 0.25% saponin, 0.5 M Pipes, 0.1 M EGTA, 0.01 mM EDTA, 0.1 mM MgCl2, 2.5% DMSO, 0.5 mM GTP, and 1.2 mg/ml brain tubulin. Cultures were then fixed by the addition of an equal quantity of 4% glutaraldehyde, and then processed and embedded for electron microscopy by conventional methods. Video-print images were obtained before sectioning, and these were used to precisely document the points along the lengths of processes at which cross-sections were made. The sections were visualized and photographed using a CM 120 electron microscope (Phillips, The Netherlands). As in our previous work, a single section taken in the midregion of each process was used for quantification.
Morphometric Analyses
For morphometry, living cells were viewed by phase-contrast microscopy using a Zeiss Axiovert 35M inverted microscope, and were photographed with a KODAK Professional DCS 420 digital camera (Eastman Kodak Company, Rochester, NY). The digital images were printed using a UPD7000 digital printer (Sony, Japan) and subsequent analyses were performed on these prints. A total of at least 234 cells from each condition were chosen at random. These cells were first determined to be either process-bearing or nonprocess-bearing, with a process defined as a cytoplasmic extension at least one cell body diameter in length. The percentage of processes that were either axon-like, dendrite-like, or ambiguous as well as the average length (mean ± SD) of each type of process were then determined. A dendrite-like process was defined as a process with a diameter greater than 5 μm as it emerges at a gradual and obtuse angle from the cell body and which tapers to at least half this diameter at sites along its length. An axon-like process was defined as a process with a proximal diameter <1.5 μm as it emerges sharply at roughly right angles from the cell body and which maintains a roughly consistent diameter along its length. Processes that could not be fit into one of these categories were termed ambiguous.
Results
Neuro-2a Cells Extend Axon-like and Dendrite-like Processes
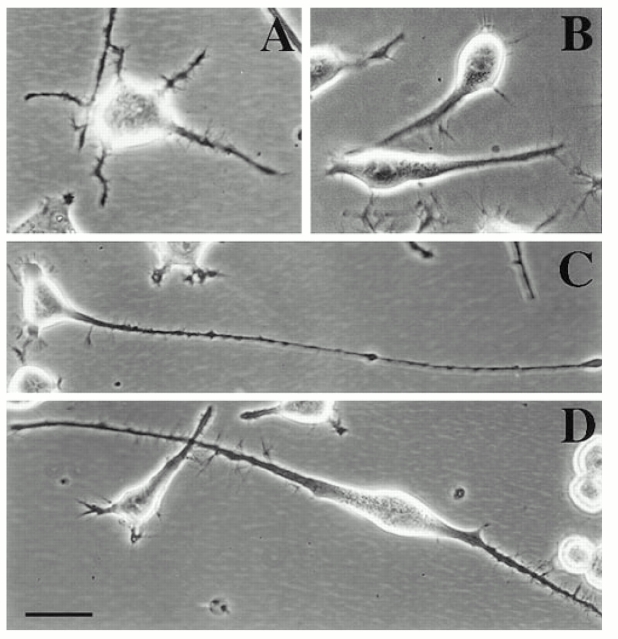
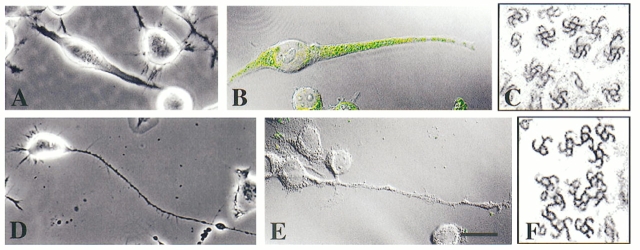
Neuro-2a cells were selected for the present study because they are both mitotic and extend short processes during interphase. The cells undergo roughly two mitotic divisions per day. The short processes are usually retracted into the cell body as the cell enters mitosis. As mitosis is completed, the daughter cells flatten somewhat and extend short processes in roughly the same pattern as the mother cell (Solomon, 1979). The morphology gradually changes with additional mitotic divisions, resulting in a great deal of morphological heterogeneity within the culture. The interphase processes are generally shorter than 50 μm, and can be classified into two categories on the basis of their morphology (see Materials and Methods). Some have the thin cylindrical shape characteristic of axons (Fig. 1 A) and others having a thicker more conical shape similar to dendrites (Fig. 1 B). Previous studies have documented that these axon-like and dendrite-like processes are also similar to bona fide axons and dendrites with regard to their ultrastructural characteristics (Ross et al., 1975). In particular, there is a clear demarcation between the cytoplasm of the cell body and that of the axon-like processes, with few or no ribosomes entering the axon-like processes. In contrast, there is no clear demarcation between the cytoplasm of the cell body and that of the dendrite-like processes, with ribosomes present throughout. An individual cell may display one or both types of processes. In one typical set of cultures, we examined 491 cells. 19% of these cells were process bearing. Of all processes extended by these cells, 58% were axon-like, 39% were dendrite-like, and 3% were ambiguous. The average lengths of these processes respectively were 36 ± 17, 37 ± 17, and 25 ± 5 μm (see Table I).
Figure 1.
Phase-contrast micrographs of cultured mouse neuroblastoma cells. A and B show normal interphase cells extending axon-like processes in A and dendrite-like processes in B. C shows an axon-like process extended by a cell in a culture treated with db cAMP. D shows dendrite-like processes extended by a cell in a culture treated with retinoic acid. Bar, 15 μm.
Table I.
