Abstract
A new technology for high-speed solution-phase parallel synthesis has been developed by the combination of microwave heating and fluorous tagging strategies. This article highlights several applications of this technology in the synthesis of library scaffolds involving Pd-catalyzed cross-coupling and multicomponent reactions.
INTRODUCTION
Increasing reaction speed and simplifying product purification are two major processes to improve the efficiency of organic synthesis. In recent years, the reaction time has been dramatically decreased by the application of microwave heating technology,1 and product separation has been facilitated by the development of polymer-supported chemistry.2 The blending of microwave heating and polymer-supported reactions is a logical consequence for further enhancement of high-throughput organic synthesis. Significant progresses have been made in this area;3 however, there are still limitations related to the physical and chemical stability of the polymer support under microwave heating and to the heterogeneous nature of solid-phase reactions.
The recent development of fluorous synthesis has provided a new opportunity to combine solution-phase reaction with phase tag-based separation.4 Perfluoroalkyl groups such C6F13 and C8F17 instead of resins are used as the phase tags to anchor substrates or reagents. Attached fluorous molecules have reasonable solubility in common organic solvents, thus reactions can be conducted in a homogeneous environment. Fluorous compounds can be isolated by passing crude products through a cartridge charged with FluroFlash® silica gel which has a C8F17 stationary phase.5 This so called fluorous solid-phase extraction (F-SPE) technique has been widely used in the separation of reaction mixtures containing fluorous catalysts, scavengers, reagents, protecting groups, and even biopolymers such as proteins and DNA fragments.4 In addition to features of homogenous reaction and simple SPE separation, fluorous synthesis also has good “combinatorial” capability. In other words, it has broad compatibility with other synthetic technologies. Described in this paper are recent developments of high-speed solution-phase parallel synthesis by combination of microwave and fluorous technologies.
MICROWAVE-ASSISTED FLUOROUS SYNTHESIS
The C-F bond of the fluorous tag has excellent chemical and physical stabilities (Teflon is a fluorous polymer), which makes fluorous molecules well suited for microwave reactions at elevated temperature. In 1997 Larhed, Hallberg and Curran introduced microwave heating in fluorous synthesis.6 We have recently focused on the development of two kinds of microwave-assisted fluorous reactions: 1) palladium-catalyzed cross-coupling reactions of fluorous benzaldehydes, and 2) fluorous multicomponent reactions. These reactions have been integrated in the synthesis of functionalized library scaffolds by using a system consisting of an automated monomode microwave reactor, a plate-to-plate SPE station, and a vacuum centrifuge for direct concentration of SPE receiving plates (Figure 1).
Figure 1.

A rapid synthesis system with microwave reactor (left), plate-to-plate SPE (middle), and a plate concentration unit (right)
Pd-CATALYZED CROSS-COUPLING REACTIONS
Palladium-catalyzed cross-coupling reactions are an important class of chemical transformations. Reactions such as Suzuki-Miyaura, Heck, and Buchwald-Hartwig reactions have been widely used in the construction of aryl C-C and C-N bonds. Aryl bromides, iodides, and trifluoromethanesulfonates (triflates) are the common substrates for these coupling reactions. We have employed perfluorooctylsulfonates as triflate alternatives, which also have good reactivity in the coupling reactions. Commercially available perfluorooctylsulfonyl fluoride can be used to convert functionalized phenols to corresponding sulfonates under solution-phase reaction conditions using K2CO3 as a base and DMF as a solvent. Fluorous sulfonates can be purified by conventional methods such as crystallization or by F-SPE. In multistep synthesis of library scaffolds, the perfluorooctylsulfonyl tag plays three roles: 1) as a protecting agent for the hydroxyl group; 2) as a fluorous tag to facilitate intermediate purification; and 3) as a hydroxy activating group for the coupling reaction.
Scheme 1 shows parallel syntheses of cyclic and acyclic amide compounds 1 and 2.7 Fluorous benzaldehydes were first subjected to reductive amination reactions. The resulting amines were then reacted with isocyanates to form substituted hydantoin rings or with benzoyl chlorides to form amides. Purified F-sulfonates were used for palladium-catalyzed cross-coupling reactions to form corresponding biaryl 1 and arylsulfide 2 products, respectively.
Scheme 1.

Synthesis of diversified cyclic and acyclic amide compounds 1 and 2
Scheme 2 illustrates another fluorous sulfonate-based synthesis of library scaffolds. The tagged substrates were taken through aldol condensation and cycloaddition reactions to form the pyrimidine ring. The intermediates were then reacted with boronic acids for Suzuki reactions to form biaryl compounds 3 or reacted with HCO2H to give traceless detagging products 4.8
Scheme 2.
