Abstract
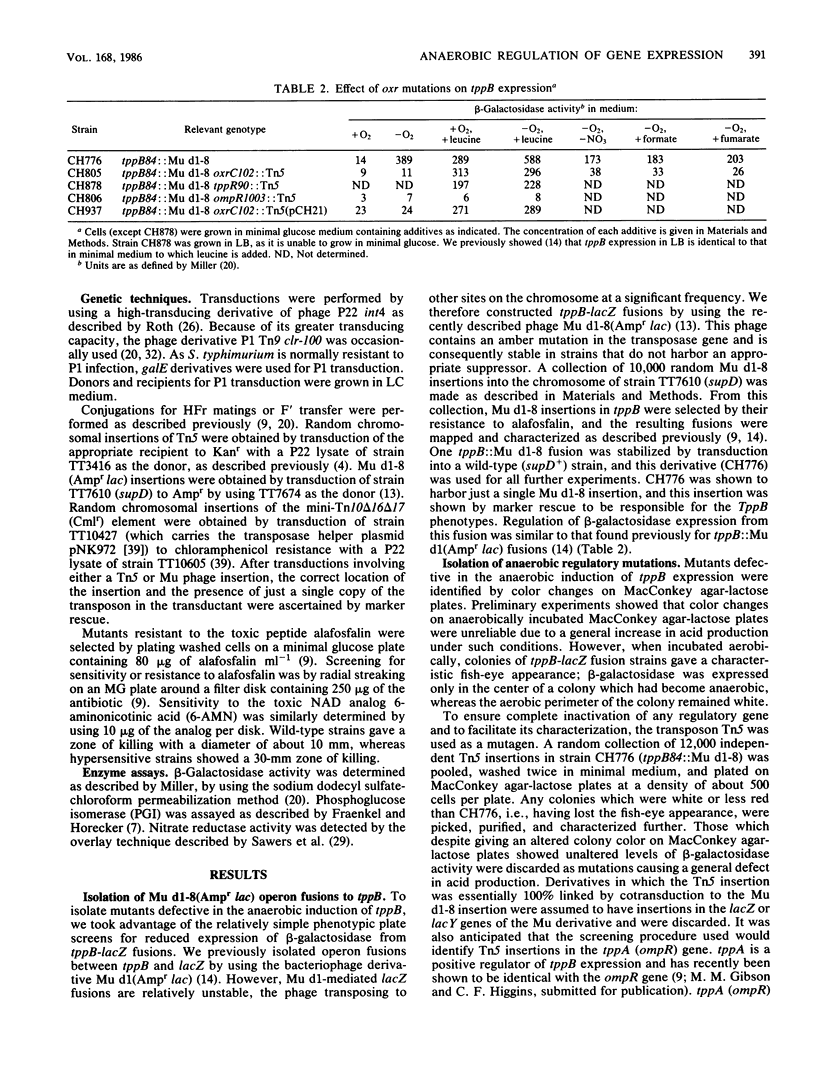
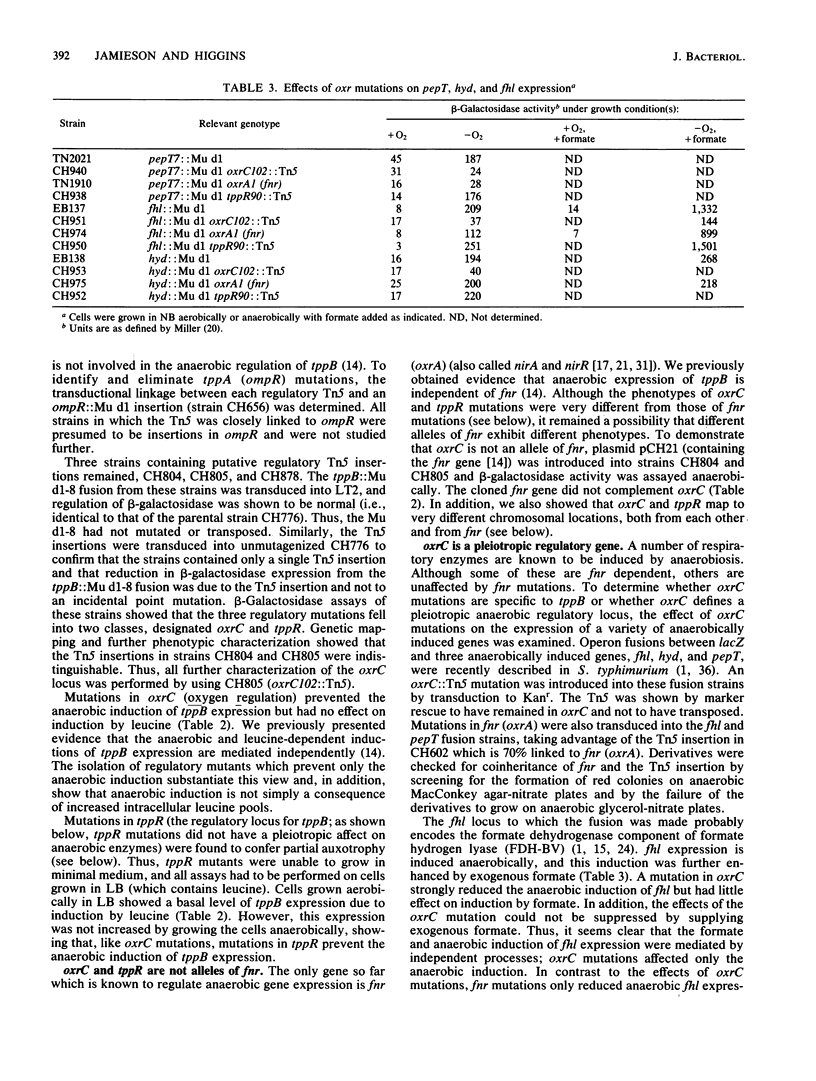
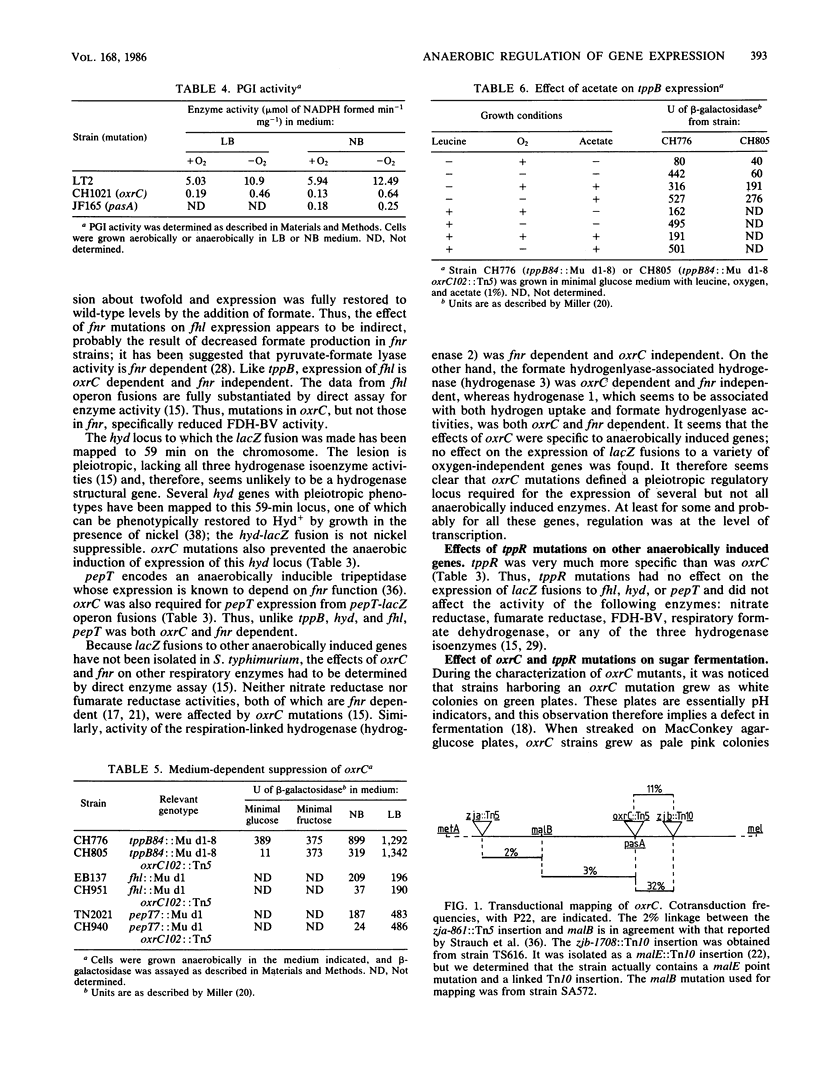
Expression of the tripeptide permease gene tppB is anaerobically induced. This induction is independent of the fnr (oxrA) gene product, which is known to be required for the anaerobic induction of several respiratory enzymes. We isolated, characterized, and mapped mutations in two genes, oxrC and tppR, which prevent the anaerobic induction of tppB expression. Mutations in oxrC were highly pleiotropic, preventing the anaerobic expression of the formate dehydrogenase component of formate hydrogen lyase (fhl), a tripeptidase (pepT), and two of the three known hydrogenase isoenzymes (hydrogenases 1 and 3). On the other hand, expression of nitrate reductase, fumarate reductase, and a number of other fnr (oxrA)-dependent enzymes was not affected by mutations in oxrC. Thus, there appeared to be at least two distinct classes of anaerobically induced genes, those which required fnr for their expression and those which required oxrC. It seems that fnr-dependent enzymes perform primarily respiratory functions, whereas oxrC-dependent enzymes served fermentative or biosynthetic roles. We found the primary defect of oxrC mutants to be a deficiency in phosphoglucose isomerase activity, implying that a product of glycolysis functions as an anaerobic regulatory signal. Mutations in tppR were specific for tppB and did not affect expression of other oxrC-dependent genes. However, tppR did exhibit phenotypes other than the regulation of tppB. Both oxrC and tppR mutants were hypersensitive to the toxic NAD analog 6-aminonicotinic acid. This suggests that oxrC and tppR may play a role in the regulation of NAD biosynthesis or, alternatively, that NAD or a related nucleotide serves as the anaerobic signal for oxrC-dependent enzymes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett E. L., Kwan H. S., Macy J. Anaerobiosis, formate, nitrate, and pyrA are involved in the regulation of formate hydrogenlyase in Salmonella typhimurium. J Bacteriol. 1984 Jun;158(3):972–977. doi: 10.1128/jb.158.3.972-977.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilous P. T., Weiner J. H. Dimethyl sulfoxide reductase activity by anaerobically grown Escherichia coli HB101. J Bacteriol. 1985 Jun;162(3):1151–1155. doi: 10.1128/jb.162.3.1151-1155.