Abstract
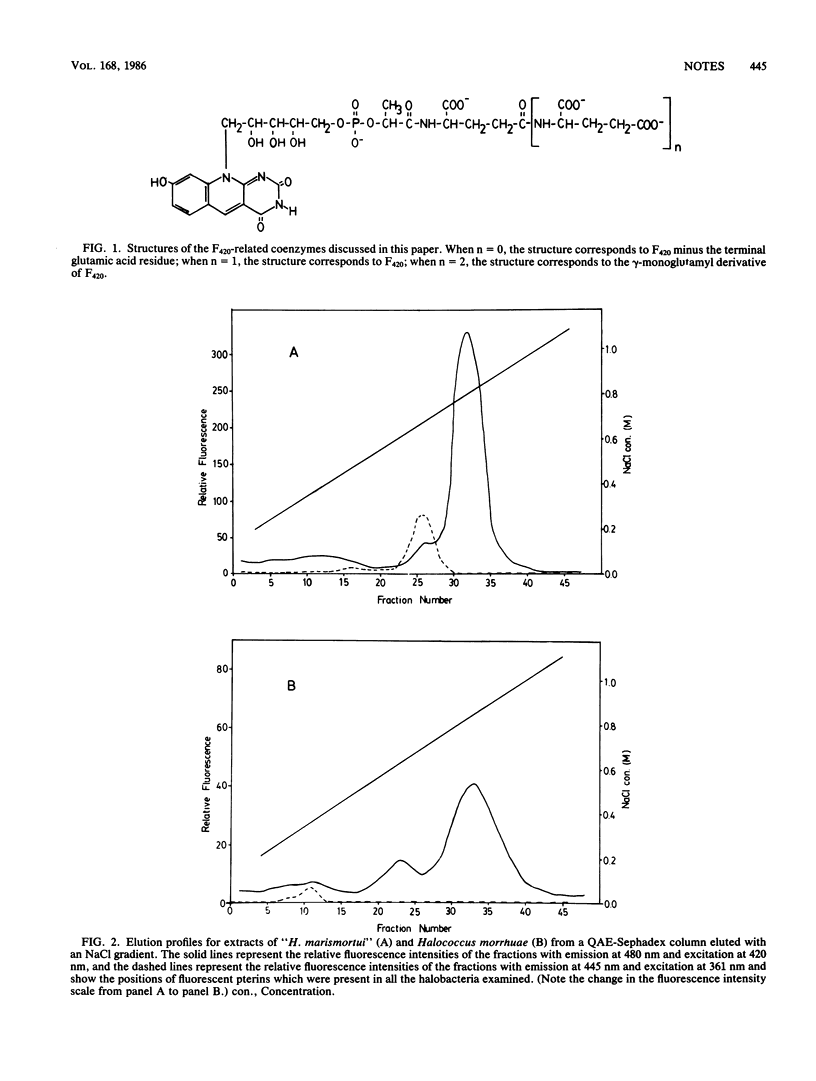
Analysis of the fluorescent compounds extracted from six different species of halobacteria and one species each of Sulfolobus and Thermoplasma revealed the universal occurrence of coenzyme F420, (N-[N-[O-[5-(8-hydroxy-5-deazaisoalloxazin-10-yl)-2,3,4-trihydroxy -4-pentoxyhydroxyphosphinyl]-L-lactyl]-L-gamma-glutamyl]-L -glutamic acid), or its gamma-monoglutamyl derivative or both. The total amount (approximately 100 pmol/mg [dry weight]) of these compounds found in the halobacteria studied was approximately 5% of the amount previously reported for methanogenic bacteria. The amount of F420 found in the Sulfolobus and Thermoplasma strains was approximately 1% of that found in the halobacteria. The major compound in all but one of the examined strains was the gamma-monoglutamyl derivative of F420; one strain of halobacteria contained only F420. For the halobacterium-derived samples, the additional glutamic acid was shown to be linked by a gamma-glutamyl peptide bond to the terminal glutamic acid of the F420 core structure by enzymatic hydrolysis of the samples with three different gamma-glutamyltranspeptidases. The product of this enzymatic hydrolysis was F420 with one less glutamic acid in the side chain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley S. T., Morton R. A. Recent developments in the molecular biology of extremely halophilic bacteria. CRC Crit Rev Microbiol. 1978;6(2):151–205. doi: 10.3109/10408417809090622. [DOI] [PubMed] [Google Scholar]
- Cheah K. S. Properties of electron transport particles from Halobacterium cutirubrum. The respiratory chain system. Biochim Biophys Acta. 1969 Jun 24;180(2):320–333. doi: 10.1016/0005-2728(69)90117-0. [DOI] [PubMed] [Google Scholar]
- Cheah K. S. The membrane-bound ascorbate oxidase system of Halobacterium halobium. Biochim Biophys Acta. 1970;205(2):148–160. doi: 10.1016/0005-2728(70)90245-8. [DOI] [PubMed] [Google Scholar]
- Duchstein H. J., Fenner H., Hemmerich P., Knappe W. R. (Photo)chemistry of 5-deazaflavin. A clue to the mechanism of flavin-dependent (de)hydrogenation. Eur J Biochem. 1979 Mar 15;95(1):167–181. doi: 10.1111/j.1432-1033.1979.tb12951.x. [DOI] [PubMed] [Google Scholar]
- Eirich L. D., Vogels G. D., Wolfe R. S. Distribution of coenzyme F420 and properties of its hydrolytic fragments. J Bacteriol. 1979 Oct;140(1):20–27. doi: 10.1128/jb.140.1.20-27.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirich L. D., Vogels G. D., Wolfe R. S. Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry. 1978 Oct 31;17(22):4583–4593. doi: 10.1021/bi00615a002. [DOI] [PubMed] [Google Scholar]