Summary of Morphometric Analyses
| Control | Sense (S1) | Antisense (AS1) | Retinoic acid (RA) | RA + AS1 | cAMP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cells (n) | 491 | 641 | 416 | 365 | 355 | 234 | ||||||
| % of cells | ||||||||||||
| bearing processes | 19% | 15% | 26% | 35% | 36% | 36% | ||||||
| % of processes that | ||||||||||||
| are dendrite-like | 39% | 44% | 2% | 65% | 2% | 5% | ||||||
| Avg. length (μm) of | ||||||||||||
| dendrite-like processes | 37 ± 17 | 48 ± 27 | 41 ± 12 | 56 ± 32 | 35 ± 13 | 29 ± 12 | ||||||
| % of processes that are | ||||||||||||
| axon-like | 58% | 51% | 88% | 27% | 76% | 88% | ||||||
| Avg. length (μm) of | ||||||||||||
| axon-like processes | 36 ± 17 | 41 ± 13 | 46 ± 22 | 41 ± 27 | 46 ± 24 | 73 ± 52 | ||||||
| % of processes that | ||||||||||||
| are ambiguous | 3% | 5% | 10% | 8% | 22% | 7% | ||||||
| Avg. length (μm) of | ||||||||||||
| ambiguous processes | 25 ± 5 | 36 ± 8 | 32 ± 3 | 30 ± 17 | 37 ± 17 | 37 ± 15 |
As previously reported, an overnight treatment with either db cAMP or retinoic acid caused the cells to stop dividing and promotes process development (Prasad et al., 1971; Shea et al., 1985; Fischer et al., 1986). Treatment with db cAMP suppressed the formation of the dendrite-like processes and promoted the formation of the axon-like processes (Fig. 1 C). Of 234 cells examined in a set of db cAMP-treated cultures, 36% were process bearing. 88% of the processes were axon-like, 5% were dendrite-like, and 7% were ambiguous. The average lengths of these processes, respectively, were 73 ± 52, 39 ± 12, and 37 ± 15 μm. Treatment with retinoic acid promoted the formation of the dendrite-like processes (Fig. 1 D). Of 365 cells examined in a set of retinoic acid-treated cultures, 35% were process bearing. 27% of the processes were axon-like, 65% were dendrite-like, and 8% were ambiguous. The average lengths of these processes, respectively, were 41 ± 27, 56 ± 32, and 30 ± 17 μm (see Table I).
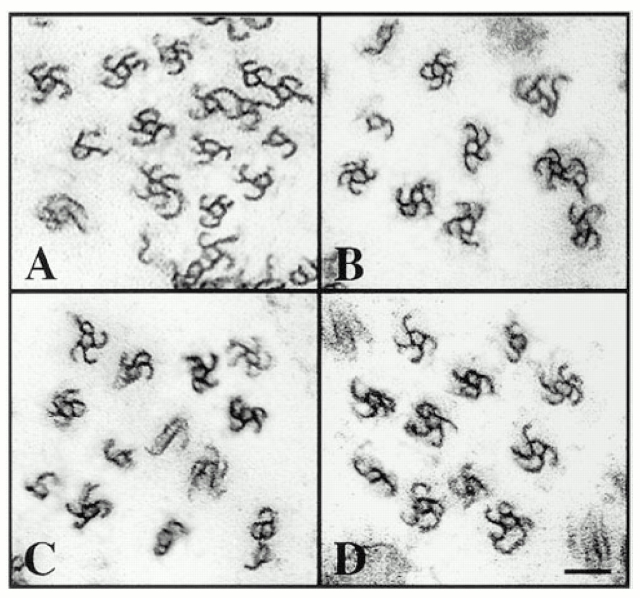
Previous studies documented that the axon-like and dendrite-like processes are similar to bona fide axons and dendrites with regard to ultrastructural features such as ribosome distribution and biochemical features such as MAP2 and tau distribution (Shea et al., 1985; Fischer et al., 1986). To determine whether these processes also show the characteristic uniform and nonuniform microtubule polarity patterns of axons and dendrites, we used the standard “hooking” method of microtubule polarity determination (see Materials and Methods). In this method, the cells are extracted in the presence of exogenous brain tubulin in a special microtubule assembly buffer that promotes the formation of lateral protofilament sheets on the existing microtubules. When viewed in cross-section, the sheets appear as hooked appendages on the microtubules. A clockwise hook observed from the tip of the process indicates that the microtubule is oriented with its plus end distal to the cell body, while a counterclockwise hook indicates the opposite. The number of microtubules within an individual cross-section taken in the midregion of the processes varied from 25–200, with the dendrite-like processes consistently showing higher numbers of microtubules than the axon-like processes. The percentage of these microtubules that displayed interpretable hooks was above 75%. Five axon-like processes from normal interphase cells all showed predominantly clockwise hooks (97 ± 3%; see Fig. 2 A), while eight dendrite-like processes showed high levels of both clockwise (58 ± 5%) and counterclockwise hooks (42 ± 5%; see Fig. 2 B). Five dendrite-like processes from cultures treated with retinoic acid showed high levels of both clockwise (46 ± 13%) and counterclockwise hooks (56 ± 9%; see Fig. 2 C). Five axon-like processes from cells treated with db cAMP showed predominantly clockwise hooks (98 ± 2%; see Fig. 2 D). Five axon-like processes from cultures treated with retinoic acid showed predominantly clockwise hooks (97 ± 2%; not shown). These data, summarized in Table II, indicate that the appropriate uniform and nonuniform microtubule polarity patterns of bona fide axons and dendrites are established within the axon-like and dendrite-like processes extended by normal interphase cells and in cells rendered postmitotic with db cAMP or retinoic acid.
