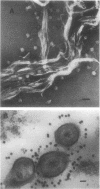
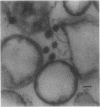
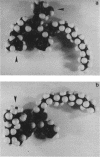
Abstract
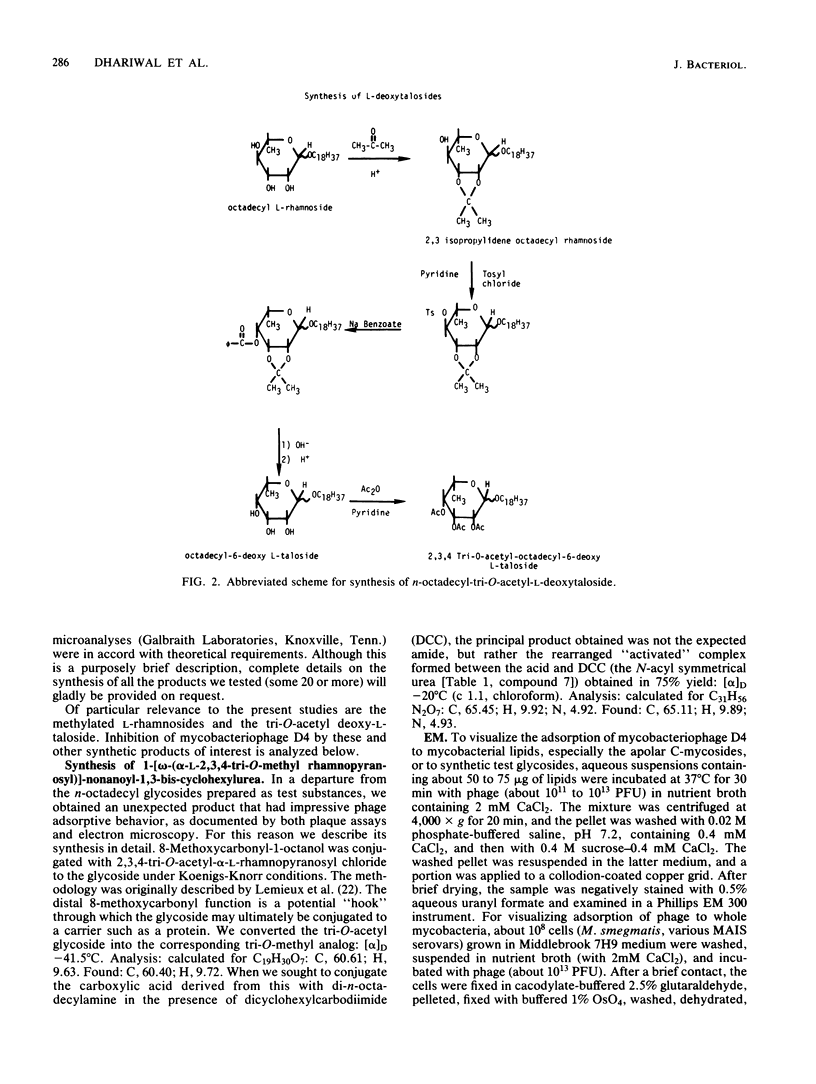
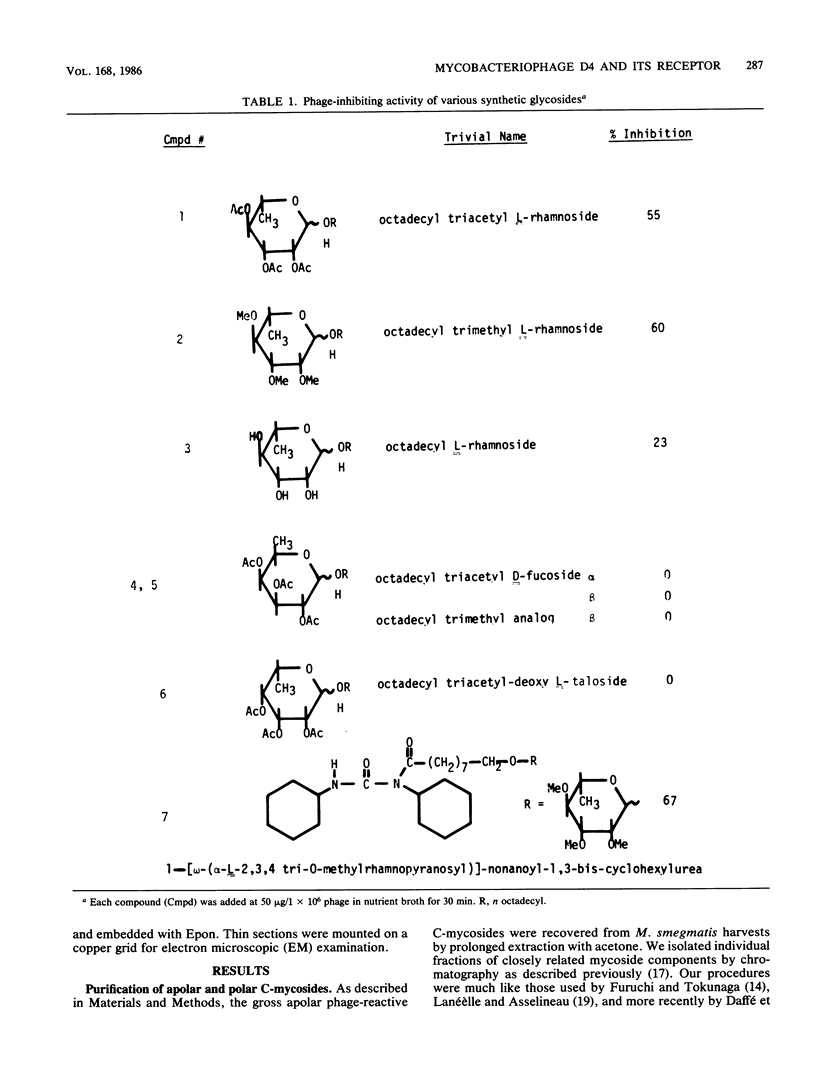
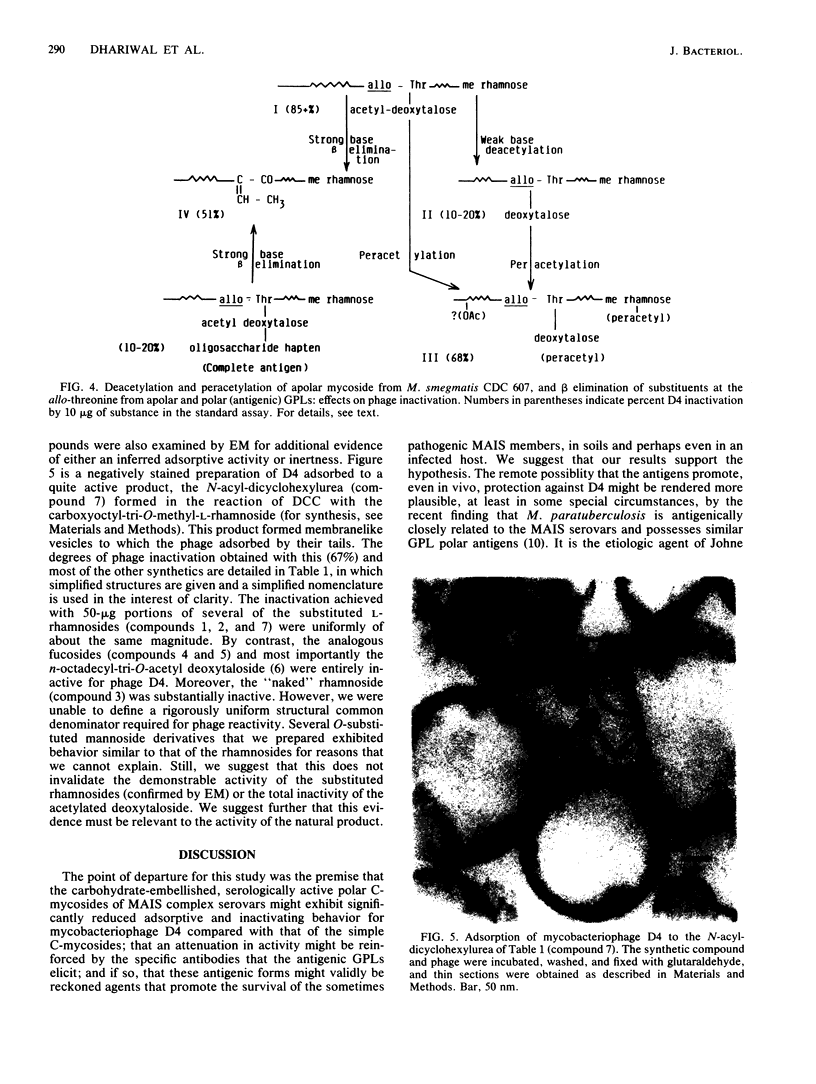
The simple apolar C-mycosides, i.e., structurally well-defined hydrophobic glycopeptidolipids of several Mycobacterium species (see diagram below), were earlier shown to behave as receptors for adsorption of mycobacteriophage D4. This phage is usually virulent for Mycobacterium smegmatis. More complex, polar C-mycosides with additional carbohydrate substituents attached solely to the deoxytalose have recently been described. They are the highly specific serotyping antigens discovered by W. B. Schaefer--lipids which characterize members of the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum (MAIS) complex. Both kinds are depicted in the structure below: (Formula: see text) where X equals H (for simple, apolar C-mycosides) and X equals small oligosaccharides (for antigenic forms; more complex, polar C-mycosides). The present investigations showed that the purified polar antigenic lipids exhibit considerably less adsorptive activity for D4 than do the apolar C-mycosides. Thus, the haptenic oligosaccharides are believed to shield the site in the molecule that the phage recognizes, and the blocking is reinforced by the specific antibodies that the antigens elicit. Although the MAIS serovars usually also produce the phage-reactive apolar C-mycosides, they are not permissive hosts for D4, nor do whole cells adsorb the phage. We suggest that in these species the apolar forms are probably "covered" at the cell surface by the antigenic lipids. Therefore, these antigenic mycosides may play a putative role in