Abstract
Kaposi’s sarcoma-associated herpesvirus origin-dependent DNA replication requires the core replication proteins plus K-Rta and K-bZIP. To determine which K-bZIP protein domains contribute to oriLyt-dependent DNA replication and facilitate suppression of K-Rta-mediated transcriptional activation, we generated a series of deletion constructs and site-directed mutations within the K-bZIP ORF. Mutation of key leucine residues within the putative leucine zipper (LZ) motif eliminated the ability of the protein to homodimerize and complement oriLyt-dependent DNA replication. Deletion of the basic amino acid region (BR) or LZ domain did not affect the ability of K-bZIP to bind to K-Rta indicating that either region contributes to heterodimerization with K-Rta. However, deletions or mutations introduced into both the LZ and BR resulted in elimination of the suppressive activity of K-bZIP even in the presence of a K-bZIP-K-Rta interaction. Interestingly, mutants that lacked the ability to suppress K-Rta transactivation were still capable of complementing oriLyt-dependent DNA replication, indicating that this activity does not contribute to the DNA synthesis-related activity of K-bZIP.
Keywords: DNA replication, herpesvirus, transactivation, leucine zipper
Introduction
Kaposi’s sarcoma-associated herpesvirus or human herpesvirus 8 (HHV8) lytic DNA replication requires 6 core replication proteins common to the herpesvirus family. These proteins: ORF6 (single-stranded DNA binding protein), ORF9 (DNA polymerase), ORF40/41 (primase-associated factor), ORF44 (helicase), ORF56 (primase), ORF59 (polymerase processivity factor) provide the enzymatic machinery for replication of viral DNA (AuCoin et al., 2004a). In addition to these core proteins, two other viral-encoded proteins are required for origin-dependent DNA replication. These proteins, ORF50 (K-Rta) and K8 (K-bZIP), supply an as yet undetermined activity.
K-Rta is the major transactivator for HHV8 and is responsible for the switch from latency to lytic replication (Byun et al., 2002; Gradoville et al., 2000; Lukac et al., 1999; Wang et al., 2001). K-Rta has autoregulatory properties, with respect to DNA binding and its own expression, and was shown to activate both immediate early as well as early genes, including K8 (K-bZIP) (Byun et al., 2002; Chang et al., 2004; Deng et al., 2000; Lukac et al., 2001). Recent studies indicated that the expression of K-Rta in the context of the viral genome is required for lytic replication and activation of several viral encoded proteins (Xu et al., 2005). With respect to the role of K-Rta in DNA replication, current data indicates that K-Rta activates a K-Rta-responsive promoter within oriLyt and suggests that initiation of DNA synthesis is triggering by a transcription event. This mode of initiation of lytic DNA synthesis would be consistent with that proposed for both Epstein Barr Virus (EBV) and human cytomegalovirus (HCMV) (Hammerschmidt et al., 1988; Schepers et al., 1996; Schepers et al., 1993).
The role of K-bZIP in DNA replication is less clear. K-bZIP interacts with K-Rta and suppresses the transactivation activity of K-Rta on some K-Rta-responsive promoters (Izumiya et al., 2003a; Liao et al., 2003). K-bZIP was also implicated in controlling the cell cycle by directly binding to cyclin-CDK2 (Izumiya et al., 2003b). Since K-bZIP has no reported transactivation function, the assumption is that a direct or indirect interaction with oriLyt provides an enzymatic activity or some other role that orchestrates initiation of DNA synthesis. Consistent with this model, K-bZIP was shown to interact with oriLyt via CCAAT enhancer binding sites within oriLyt through an indirect or “piggyback” interaction with CCAAT enhancer binding protein alpha (C/EBPα) in BCBL-1 cells (Wang et al., 2003a; Wang et al., 2003b).
Our previous studies demonstrated that the oriLyt-dependent DNA replication-associated activity of K-bZIP requires the presence of an intact leucine zipper motif (AuCoin et al., 2004a). Apparently, the region containing the leucine zipper is required for multimerization, but does not form the traditional coiled-coil structure (Al Mehairi et al., 2005). The BR plus the leucine zipper motif were demonstrated to comprise the region of interaction between K-bZIP and C/EBPα (Wang et al., 2003a). Consequently, the K-bZIP leucine zipper and BR amino acid motifs are key protein domains essential for the interaction with cellular and other viral encoded proteins. This suggests that the replication function supplied by K-bZIP may be distinct from that supplied by the closely related Zta protein of Epstein Barr Virus (EBV). In EBV, Zta provides a transactivation function as well as a replication activity within oriLyt by interacting with several defined Zta response elements (ZREs) (Fixman et al., 1992; Sarisky et al., 1996).