Figure 2.
Microtubule polarity analyses performed on processes extended by neuroblastoma cells. In the axon-like processes extended by normal interphase cells, the hooked appendages on the microtubules are predominantly clockwise (A). In the dendritelike processes extended by these cells, roughly equal proportions of the hooks are clockwise and counterclockwise (B). In the dendrite-like processes extended in the presence of retinoic acid, roughly equal proportions of the hooks are clockwise and counterclockwise (C). In the axon-like processes extended in the presence of db cAMP, the hooks are predominantly clockwise (D). Bar, 0.08 μm.
Table II.
Summary of Microtubule Polarity Analyses
| Axon-like processes* | Dendrite-like processes* | |||
|---|---|---|---|---|
| Control | 97 ± 3% (n = 5) | 58 ± 5% (n = 8) | ||
| Sense (S1) | 96 ± 2% (n = 7) | 59 ± 13% (n = 7) | ||
| Antisense (AS1) | 99 ± 1% (n = 8) | N/A | ||
| Retinoic acid (RA) | 97 ± 2% (n = 5) | 46 ± 13% (n = 5) | ||
| RA + AS1 | 97 ± 3% (n = 8) | N/A | ||
| cAMP | 98 ± 2% (n = 5) | N/A |
Percentage of hooked microtubules with clockwise curvature as viewed from tip of process (mean ± SD). n, number of processes examined.
CHO1/MKLP1 Is Redistributed during Process Formation
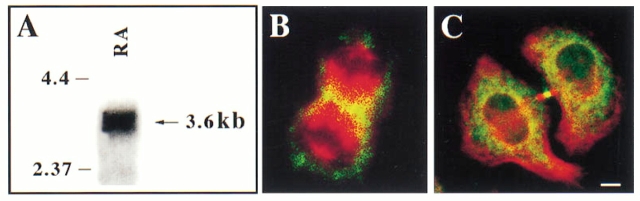
If the mitotic motor CHO1/MKLP1 plays a role in process formation, we would expect its expression to continue as the cell becomes postmitotic. Northern blot analyses indicate that the motor is expressed in mitotic cells, as expected, and also that expression continues in cells treated with either db cAMP or retinoic acid (see Fig. 3 A). Similar to our previous experience with mitotic cells (Sellitto and Kuriyama, 1988), we had difficulty in detecting the protein on Western blots. The reasons for this are unclear, but apparently relate to factors other than simply the low abundance of CHO1/MKLP1 within these cells (Kuriyama, R., unpublished observations). Because of this difficulty, we used quantitative immunofluorescence to analyze the levels as well as the distribution of the CHO1/MKLP1 protein with these cells. Our attention was initially focused on the mitotic cells to ascertain whether the monoclonal antibody previously used in Chinese hamster ovary cells effectively recognizes CHO1/MKLP1 in mouse neuroblastoma cells. The distribution of labeling was entirely similar to that reported for CHO and other mitotic cells types. Staining was concentrated along the microtubules of each halfspindle during metaphase (not shown), in the midzone region during anaphase (Fig. 3 B) and within the residual body during telophase (Fig. 3 C).
Figure 3.
A shows a Northern blot analysis designed to detect the presence of the 3.6-kb CHO1 message in neuroblastoma cultures. Messenger RNA from cultures treated with either retinoic acid (RA), db cAMP, or neither reagent were analyzed. Detectable levels of CHO1 message were found under all three conditions. The RA condition is shown here. B and C show immunofluorescence images of mitotic neuroblastoma cells immunostained for tubulin (red) and CHO1/MKLP1 (green). B shows a cell in anaphase, with CHO1/MKLP1 staining concentrated in the midzone. C shows a cell in telophase, with CHO1/ MKLP1 staining concentrated in the midbody. Diffuse staining for CHO1/MKLP1 is also present throughout the cells. Bar, 5 μm.
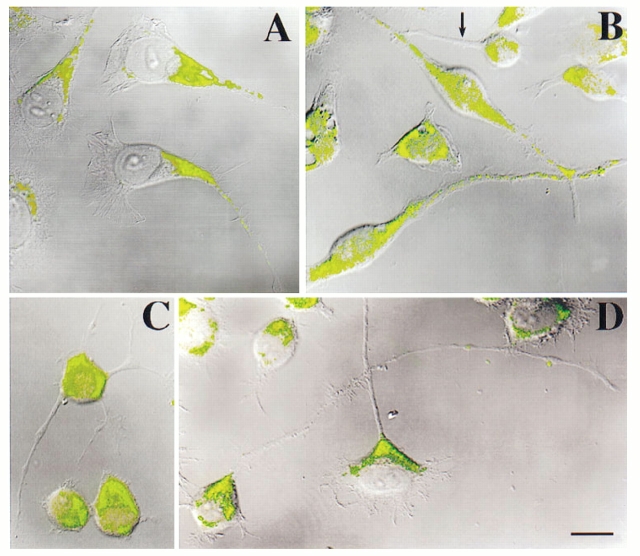
Having obtained these results as a positive control, we turned our attention to the process-bearing cells. In normal interphase cells, the staining for CHO1/MKLP1 was diffuse within the cell body, sometimes also appearing in nuclei. This contrasts with typical nonneuronal interphase cells in which staining is generally concentrated only at the centrosome and nucleus. In the neuroblastoma cells, the staining also extended into the dendrite-like processes. Staining was especially high in the more proximal onethird to one-half of the processes and then diminished with distance from the cell body (Fig. 4 A). This pattern was also displayed by the dendrite-like processes extended in the presence of retinoic acid (Fig. 4 B). In contrast, the axon-like processes of normal interphase cells (Fig. 4 C), cells treated with retinoic acid (Fig. 4 B; arrow), and cells treated with db cAMP (Fig. 4 D) showed virtually no staining.