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey L. B., Ingraham J. L. A regulatory gene (use) affecting the expression of pyrA and certain other pyrimidine genes. J Bacteriol. 1982 Jul;151(1):144–152. doi: 10.1128/jb.151.1.144-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J., Higgins C. F., Booth I. R. Proline uptake through the major transport system of Salmonella typhimurium is coupled to sodium ions. J Bacteriol. 1984 Oct;160(1):22–27. doi: 10.1128/jb.160.1.22-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., HORECKER B. L. PATHWAYS OF D-GLUCOSE METABOLISM IN SALMONELLA TYPHINMURIUM. A STUDY OF A MUTANT LACKING PHOSPHOGLUCOSE ISOMERASE. J Biol Chem. 1964 Sep;239:2765–2771. [PubMed] [Google Scholar]
- Foster J. W., Holley E. A., Mya S. NAD metabolism in Salmonella typhimurium: isolation of pyridine analogue supersensitive (pas) and pas suppressor mutants. J Gen Microbiol. 1984 Nov;130(11):2873–2881. doi: 10.1099/00221287-130-11-2873. [DOI] [PubMed] [Google Scholar]
- Gharbi S., Belaich A., Murgier M., Lazdunski A. Multiple controls exerted on in vivo expression of the pepN gene in Escherichia coli: studies with pepN-lacZ operon and protein fusion strains. J Bacteriol. 1985 Sep;163(3):1191–1195. doi: 10.1128/jb.163.3.1191-1195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. M., Price M., Higgins C. F. Genetic characterization and molecular cloning of the tripeptide permease (tpp) genes of Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):122–130. doi: 10.1128/jb.160.1.122-130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M., Jamieson D., Powell L. M. Genetic map of the opp (Oligopeptide permease) locus of Salmonella typhimurium. J Bacteriol. 1983 Feb;153(2):830–836. doi: 10.1128/jb.153.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley E. A., Spector M. P., Foster J. W. Regulation of NAD biosynthesis in Salmonella typhimurium: expression of nad-lac gene fusions and identification of a nad regulatory locus. J Gen Microbiol. 1985 Oct;131(10):2759–2770. doi: 10.1099/00221287-131-10-2759. [DOI] [PubMed] [Google Scholar]
- Hughes K. T., Cookson B. T., Ladika D., Olivera B. M., Roth J. R. 6-Aminonicotinamide-resistant mutants of Salmonella typhimurium. J Bacteriol. 1983 Jun;154(3):1126–1136. doi: 10.1128/jb.154.3.1126-1136.