virulence of the MAIS members by protecting these mycobacteria from their own potential pathogen. The results of chemical transformations at specific sites of the mycoside core coupled with studies of simple synthetic lipid glycosides indicated that the principal phage receptor activity resides in the terminal methylated rhamnose (see diagram). It is this sugar which is evidently masked by the (seemingly remote) haptenic oligosaccharides.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L., Kim K. S. Mycobacterium. Bacteriol Rev. 1977 Mar;41(1):217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Aspinall G. O., Shin J. E. Structure of the specific oligosaccharides from the glycopeptidolipid antigens of serovars in the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex. J Biol Chem. 1981 Jul 10;256(13):6817–6822. [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Brennan P. J., Mayer H., Aspinall G. O., Nam Shin J. E. Structures of the glycopeptidolipid antigens from serovars in the Mycobacterium avium/Mycobacterium intracellulare/Mycobacterium scrofulaceum serocomplex. Eur J Biochem. 1981 Mar 16;115(1):7–15. doi: 10.1111/j.1432-1033.1981.tb06190.x. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Souhrada M., Ullom B., McClatchy J. K., Goren M. B. Identification of atypical mycobacteria by thin-layer chromatography of their surface antigens. J Clin Microbiol. 1978 Oct;8(4):374–379. doi: 10.1128/jcm.8.4.374-379.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camphausen R. T., Jones R. L., Brennan P. J. A glycolipid antigen specific to Mycobacterium paratuberculosis: structure and antigenicity. Proc Natl Acad Sci U S A. 1985 May;82(10):3068–3072. doi: 10.1073/pnas.82.10.