Figure 4.
Immunofluorescence analyses of CHO1/MKLP1 distribution in neuroblastoma cells. Staining is shown in green, while cellular morphology is revealed by overlays of the DIC (differential-interference contrast) images of the cells. A shows dendrite-like processes of normal interphase cells. B shows dendrite-like processes of retinoic acid–treated cultures. One axonlike process is also apparent in B, and is marked with an arrow. C shows axon-like processes extended by a normal interphase cell. D shows axon-like processes extended by cells treated with db cAMP. In all cases, the cytoplasm within the cell body stains for CHO1/MKLP1. In some but not all cases, nuclear staining is also apparent. In all cases, the dendrite-like processes stain for CHO1/MKLP1, with high levels of staining proximally and gradually decreasing levels with distance from the cell body. The axonlike processes show virtually no staining for CHO1/MKLP1. Bar, 10 μm.
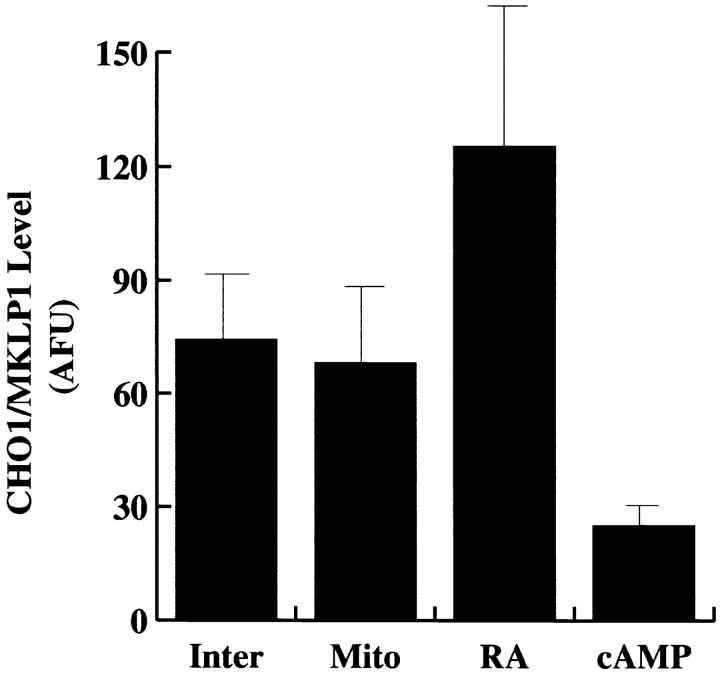
Fig. 5 shows data derived from quantitative immunofluorescence analyses on the total levels of CHO1/MKLP1 in normal interphase cells, cells undergoing mitotis, cells treated with retinoic acid, and cells treated with cAMP. The levels were statistically indistinguishable between normal interphase cells and cells undergoing mitotis, but showed a dramatic increase in the presence of retinoic acid and a dramatic decrease in the presence of cAMP (see figure legend for details). These data indicate a direct correlation between the levels of CHO1/MKLP1 and whether or not the cells generate dendrite-like processes.
Figure 5.
Quantitative immunofluorescence analyses on the levels of CHO1/MKLP1 within interphase neuroblastoma cells (Inter), cells undergoing mitotis (Mito), cells treated with retinoic acid (RA), and cells treated with cAMP (cAMP). Protein levels were measured in arbitrary fluorescence units (AFU). Ten cells were examined under each condition, and a mean ± SD was obtained for each condition. Statistical analyses using the student's t test indicate no statistical difference between interphase and mitotic cells (P < 0.687), but marked differences between retinoic acid– treated cells and the other experimental conditions (P < 0.001) and between cAMP-treated cells and the other experimental conditions (P < 0.0005). Retinoic acid–treated cells had significantly higher levels of CHO1/MKLP1 while cAMP-treated cultures had significantly lower levels of CHO1/MKLP1.
Inhibition of CHO1/MKLP1 Expression Affects Process Formation
The fact that CHO1/MKLP1 is concentrated within the dendrite-like processes is consistent with the idea that it might play a role in the differentiation of these processes. To test this, we used antisense oligonucleotides to suppress the expression of CHO1/MKLP1. Two different non-overlapping 19-base antisense oligonucleotide sequences corresponding to sites near the amino-terminal portion of the molecule were fabricated. As controls, we also obtained sense oligonucleotide sequences corresponding to both of these antisense sequences. The sequences, which were termed AS1, AS2, S1, and S2, were added directly to the culture medium at various concentrations, and were renewed every 6 h. The cultures were then prepared for quantitative immunofluorescence analyses on CHO1/MKLP1 levels every 24 h up to 72 h. To test for specificity, cultures were also prepared for quantitative analyses on the levels of β-tubulin, cytoplasmic dynein, and MAP2. MAP2 was of particular interest because it is a dendrite-enriched protein that may have a role of its own in dendritic differentiation (see for example Fischer et al., 1986). For every data point, 100 cells were randomly selected, and mean ± SD was obtained for the group.