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Sawers R. G., Rugman P. A., Boxer D. H., Higgins C. F. Effects of anaerobic regulatory mutations and catabolite repression on regulation of hydrogen metabolism and hydrogenase isoenzyme composition in Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):405–411. doi: 10.1128/jb.168.1.405-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritzkes D. R., Zhang X. Y., Lin E. C. Use of phi(glp-lac) in studies of respiratory regulation of the Escherichia coli anaerobic sn-glycerol-3-phosphate dehydrogenase genes (glpAB). J Bacteriol. 1984 Feb;157(2):591–598. doi: 10.1128/jb.157.2.591-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE M., CURTISS R. Genetic fine structure of the C region and the linkage map of phage P22. Genetics. 1961 Dec;46:1573–1580. doi: 10.1093/genetics/46.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Merberg D., Datta P. Altered expression of biodegradative threonine dehydratase in Escherichia coli mutants. J Bacteriol. 1982 Apr;150(1):52–59. doi: 10.1128/jb.150.1.52-59.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman B. M., Cole J. A. The chromosomal location and pleiotropic effects of mutations of the nirA+ gene of Escherichia coli K12: the essential role of nirA+ in nitrite reduction and in other anaerobic redox reactions. J Gen Microbiol. 1978 May;106(1):1–12. doi: 10.1099/00221287-106-1-1. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Liljeström P., Harayama S. Cosmid cloning and transposon mutagenesis in Salmonella typhimurium using phage lambda vehicles. Mol Gen Genet. 1981;181(2):153–157. doi: 10.1007/BF00268420. [DOI] [PubMed] [Google Scholar]
- Pascal M. C., Burini J. F., Chippaux M. Regulation of the trimethylamine N-oxide (TMAO) reductase in Escherichia coli: analysis of tor::Mud1 operon fusion. Mol Gen Genet. 1984;195(1-2):351–355. doi: 10.1007/BF00332770. [DOI] [PubMed] [Google Scholar]
- Pecher A., Zinoni F., Böck A. The seleno-polypeptide of formic dehydrogenase (formate hydrogen-lyase linked) from Escherichia coli: genetic analysis. Arch Microbiol. 1985 May;141(4):359–363. doi: 10.1007/BF00428850. [DOI] [PubMed] [Google Scholar]
- Pecher A., Zinoni F., Jatisatienr C., Wirth R., Hennecke H., Böck A. On the redox control of synthesis of anaerobically induced enzymes in enterobacteriaceae. Arch Microbiol. 1983 Nov;136(2):131–136. doi: 10.1007/BF00404787. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers R. G., Ballantine S. P., Boxer D. H. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: evidence for a third isoenzyme. J Bacteriol. 