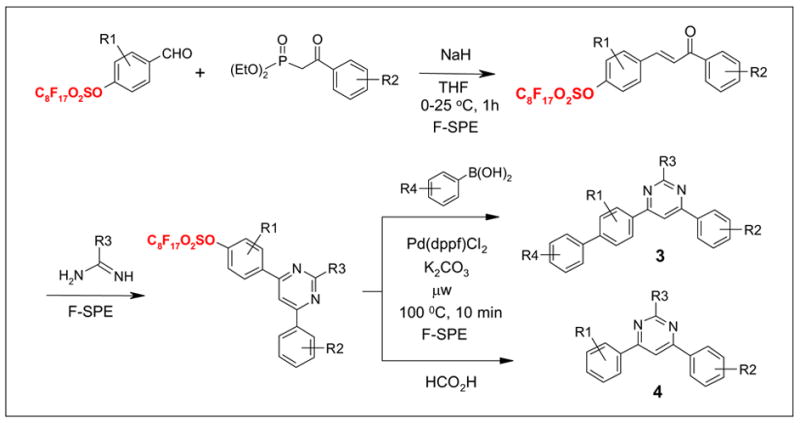
Multi-step synthesis of substituted pyrimidines 3 and 4
MULTICOMPONENT REACTIONS
Fluorous benzaldehydes have been used in the multicomponent reaction (MCR) for substituted 3-aminoimidazo[1,2-a]pyridines and 3-aminoimidazo[1,2-a]pyrazines 5 and 6 (Scheme 3).9 The condensation products generated by the MCR of fluorous benzaldehydes, isonitriles, 2-aminopyridines or 2-aminopyrazines were used for Pd-catalyzed cross-coupling reactions with boronic acids or thiols to produce compounds 5 or 6, respectively.
Scheme 3.

MCRs for synthesis of heterocyclic libraries 5 and 6
We recently collaborated with a research group at Amgen to optimize the Ugi/de-Boc/cyclization synthesis for quinoxalinones 7 and benzimidazoles 8 (Scheme 4).10a The original thermal reactions required 36-48 h for the Ugi reaction and the condensation products were purified by double scavenging with an immobilized tosylhydrazine and diisopropylethylamine to remove excess aldehydes and unreacted acids.10b We used a fluorous Boc-protected diamine, and the Ugi reactions were conducted under microwave irradiation for 20 min. The double scavenging step was replaced by a simple F-SPE. The final reaction step was also conducted by microwave heating and products were also purified by F-SPE.
Scheme 4.
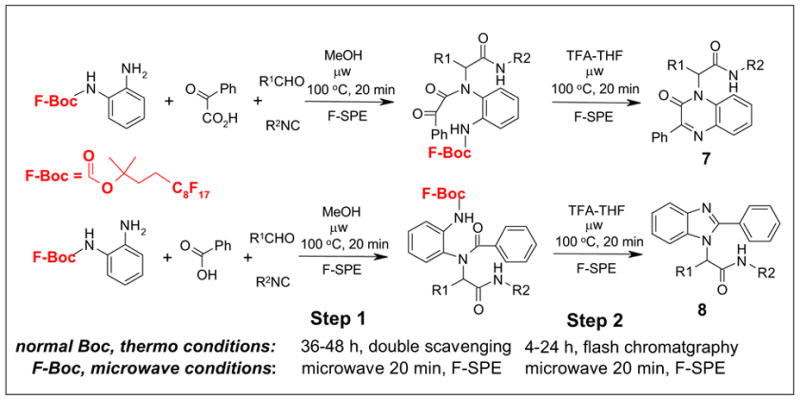
Synthesis of quinoxalinones 7 and benzimidazoles 8
Parallel synthesis of bicyclic proline scaffolds has been developed by a two-step synthesis involving 1,3-dipolar cycloaddition of fluorous benzaldehydes followed by Pd-catalyzed Suzuki coupling reaction with boronic acids (Scheme 5).11 Both reactions were conducted under microwave irradiation and reaction mixtures were purified by F-SPE without performing chromatography.
Scheme 5.

