Abstract
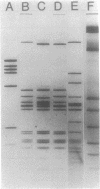
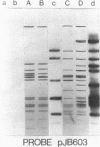
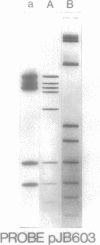
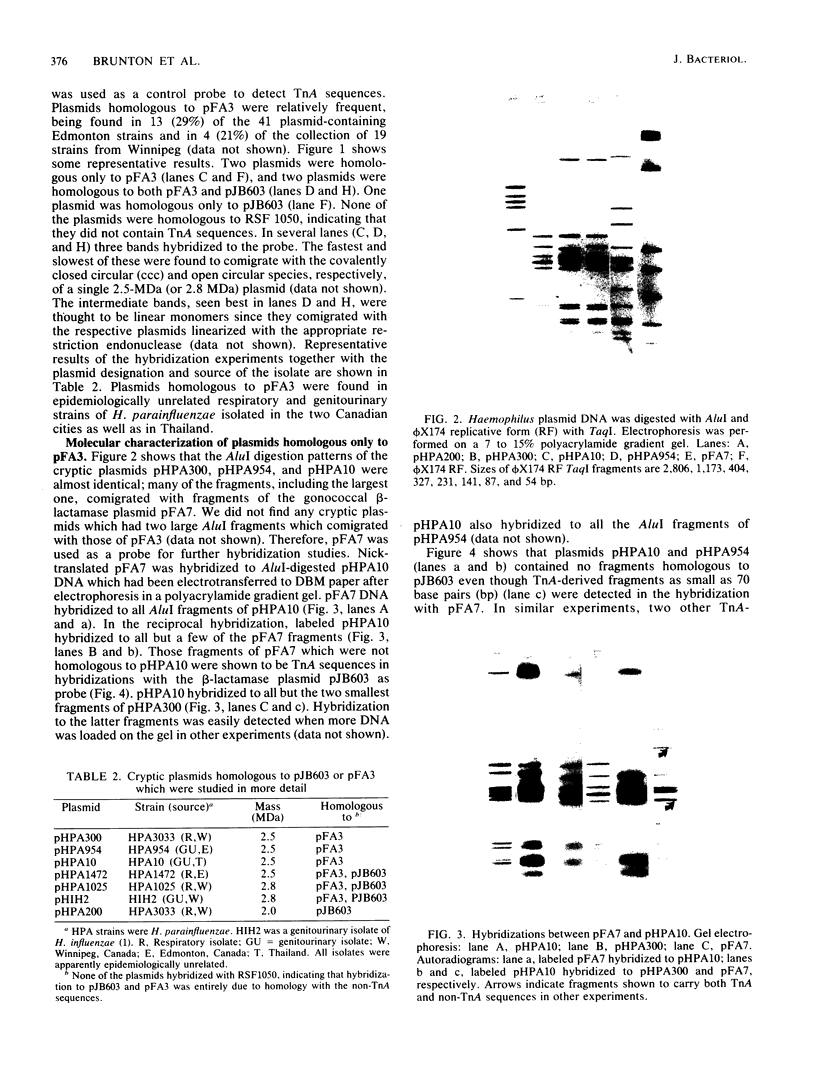
Fifty-nine percent of unselected strains of Haemophilus parainfluenzae were found to carry small, phenotypically cryptic plasmid DNA species. Using filter blot hybridization, we found several plasmids which were homologous to the small beta-lactamase-specifying plasmids pJB1 and pFA7, which were originally isolated from Haemophilus ducreyi and Neisseria gonorrhoeae, respectively. Detailed filter hybridization studies combined with electron microscope heteroduplex analysis suggested that three cryptic plasmids are completely homologous to the non-TnA sequences of pJB1. One cryptic plasmid was found to be highly homologous to pJB603, a small beta-lactamase plasmid previously found in two isolates of H. influenzae. A second group of plasmids were found to carry sequences homologous to pJB1 and other sequences homologous to pJB603. These results strongly suggest that small beta-lactamase plasmids found in Haemophilus species and N. gonorrhoeae may have arisen by insertion of the transposable beta-lactamase-specifying element TnA into small, phenotypically cryptic replicons resident in H. parainfluenzae. Attempts to reproduce such a recombination event in the laboratory were not successful.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L., Brunton J. L., Meier M., Bowman M. N., Slaney L. A. Haemophilus influenzae: comparison of respiratory tract isolates with genitourinary tract isolates. J Clin Microbiol. 1982 Nov;16(5):826–831. doi: 10.1128/jcm.16.5.826-831.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton W. L., Brunton J. L., Slaney L., MacLean I. Plasmid-mediated sulfonamide resistance in Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Jan;21(1):159–165. doi: 10.1128/aac.21.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton W. L., Penner S., Slaney L., Brunton J. Biochemical characteristics of Haemophilus influenzae in relationship to source of isolation and antibiotic resistance. J Clin Microbiol. 1978 Jun;7(6):519–523. doi: 10.1128/jcm.7.6.519-523.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. M., Grinsted J., Richmond M. H. Transposition of TnA does not generate deletions. Mol Gen Genet. 1977 Jul 20;154(2):205–211. doi: 10.1007/BF00330839. [DOI] [PubMed] [Google Scholar]
- Brunton J. L., Maclean I., Ronald A. R., Albritton W. L. Plasmid-mediated ampicillin resistance in Haemophilus ducreyi. Antimicrob Agents Chemother. 1979 Feb;15(2):294–299. doi: 10.1128/aac.15.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J., Bennett P., Grinsted J. Molecular nature of a plasmid specifying beta-lactamase production in Haemophilus ducreyi. J Bacteriol. 1981 Dec;148(3):788–795. doi: 10.1128/jb.148.3.788-795.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J., Meier M., Ehrman N., Maclean I., Slaney L., Albritton W. L. Molecular epidemiology of beta-lactamase-specifying plasmids of Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Jun;21(6):857–863. doi: 10.1128/aac.21.