- Eker A. P., Dekker R. H., Berends W. Photoreactivating enzyme from Streptomyces griseus-IV. On the nature of the chromophoric cofactor in Streptomyces griseus photoreactivating enzyme. Photochem Photobiol. 1981 Jan;33(1):65–72. doi: 10.1111/j.1751-1097.1981.tb04298.x. [DOI] [PubMed] [Google Scholar]
- Fitt P. S., Sharma N., Castellanos G. A comparison of liquid-holding recovery and photoreactivation in halophilic and non-halophilic bacteria. Biochim Biophys Acta. 1983 Jan 20;739(1):73–78. doi: 10.1016/0167-4781(83)90046-5. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Hemmerich P., Massey V. Flavin and 5-deazaflavin: a chemical evaluation of 'modified' flavoproteins with respect to the mechanisms of redox biocatalysis. FEBS Lett. 1977 Dec 1;84(1):5–21. doi: 10.1016/0014-5793(77)81047-8. [DOI] [PubMed] [Google Scholar]
- Hescox M. A., Carlberg D. M. Photoreactivation in Halobacterium cutirubrum. Can J Microbiol. 1972 Jul;18(7):981–985. doi: 10.1139/m72-152. [DOI] [PubMed] [Google Scholar]
- Javor B. J. Growth potential of halophilic bacteria isolated from solar salt environments: carbon sources and salt requirements. Appl Environ Microbiol. 1984 Aug;48(2):352–360. doi: 10.1128/aem.48.2.352-360.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Stadtman T. C. Reconstitution of a formate-NADP+ oxidoreductase from formate dehydrogenase and a 5-deazaflavin-linked NADP+ reductase isolated from Methanococcus vannielii. J Biol Chem. 1980 Feb 10;255(3):1049–1053. [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. I. Spectrophotometric identification of the cytochromes of Halobacterium cutirubrum. Arch Biochem Biophys. 1968 Dec;128(3):716–724. doi: 10.1016/0003-9861(68)90080-5. [DOI] [PubMed] [Google Scholar]
- Massey V., Hemmerich P. Photoreduction of flavoproteins and other biological compounds catalyzed by deazaflavins. Biochemistry. 1978 Jan 10;17(1):9–16. doi: 10.1021/bi00594a002. [DOI] [PubMed] [Google Scholar]
- Reed B., Scott J. M. Identification of the intracellular folate coenzymes of different cell types. Methods Enzymol. 1980;66:501–507. doi: 10.1016/0076-6879(80)66493-3. [DOI] [PubMed] [Google Scholar]
- Stankovich M. T., Massey V. Determination of the redox potential of deazariboflavin by equilibration with flavins. Biochim Biophys Acta. 1976 Dec 8;452(2):335–344. doi: 10.1016/0005-2744(76)90183-2. [DOI] [PubMed] [Google Scholar]
- Tzeng S. F., Wolfe R. S., Bryant M. P. Factor 420-dependent pyridine nucleotide-linked hydrogenase system of Methanobacterium ruminantium. J Bacteriol. 1975 Jan;121(1):184–191. doi: 10.1128/jb.121.1.184-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