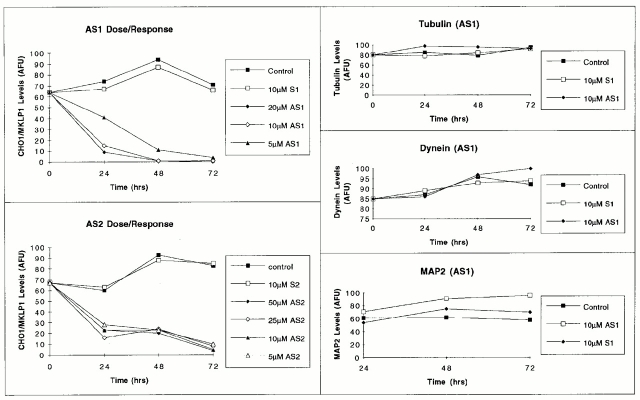
Marked effects on the levels of CHO1/MKLP1 were observed in cultures treated with either of the antisense sequences. By the first 24 h in the 5 μM concentration of AS1, the protein levels were reduced to <60% of control levels and were further reduced to 10–20% of control levels by 48 h. When the concentration of antisense was increased to 10 or 20 μM, the protein levels were reduced to 15–20% of control levels, and were further reduced to undetectable levels by 48 h. The AS2 antisense sequence also resulted in severely diminished protein levels, but was less effective than AS1, even when used at somewhat higher concentrations. Neither of the sense sequences detectably altered CHO1/MKLP1 levels. Neither of the antisense sequences and neither of the sense sequences resulted in diminutions in the levels of β-tubulin, MAP2, or cytoplasmic dynein. These results, shown in Figs. 6 and 7, indicate that the antisense sequences are effective in diminishing CHO1/MKLP1 levels, and strongly suggest that this response is not due to nonspecific ill effects of the oligonucleotides.
Figure 6.
Quantitative analyses on the levels of CHO1/MKLP1 (left two panels), and the levels of tubulin, dynein, and MAP2 (right three panels) in cultures treated with antisense and sense oligonucleotides. Protein levels were determined using quantitative immunofluorescence microscopy, and were expressed in arbitrary fluorescence units (AFU). Only process-bearing cells were scored in these analyses, and 100 cells were analyzed for each experimental condition and time point. Standard deviations were generally low, on the order of 15 AFU, and could not be included on the graph because the error bars would be short and blur together. Both AS1 and AS2 treatments resulted in diminished staining for CHO1/MKLP1 in a dose- and time-dependent manner. Sense controls did not affect CHO1/ MKLP1 levels. Neither antisense nor sense oligonucleotides resulted in diminutions in the levels of β-tubulin, dynein, or MAP2.
Figure 7.
Effects of CHO1/MKLP1 sense and antisense oligonucleotides on morphology, CHO1/MKLP1 immunofluorescence, and microtubule polarity orientation. A–C are cells treated with sense oligonucleotides, while D–F are cells treated with antisense oligonucleotides. The sense-treated cultures were entirely similar to controls in that they showed many dendrite-like processes (A) that stained for CHO1/MKLP1 (B) and contained microtubules of both orientations (C). The antisense-treated cultures contained almost exclusively axon-like processes (D). The staining for CHO1/MKLP1 was virtually obliterated from these cultures (E), and the microtubules within the processes were of uniform polarity orientation (F). Bars: (A, B, D, and E) 20 μm; (C and F) 0.1 μm.
Within the first 24 h in either antisense sequence, it was apparent that the number of cells in the culture had not increased, suggesting that mitotic division had ceased. This interpretation is supported by the fact that mitotic cells were never observed in cultures immunostained for tubulin or CHO1/MKLP1. Sense-treated cultures were entirely similar to controls with regard to cell proliferation and the appearance of mitotic profiles in immunostained cultures. Marked effects on process morphology were also observed in the cultures treated with either of the antisense sequences but neither of the sense sequences (see Fig. 7 and Table I). Specifically, the formation of dendrite-like processes was virtually obliterated in the presence of antisense, even when retinoic acid was also included in the culture. Diminution in the number of dendrite-like processes was apparent at 24 h in all concentrations of the antisense. For quantitative analyses, we used the 10 μm concentration of AS1 for 48 h, the lowest concentration and timeframe for either of the two antisense sequences that virtually obliterated detectable CHO1/MKLP1 staining. Out of 416 cells examined, 26% were process bearing. 88% of the processes were axon-like, 10% were ambiguous, and only 2% were dendrite-like. In cultures treated with antisense and retinoic acid, 36% of 355 cells were process bearing. 76% of the processes were axon-like, 22% were ambiguous, and only 2% were dendrite-like. The average lengths of the processes were not notably different from the lengths of processes of untreated cultures. In addition, sense-treated cultures were not notably different from untreated cultures with regard to any morphological criterion. Fig. 7 A shows a sense-treated culture with predominantly dendrite-like processes, while Fig. 7 D shows an antisense-treated culture with only axon-like processes. Fig. 7, B and E show that the dendrite-like processes of sense-treated cultures are immunoreactive for CHO1/ MKLP1 while the processes in the antisense-treated cultures are not. Cultures rinsed free of antisense or not replenished with antisense every 6 h showed the reappearance of CHO1/MKLP1-immunoreactivity, mitotic activity, and dendrite-like processes within 24 h (data not shown).