There is an apparent discrepancy surrounding which motif is required for interaction with, and for suppression of, K-Rta-mediated transcriptional activation. Two contradicting reports show that the either the BR or the LZ region is the point of interaction between K-bZIP and K-Rta (Izumiya et al., 2003a; Liao et al., 2003). Liao et al reports that a deletion of the leucine zipper region of K-bZIP abrogates its suppressive affects; however, Izumiya et al report that deletion of the BR region resulted in a loss of suppression of K-Rta-mediated transactivation (deletion of the LZ had no effect). This controversy served as a major impetus for our investigation of the binding domains of K-bZIP and how repression of K-Rta-mediated transactivation relates to DNA synthesis.
In this report we defined the K-bZIP point of interaction required for both homodimerization of K-bZIP and heterodimerization with K-Rta. We show that point mutations introduced into the leucine zipper motif disrupt the ability of K-bZIP to homodimerize and fail to complement oriLyt-dependent DNA replication. In addition, K-bZIP LZ mutants that fail to homodimerize no longer inhibit the transactivation activity of K-Rta. Deletion of both the LZ and BR domains of K-bZIP is required to eliminate a K-bZIP-K-Rta interaction, indicating that either structure can interact with K-Rta. Mutations introduced into the BR region of K-bZIP did not interfere with binding to K-Rta, but affect the ability of K-bZIP to repress K-Rta-mediated transactivation and oriLyt amplification. Finally, we show that the suppression of K-Rta transactivation by K-bZIP does not contribute to oriLyt-dependent DNA replication.
Results and discussion
The K-bZIP leucine zipper and the basic amino acid region contribute to homodimerization
The first step to defining a region within the K-bZIP protein that contributes to a self-interaction is to identify a deletion mutant that no longer forms a dimer. We generated 7 HA-tagged expression plasmids each with a different 33 amino acid deletion in the K-bZIP ORF (Fig. 1A). Deletion plasmids were each cotransfected with a full-length wt K-bZIP expression plasmid that was FLAG tagged. Protein extracts were prepared and immunoprecipitated with anti-FLAG antibody. An interaction between the two K-bZIP species was detected by reacting Western blot of the immunoprecipitated protein with an anti-HA antibody. All of the K-bZIP deletion proteins interacted with wt K-bZIP except deletion number 6 (D6) (Fig. 1B, lane 6). K-bZIP D6 has a 33f amino acid deletion that removed the entire BR region and most of the LZ motif, leaving leucine residues 205, 212 and 219 of the LZ domain. All FLAG and HA-tagged K-bZIP proteins were similarly expressed (Fig. 6B, see FLAG and HA Lysates) and all FLAG-tagged protein species were efficiently immunoprecipitated (Fig. 6B, see FLAG IP FLAG West). These results show that the region between amino acids 169 and 202 of the K-bZIP ORF contribute to homodimerization and confirm earlier reports implicating the LZ and BR domains.
Figure 1. K-bZIP protein lacking the BR and LZ domains fail to homodimerize.
(A) Genomic arrangements of nucleotide coordinates for ORF50 and K-bZIP and a schematic of the K-bZIP ORF showing the basic amino acid region (BR) and leucine zipper motif (LZ) and HA tag. Also shown are the 7 in frame deletion mutants generated for K-bZIP and the designated amino acids that were deleted. (B) Western blot of co-immunoprecipitation assay of K-bZIP deletion mutants and wt K-bZIP. Cells were transfected and treated as described in materials and methods. Lanes: wt-K-bZIP-FLAG, transfection and immunoprecipitation of control K-bZIP-FLAG protein; wt K-bZIP-FLAG and wt K-bZIP-HA, cotransfection control plasmids and immunoprecipitation of FLAG and HA tagged wt K-bZIP protein; 1, protein samples from cotransfection of wt K-bZIP-HA and D1; 2, protein samples from cotransfection of wt K-bZIP-HA and D2; 3, protein samples from cotransfection of wt K-bZIP-HA and D3; 4, protein samples from cotransfection of wt K-bZIP-HA and D4; 5, protein samples from cotransfection of wt K-bZIP-HA and D5; 6, protein samples from cotransfection of wt K-bZIP-HA and D6; 7, protein samples from cotransfection of wt K-bZIP-HA and D7. FLAG Lysate: protein samples from transfections reacted with anti-FLAG antibody, HA Lysate: protein samples from transfections reacted with anti-HA antibody, FLAG IP HA West: cotransfections were immunoprecipitated with anti-FLAG antibody and western blots were reacted with anti-HA antibody, FLAG IP FLAG West: cotransfections were immunoprecipitated with anti-FLAG antibody and western blots were reacted with anti-FLAG antibody.