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffe M., Laneelle M. A., Puzo G. Structural elucidation by field desorption and electron-impact mass spectrometry of the C-mycosides isolated from Mycobacterium smegmatis. Biochim Biophys Acta. 1983 May 16;751(3):439–443. doi: 10.1016/0005-2760(83)90304-1. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Furuchi A., Tokunaga T. Nature of the receptor substance of Mycobacterium smegmatis for D4 bacteriophage adsorption. J Bacteriol. 1972 Aug;111(2):404–411. doi: 10.1128/jb.111.2.404-411.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B. Immunoreactive substances of mycobacteria. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):50–69. doi: 10.1164/arrd.1982.125.3P2.50. [DOI] [PubMed] [Google Scholar]
- Goren M. B., McClatchy J. K., Martens B., Brokl O. Mycosides C: behavior as receptor site substance for mycobacteriophage D4. J Virol. 1972 Jun;9(6):999–1003. doi: 10.1128/jvi.9.6.999-1003.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., Toubiana R. A facile permethylation of cord factor. Biochim Biophys Acta. 1979 Jul 27;574(1):64–69. doi: 10.1016/0005-2760(79)90085-7. [DOI] [PubMed] [Google Scholar]
- Laneelle G., Asselineau J., Chamoiseau G. Presence de mycosides C' (formes simplifiees de mycoside C) dans les bacteries isolees de bovins atteints du farcin. FEBS Lett. 1971 Dec 1;19(2):109–111. doi: 10.1016/0014-5793(71)80490-8. [DOI] [PubMed] [Google Scholar]
- Laneelle G., Asselineau J. Structure d'un glycoside de peptidolipide isolé d'une mycobactérie. Eur J Biochem. 1968 Sep 24;5(4):487–491. doi: 10.1111/j.1432-1033.1968.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Lemieux R. U., Bundle D. R., Baker D. A. The properties of a "synthetic" antigen related to the human blood-group Lewis a. J Am Chem Soc. 1975 Jul 9;97(14):4076–4083. doi: 10.1021/ja00847a035. [DOI] [PubMed] [Google Scholar]