Microtubule Polarity Studies
Given the properties of CHO1/MKLP1 in the mitotic spindle, it is reasonable to speculate that the primary role of the motor during process formation is to establish the nonuniform microtubule polarity pattern of the dendrite-like processes. To investigate this, microtubule polarity orientation was assessed in the processes of cells treated with AS1 both in the absence and presence of retinoic acid. Since antisense treatment virtually obliterates the formation of processes with the dendrite-like morphology, processes from eight different cells were chosen at random for microtubule polarity analyses. All of these processes had an axon-like morphology. In the eight processes from antisense-treated cultures which were not treated with retinoic acid, 99 ± 1% of the hooks were clockwise (Fig. 7 F). In the eight processes from antisense-treated cultures which were also treated with retinoic acid, 97 ± 3% of the hooks were clockwise. In S1-treated cultures, seven axon-like processes displayed 96 ± 2% clockwise hooks, and seven dendrite-like processes displayed 59 ± 13% clockwise hooks (Fig. 7 C). These analyses indicate that the antisense treatment, but not the sense treatment, compromises the establishment of nonuniformly oriented microtubule arrays within developing processes.
Discussion
A principal goal over these past several years has been to elucidate the molecules and mechanisms that are essential for the establishment of the axonal and dendritic microtubule arrays (for reviews see Baas and Yu, 1996; Heidemann, 1996). In terms of the specific molecules that underlie the distinct features of these arrays, most efforts have focused on the fibrous microtubule-associated proteins (MAPs). Tau and MAP2 have been of particular interest because the former is concentrated within axons and the latter is concentrated within dendrites. A fundamental role for these MAPs in the differentiation of axons and dendrites was strongly suggested by studies in which these MAPs were expressed in nonprocess-bearing nonneuronal Sf9 cells. As a result, the cells acquired dense bundles of microtubules, and extended elongate processes with many of the morphological characteristics of axons and dendrites (Knops et al., 1991; LeClerc et al., 1993, 1995). However, irrespective of whether tau or any of the MAP2 isoforms was expressed, the processes contained uniformly plus-end-distal microtubules, and never showed the distinct nonuniform microtubule polarity pattern of dendrites (Baas et al., 1991; Chen et al., 1992; LeClerc et al., 1993). These results suggest that MAPs contribute to the formation of processes, but that some additional factor is needed to establish their distinct microtubule polarity patterns, at least in the case of the dendrite. Recent studies from our laboratory strongly suggest that this “missing link” is a molecular motor (Sharp et al., 1996). Specifically, we showed that expression in Sf9 cells of a fragment of a motor that transports microtubules with minus-ends-leading against microtubules of the opposite orientation resulted in the formation of dendrite-like processes with nonuniform microtubule polarity orientation.
The results of the present study provide the first indications that this particular molecular motor protein, CHO1/ MKLP1, might be the actual motor that establishes nonuniform microtubule polarity orientation within the dendrites of neuronal cells. We chose to use neuro-2a cells for this study because CHO1/MKLP1 is a mitotic motor, and these cells rapidly shift from mitotic division to process outgrowth. Thus, these cells are ideal for testing whether a redistribution of CHO1/MKLP1 occurs during the transformation of a dividing neuroblast into a process-bearing neuronal cell. Neuro-2a cells were chosen also because of technical advantages over primary neurons. For example, we could use the mitotic cells as a positive control for the specificity of our immunostains before drawing conclusions about CHO1/MKLP1 levels or distribution based on immunostains of process-bearing cells. In addition, and particularly relevant to the antisense experiments, these neuroblastoma cells differentiate dendrite-like processes far more rapidly than primary neurons, and do so in the absence of growth factors from nonneuronal cells that might be adversely effected by the antisense. Our studies indicate that the axon-like and dendrite-like processes extended by these cells have the appropriate uniform and nonuniform microtubule polarity patterns of bona fide neurons, that CHO1/MKLP1 continues to be expressed after the cells have been rendered postmitotic, that CHO1/ MKLP1 becomes concentrated specifically within the dendrite-like processes both during normal interphase and in cells induced to differentiate with retinoic acid, and that inhibition of CHO1/MKLP1 expression with antisense oligonucleotides suppresses the formation of the dendritelike processes. The simplest interpretation of these findings is that CHO1/MKLP1 is used for mitotic progression when the cell is dividing and then for the establishment of the dendritic microtubule array when the cell is undergoing process formation.
Antisense oligonucleotides have been used successfully in previous studies on motor protein function in neuronal cells (Ferreira et al., 1992). Nevertheless, antisense experiments are notoriously difficult to interpret, and therefore a great deal of caution must be exercised in interpreting the results of the present experiments. One must consider the possibility that the antisense oligonucleotides inhibited the expression of other essential proteins or in some other way had deleterious effects on the health of the cells. Several lines of evidence suggest that this is not the case. First, two different nonoverlapping antisense oligonucleotides produced essentially the same results. Second, the corresponding sense oligonucleotides to each of these sequences produced no detectable changes in the cultures. Third, BLAST searches revealed no known proteins whose expression should be altered by either the antisense or sense sequences. Fourth, a dose-response curve indicates specificity in the effects of the antisense and efficacy of low concentrations of the antisense to almost completely obliterate the protein from the cells. Fifth, the cells appeared to be healthy in the presence of the antisense, sometimes producing longer and more robust axon-like processes than control cells. Sixth, no deleterious effects in the expression of three other proteins, β-tubulin, dynein, and MAP2, were detected. MAP2 is a particularly good control for these experiments given that it is a dendrite- enriched protein thought to play a critical role of its own in dendritic differentiation (see for example Fischer et al., 1986). Seventh, removal of the antisense resulted in a recovery in the expression of CHO1/MKLP1 and a corresponding return in the development of dendrite-like processes. Eighth, the antisense inhibited mitotic progression as expected given its role in cell division and this was also reversed upon removal of the antisense. Finally, all of the various results on the localization of the protein and the effects of the antisense treatment on cell division and process formation are entirely consistent with the known in vivo and in vitro properties of the molecule.