Synthesis of biaryl substituted pralines 9
In addition to Pd-catalyzed cross coupling and MCRs, fluorous aminoesters were also employed in the synthesis of an N-alkylated dihydropteridinones 10 (Scheme 6).9 The 4,6-dichloro-5-nitropyrimidine was first displaced with fluorous aminoesters. The resulting products were then displaced with secondary amines. The reduction of the nitro group was conducted by hydrogenation using Pd/C as a catalyst. The cyclization reactions were promoted by microwave irradiation. The N-alkylation reaction of the cyclized products with benzyl halides gave mono products 10 in high selectivity. A library containing 89 compounds was prepared. All the intermediate and final products were purified by F-SPE or crystallization. Purities of most final products were greater than 90%.
Scheme 6.
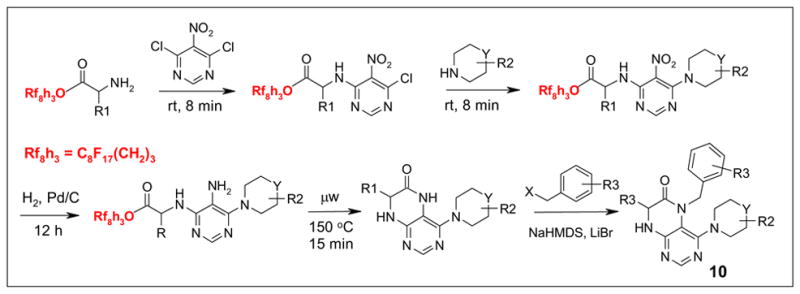
Fluorous synthesis of N-alkylated dihydropteridinones 10
CONCLUSIONS
Microwave-enhanced fluorous synthesis successfully addresses two important aspects of organic synthesis: the speed of reaction and the efficiency of product purification. It is a promising new technology for solution-phase parallel synthesis.
Acknowledgments
The author thanks Dr. Tadamichi Nagashima, Yimin Lu, and Christine H.-T. Chen for thier research contributions. Parts of our work were supported by National Institutes of General Medical Sciences SBIR Grants (2R44GM062717-02 and 2R44GM067326-02A1). The Pd-catalyzed microwave reactions were conducted under license to US patent 6,136,157 and European patent No. 0901453 held by Personal Chemistry, now Biotage.
References
- 1.a. Loupy A, editor. Microwaves in Organic Synthesis. Wiley-VCH; Weinheim: 2002. [Google Scholar]; b. Kappe OC. Angew Chem Int Ed. 2004;43:6250. doi: 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaou KC, Hanko R, Hartwig W, editors. Handbook of Combinatorial Chemistry. Wiley-VCH; Weinheim: 2002. [Google Scholar]
- 3.Swamy KMK, Yeh W-B, Lin M-J, Sun C-M. Curr Med Chem. 2003;10:2403. doi: 10.2174/0929867033456594. [DOI] [PubMed] [Google Scholar]
- 4.a. Gladysz JA, Curran DP, Horvath IT, editors. Handbook of Fluorous Chemistry. Wiley-VCH; Weinheim: 2004. [Google Scholar]; b. Zhang W. Chem Rev. 2004;104:2531. doi: 10.1021/cr030600r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c. Zhang W. Tetrahedron. 2003;59:4475. [Google Scholar]
- 5.Curran DP. Synlett. 2001:1488. [Google Scholar]
- 6.a. Larhed M, Hoshino M, Hadida S, Curran DP, Hallberg A. J Org Chem. 1997;62:5583. [Google Scholar]; b. Olofeeson K, Kim SY, Larhed M, Curran DP, Hallberg A. J Org Chem. 1999;64:4539. [Google Scholar]
- 7.a. Zhang W, Lu Y, Chen CH-T. Molecular Diversity. 2003;7:199. doi: 10.1023/b:modi.0000006825.12186.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]; b. Zhang W, Chen CH-T, Lu Y, Nagashima T. Org Lett. 2004;6:1473. doi: 10.1021/ol0496428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Nagashima T, Lu Y, Chen CHT. Tetrahedron Lett. 2004;45:461. [Google Scholar]
- 9.Lu Y, Zhang W. QSAR Comb Sci. 2004;23:827. doi: 10.1901/jaba.2004.23-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a. Zhang W, Tempest P. Tetrahedron Lett. 2004;45:6757. [Google Scholar]; b. Nixey T, Tempest P, Hulme C. Tetrahedron Lett. 2002;43:1637. [Google Scholar]
- 11.Zhang W, Chen CHT. Tetrahedron Lett. 2005;46:1807. [Google Scholar]
- 12.Nagashima T, Zhang W. J Comb Chem. 2004;6:942. doi: 10.1021/cc049885r. [DOI] [PubMed] [Google Scholar]