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R., Witchitz J. L., Gerbaud G. R., Bouanchaud D. H. Incompatibility groups and the classification of fi - resistance factors. J Bacteriol. 1972 Nov;112(2):666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Slaney L., Maclean I. W., Albritton W. L. Mobilization of nonconjugative antibiotic resistance plasmids in Haemophilus ducreyi. J Bacteriol. 1982 Feb;149(2):726–732. doi: 10.1128/jb.149.2.726-732.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dickgiesser N., Bennett P. M., Richmond M. H. Penicillinase-producing Neisseria gonorrhoeae: a molecular comparison of 5.3-kb and 7.4-kb beta-lactamase plasmids. J Bacteriol. 1982 Sep;151(3):1171–1175. doi: 10.1128/jb.151.3.1171-1175.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Saunders J. R., Richmond M. H., Falkow S. Relationships among some R plasmids found in Haemophilus influenzae. J Bacteriol. 1977 Jul;131(1):356–362. doi: 10.1128/jb.131.1.356-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Bedinger P., Champoux J. J., Falkow S. Deletions affecting the transposition of an antibiotic resistance gene. Proc Natl Acad Sci U S A. 1977 Feb;74(2):702–706. doi: 10.1073/pnas.74.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs R., Kaulfers P. M., Jahn G., Teschner U. Molecular characterization of a small Haemophilus influenzae plasmid specifying beta-lactamase and its relationship to R factors from Neisseria gonorrhoeae. J Gen Microbiol. 1979 Mar;111(1):223–231. doi: 10.1099/00221287-111-1-223. [DOI] [PubMed] [Google Scholar]
- Laufs R., Riess F. C., Jahn G., Fock R., Kaulfers P. M. Origin of Haemophilus influenzae R factors. J Bacteriol. 1981 Aug;147(2):563–568. doi: 10.1128/jb.147.2.563-568.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund M. E., Blazevic D. J. Rapid speciation of Haemophilus with the porphyrin production test versus the satellite test for X. J Clin Microbiol. 1977 Feb;5(2):142–144. doi: 10.1128/jcm.5.2.142-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perine P. L., Thornsberry C., Schalla W., Biddle J., Siegel M. S., Wong K. H., Thompson S. E. Evidence for two distinct types of penicillinase-producing Neisseria gonorrhoeae. Lancet. 1977 Nov 12;2(8046):993–995. doi: 10.1016/s0140-6736(77)92891-4. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Bennett P. M., Choi C. L., Brown N., Brunton J., Grinsted J., Wallace L. The genetic basis of the spread of beta-lactamase synthesis among plasmid-carrying bacteria. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):349–359. doi: 10.1098/rstb.1980.0052. [DOI] [PubMed] [Google Scholar]
- Roberts M., Elwell L. P., Falkow S. Molecular characterization of two beta-lactamase-specifying plasmids isolated from Neisseria gonorrhoeae. J Bacteriol. 1977 Aug;131(2):557–563. doi: 10.1128/jb.131.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sox T. E., Mohammed W., Sparling P. F. Transformation-derived Neisseria gonorrhoeae plasmids with altered structure and function. J Bacteriol. 1979 May;138(2):510–518. doi: 10.1128/jb.138.2.510-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]