- Liav A., Goren M. B. A facile synthesis of 3,6-di-O-methyl-D-glucose. Carbohydr Res. 1986 Jul 1;149(2):C13–C16. doi: 10.1016/s0008-6215(00)90070-3. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Redmond W. B., Ward D. M. Media and methods for phage-typing mycobacteria. Bull World Health Organ. 1966;35(4):563–568. [PMC free article] [PubMed] [Google Scholar]
- SMITH D. W., RANDALL H. M., MACLENNAN A. P., LEDERER E. Mycosides: a new class of type-specific glycolipids of Mycobacteria. Nature. 1960 Jun 11;186:887–888. doi: 10.1038/186887a0. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B. Serologic identification and classification of the atypical mycobacteria by their agglutination. Am Rev Respir Dis. 1965 Dec;92(6):85–93. doi: 10.1164/arrd.1965.92.6P2.85. [DOI] [PubMed] [Google Scholar]
- Tereletsky M. J., Barrow W. W. Postphagocytic detection of glycopeptidolipids associated with the superficial L1 layer of Mycobacterium intracellulare. Infect Immun. 1983 Sep;41(3):1312–1321. doi: 10.1128/iai.41.3.1312-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga T., Kataoka T., Suga K. Phage inactivation by an ethanol-ether extract of Mycobacterium smegmatis. Am Rev Respir Dis. 1970 Feb;101(2):309–313. doi: 10.1164/arrd.1970.101.2.309. [DOI] [PubMed] [Google Scholar]
- Vilkas E., Lederer E. N-methylation de peptides par a methode de Hakomori. Structure du mycoside Cbl. Tetrahedron Lett. 1968 May;(26):3089–3092. doi: 10.1016/s0040-4039(00)89603-3. [DOI] [PubMed] [Google Scholar]
- Voiland A., Bruneteau M., Michel G. Etude du mycoside C 2 de Mycobacterium avium. Détermination de la structure. Eur J Biochem. 1971 Jul 29;21(2):285–291. doi: 10.1111/j.1432-1033.1971.tb01468.x. [DOI] [PubMed] [Google Scholar]