It is curious that these findings on CHO1/MKLP1 were obtained after having learned that a fragment of the motor can induce Sf9 cells to form dendrite-like processes (Kuriyama et al., 1994; Sharp et al., 1996). In contrast, MAP localization was discovered first, after which the functional properties of tau and MAP2 were revealed by expression in nonneuronal cells. One feature of the expression studies on CHO1/MKLP1 merits some discussion, namely the fact that expression of the full-length motor did not, in fact, induce process formation. This result was only obtained when roughly half of the molecule containing its NH2-terminal motor domain was expressed. On the basis of this result, we suggested that the actual dendritic motor may be a splice variant of CHO1/MKLP1 or that the COOHterminal region of the motor must be removed before its participation in process formation. The present studies provide no evidence for these possibilities. The Northern blot analyses revealed no evidence for splice variants, and although we were unable to obtain a reliable Western blot for determination of its molecular weight, it is clear that the molecule that we are detecting with the antibody is longer than the fragment expressed in our previous studies because our monoclonal antibody recognizes a site within the stalk region of the molecule not included within the NH2-terminal fragment. In addition, the fact that staining with this antibody is obliterated as a result of treatment with antisense to a very different part of the molecule is strong evidence that we are dealing with the same molecule, both for mitosis and process formation. We now suspect that the COOH-terminal half of the molecule is altered by a posttranslational modification such as phosphorylation, and/or that the protein must somehow work in conjunction with one or more other proteins to participate in process formation. MAP2 is a provocative candidate for such a cofactor in that it is dendrite-enriched and also has bound kinase activity (Obar et al., 1989; Rabino et al., 1989).
Current efforts are aimed at determining whether CHO1/ MKLP1 is expressed in primary neurons, and if so, whether it plays a critical role in the development of bona fide dendrites. It may be that a redistribution of mitotic motors is capable of establishing dendrite-like processes in neuroblastoma cells, but that a different motor with similar properties is used for the formation of bona fide dendrites. Some support for this perspective lies in the fact that dendritic development from primary neurons requires special growth factors and significant levels of new protein expression (Lein and Higgins, 1989; Lein et al., 1995). In addition, it appears that the transport of microtubules with minus-ends-leading occurs at a discrete critical point in development (Baas et al., 1989; Sharp et al., 1996), and it is attractive to hypothesize that this developmental milestone might relate to the expression of a novel motor protein. If this reasoning is correct, the ability of a mitotic motor to form primitive dendrites in neuroblastoma cells may reflect the kinds of events that occurred early in the evolution of the neuron, before the appearance of a more specialized motor. Alternatively, it is fascinating to consider the possibility that a single motor protein might serve one function during mitotis and another function during dendritic development, both related to the organization of microtubules into a nonuniform polarity pattern. Some support for this latter idea derives from the fact that retinoic acid, a factor that promotes dendritic development in the neuroblastoma cultures, induces a notable increase in the levels of CHO1/MKLP1, presumably by enhancing its expression. In bona fide neurons, a similar increase in expression may occur during the onset of dendritic differentiation. Whichever of these alternatives is the case, the present results provide compelling support for the view that the establishment of the mitotic spindle and the postmitotic neuronal microtubule arrays may be variations on the same theme.
Acknowledgments
We thank Dr. K. Kevin Pfister for providing the antibody against dynein and Dr. Itzhak Fischer for providing the antibody against MAP2. We especially thank Dr. Kenneth Kosik for his invaluable advice on the use of antisense.
This work was supported by National Institutes of Health (NIH) grants NS28785 and NS34270 to P.W. Baas, and NIH grant GM-41350 and Council for Tobacco Research Grant 3157 to R. Kuriyama. P.W. Baas is the recipient of a Research Career Development Award from the NIH.
Abbreviations used in this paper
- db cAMP
dibutyryl cyclic AMP
- MAP2
microtubule-associated protein-2
Footnotes
Please address all correspondence to P.W. Baas, Department of Anatomy, The University of Wisconsin Medical School, 1300 University Avenue, Madison, WI 53706. Tel.: (608) 262-7307. Fax: (608) 262-7306. E-Mail: pwbaas@facstaff.wisc.edu
References
- Baas PW, Yu W. A composite model for establishing the axonal and dendritic microtubule arrays. Mol Neurobiol. 1996;12:145–161. doi: 10.1007/BF02740651. [DOI] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci USA. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Black MM, Banker GA. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J Cell Biol. 1989;109:3085–3094. doi: 10.1083/jcb.109.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Pienkowski TP, Kosik KS. Processes induced by tau expression in Sf9 cells have an axon-like microtubule organization. J Cell Biol. 1991;115:1333–1344. doi: 10.1083/jcb.115.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NR, Goldstein LSB. Going mobile: microtubule motors and chromosome segregation. Proc Natl Acad Sci USA. 1996;93:1735–1742. doi: 10.1073/pnas.93.