1985 Dec;164(3):1324–1331. doi: 10.1128/jb.164.3.1324-1331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers R. G., Jamieson D. J., Higgins C. F., Boxer D. H. Characterization and physiological roles of membrane-bound hydrogenase isoenzymes from Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):398–404. doi: 10.1128/jb.168.1.398-404.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreyer R., Böck A. Phosphoglucose isomerase from Escherischia coli K 10: purification, properties and formation under aerobic and anaerobic condition. Arch Microbiol. 1980 Oct;127(3):289–298. doi: 10.1007/BF00427206. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Rice D. W., Guest J. R. Homology between CAP and Fnr, a regulator of anaerobic respiration in Escherichia coli. J Mol Biol. 1983 May 15;166(2):241–247. doi: 10.1016/s0022-2836(83)80011-4. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., MacGregor C. H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982 Aug;151(2):788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V. Requirement of Fnr and NarL functions for nitrate reductase expression in Escherichia coli K-12. J Bacteriol. 1982 Sep;151(3):1320–1325. doi: 10.1128/jb.151.3.1320-1325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Lenk J. B., Gamble B. L., Miller C. G. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. D., Doelle H. W., Westwood A. W., Gordon G. L. Effect of oxygen on several enzymes involved in the aerobic and anaerobic utilization of glucose in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1099–1105. doi: 10.1128/jb.112.3.1099-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R., Boxer D. H. Pleiotropic hydrogenase mutants of Escherichia coli K12: growth in the presence of nickel can restore hydrogenase activity. Biochimie. 1986 Jan;68(1):157–166. doi: 10.1016/s0300-9084(86)81080-x. [DOI] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Firth A. Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J Bacteriol. 1972 Jul;111(1):24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes J. H., Casson L. P., Honkanen A. K., Walker G. C. Anaerobiosis induces expression of ant, a new Escherichia coli locus with a role in anaerobic electron transport. J Bacteriol. 1984 Apr;158(1):180–186. doi: 10.1128/jb.158.1.180-186.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoni F., Beier A., Pecher A., Wirth R., Böck A. Regulation of the synthesis of hydrogenase (formate hydrogen-lyase linked) of E. coli. Arch Microbiol. 1984 Nov;139(4):299–304. doi: 10.1007/BF00408370. [DOI] [PubMed] [Google Scholar]