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP-2 and tau determine spacings between microtubules in dendrites and axons. Nature (Lond) 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- Euteneuer U, McIntosh JR. Structural polarity of kinetochore microtubules in PTK2 cells. J Cell Biol. 1982;98:338–345. doi: 10.1083/jcb.89.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Niclas J, Vale RD, Banker G, Kosik KS. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J Cell Biol. 1992;117:595–606. doi: 10.1083/jcb.117.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer I, Shea TB, Kosik KS, Sapirstein VS. Retinoic acid induces MAP-2 containing neurites in mouse neuroblastoma cells. Ann NY Acad Sci. 1986;466:429–430. [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature (Lond) 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heidemann SR. Cytoplasmic mechanisms of axonal and dendritic growth in neurons. International Rev Cytol. 1996;165:235–296. doi: 10.1016/s0074-7696(08)62224-x. [DOI] [PubMed] [Google Scholar]
- Heidemann SR, McIntosh JR. Visualization of the structural polarity of microtubules. Nature (Lond) 1980;286:517–519. doi: 10.1038/286517a0. [DOI] [PubMed] [Google Scholar]
- Heidemann SR, Landers JM, Hamborg MA. Polarity orientation of axonal microtubules. J Cell Biol. 1981;91:661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt MA. Cellular roles of kinesin and related proteins. Curr Opin Cell Biol. 1994;6:63–68. doi: 10.1016/0955-0674(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Knops J, Kosik KS, Lee G, Pardee JD, Cohen-Gould L, McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991;114:725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Dragas-Granoic S, Maekawa T, Vassilev A, Khodjakov A, Kobayashi H. Heterogeneity and microtubule interaction of the CHO1 antigen, a mitosis-specific kinesin-like protein. Analysis of subdomains expressed in insect Sf9 cells. J Cell Sci. 1994;107:3485–3499. doi: 10.1242/jcs.107.12.3485. [DOI] [PubMed] [Google Scholar]
- LeClerc N, Kosik KS, Cowan N, Pienkowski TP, Baas PW. Process formation in Sf9 cells induced by the expression of a microtubule-associated protein 2c-like construct. Proc Natl Acad Sci USA. 1993;90:6223–6227. doi: 10.1073/pnas.90.13.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc N, Baas PW, Garner CG, Kosik KS. Juvenile and mature MAP2 isoforms induce distinct cellular morphologies. Mol Biol Cell. 1995;3:435–442. doi: 10.1091/mbc.7.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein PJ, Higgins D. Laminin and a basement membrane extract have different effects on axonal and dendritic outgrowth from embryonic rat sympathetic neurons in vitro. . Dev Biol. 1989;136:330–345. doi: 10.1016/0012-1606(89)90260-1. [DOI] [PubMed] [Google Scholar]
- Lein P, Johnson M, Guo X, Rueger D, Higgins D. Osteogenic Protein-1 induces dendritic growth in rat sympathetic neurons. Neuron. 1995;15:597–605. doi: 10.1016/0896-6273(95)90148-5. [DOI] [PubMed] [Google Scholar]
- McIntosh, J.R. 1994. The roles of microtubules in chromosome movement. In Microtubules. J.S. Hyams and C.W. Lloyd, editors. Wiley-Liss, New York. pp. 413–434.
- Moore JD, Endow SE. Kinesin proteins: a phylum of motors for microtubule-based motility. BioEssays. 1996;18:207–219. doi: 10.1002/bies.950180308. [DOI] [PubMed] [Google Scholar]
- Obar RA, Dingus J, Bayley H, Vallee RB. The RII subunit of cAMP-dependent protein kinase binds to a common amino-terminal domain on microtubule-associated proteins 1A, 2B, and 2C. Neuron. 1989;3:639–645. doi: 10.1016/0896-6273(89)90274-2. [DOI] [PubMed] [Google Scholar]
- Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. A plus-enddirected motor that moves anti-parallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature (Lond) 1992;359:543–547. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- Pfister KK, Salata MW, Dillman JF, III, Torre E, Lye RJ. Identification and developmental regulation of a neuron-specific subunit of cytoplasmic dynein. Mol Biol Cell. 1996;7:331–343. doi: 10.1091/mbc.7.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KN, Hsie AW. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3′:5′-cyclic monophosphate. Nature (Lond) 1971;233:141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Rabino HM, Dammerman M, Shafit-Zagardo B, Erlichman J. Localization and characterization of the binding site for the regulatory subunit of type II cAMP-dependent protein kinase of MAP2. Neuron. 1989;3:631–638. doi: 10.1016/0896-6273(89)90273-0. [DOI] [PubMed] [Google Scholar]
- Ross J, Olmsted JB, Rosenbaum JL. The ultrastructure of mouse neuroblastoma cells in tissue culture. Tissue and Cell. 1975;7:107–156. doi: 10.1016/s0040-8166(75)80010-3. [DOI] [PubMed] [Google Scholar]
- Sellito C, Kuriyama R. Distribution of a matrix component of the midbody during the cell cycle in Chinese hamster ovary cells. J Cell Biol. 1988;106:431–439. doi: 10.1083/jcb.106.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Yu W, Baas PW. Transport of dendritic microtubules establishes their nonuniform polarity orientation. J Cell Biol. 1995;130:93–104. doi: 10.1083/jcb.130.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Kuriyama R, Baas PW. Expression of a kinesin-related protein induces Sf9 cells to form dendrite-like processes with nonuniform microtubule polarity orientation. J Neurosci. 1996;16:4370–4375. doi: 10.1523/JNEUROSCI.16-14-04370.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea TB, Fischer I, Sapirstein VS. Effect of retinoic acid on growth and morphological differentiation of mouse NB2a neuroblastoma cells in culture. Dev Brain Res. 1985;21:307–314. doi: 10.1016/0165-3806(85)90220-2. [DOI] [PubMed] [Google Scholar]
- Solomon F. Detailed morphologies of sister neuroblastoma cells are related. Cell. 1979;16:165–169. doi: 10.1016/0092-8674(79)90197-1. [DOI] [PubMed] [Google Scholar]