The leucine zipper domain of K-bZIP mediates homodimerization
The co-immunoprecipitation experiment showed that the region of K-bZIP containing the LZ and BR domains is required for homodimerization. In addition, since K-bZIP is known to exist as a dimer in transfected and infected cells (Al Mehairi, Cerasoli, and Sinclair, 2005; Izumiya et al., 2003a) we wanted to investigate if dimerization of K-bZIP was involved in, or required for, the DNA replication function conferred by the protein.
Figure 2A is a schematic of the ORF50 and K-bZIP genomic region. Also shown are the domains of interest within the K-bZIP ORF (Fig. 2A). The putative basic region (BR) for K-bZIP was identified as containing six basic amino acids (Fig. 2A) and the putative leucine zipper (LZ) encompasses leucine residues and an isoleucine residue at amino acids 191, 198, 205, 212 and 219 (Fig. 2A). In order to study the contribution of these domains to homodimerization we altered several leucine residues within the LZ using site-directed mutagenesis and assayed the ability of these mutants to interact with wt K-bZIP using the co-immunoprecipitation assay (Fig 2C).
Figure 2. K-bZIP homodimerization requires an intact LZ.
(A) Schematic showing the K-bZIP ORF and amino acid residues comprising the BR (basic region) and LZ (leucine zipper). (B) Amino acid sequence of the leucine zipper motif and the location of mutations introduced into key leucine residues to generate K-bZIP mutants LZ-1, LZ-2 and LZ-3 and LZ-205. (C) Cotransfection and immunoprecipitation of wt K-bZIP with leucine zipper mutants. Assays were performed as described previously.
293T cells were cotransfected with FLAG tagged LZ mutants (Fig. 2B) along with wild type HA tagged K-bZIP. The differentially tagged proteins allowed us to detect binding between mutant and wild type K-bZIP species upon co-immunoprecipitation by anti-FLAG antibodies followed by Western blot analysis using HA specific antibodies. Under these cotransfection conditions leucine zipper mutants LZ-1 and LZ-2 failed to interact with K-bZIP-HA (Fig. 2C). Control lysates showed that all proteins were expressed efficiently and at similar levels (Fig. 2C, FLAG and HA lysates). These results indicated that the LZ motif of K-bZIP, especially residues 198 and 205, is essential for efficient homodimerization.
K-bZIP requires an intact BR/LZ region to interact with K-Rta
Since it was previously reported that there is an interaction between K-bZIP and the viral transactivator ORF50 (K-Rta), we next wanted to evaluate the ability of the K-bZIP deletion mutants to interact with K-Rta. Cells were cotransfected with plasmids expressing each K-bZIP deletion mutant (from Fig. 1A) and a K-Rta expression plasmid containing an in-frame FLAG epitope. Co-immunoprecipitation assays were performed and precipitated protein complexes were analyzed by Western blot. Consistent with the K-bZIP homodimerization results, K-bZIP deletion mutant D6 failed to interact with K-Rta (Fig. 3C).
Figure 3. Interaction of K-bZIP BR and LZ mutants with K-Rta.
(A) Schematic of expression plasmids where either the LZ or the BR of K-bZIP was deleted from the ORF and (B) expression plasmids where amino acid residue changes were introduced into the K-bZIP BR or LZ domains in the backbone of BR or LZ deletion mutants. (C) Co-immunoprecipitation of K-Rta with K-bZIP deletion mutants. Lanes: Wt K-bZIP-HA Wt K-Rta-FLAG, cotransfection and immunoprecipitation of Wt K-bZIP and wt K-Rta; 1, cotransfection and immunoprecipitation of D1 and wt K-Rta; 2, cotransfection and immunoprecipitation of D2 and Wt K-Rta; 3, cotransfection and immunoprecipitation of D3 and Wt K-Rta; 4, cotransfection and immunoprecipitation of D4 and wt K-Rta; 5, cotransfection and immunoprecipitation of D5 and Wt K-Rta; 6, cotransfection and immunoprecipitation of D6 and wt K-Rta; 7, cotransfection and immunoprecipitation of D7 and wt K-Rta. (D) Amino acid residue mutations introduced into the BR domain of K-bZIP have no effect on K-Rta binding. Western blot of co-immunoprecipitated protein-protein interactions between various K-bZIP BR mutants and wt K-RTA. (E) K-bZIP leucine zipper mutants interact with K-Rta. Western blot of co-immunoprecipitated protein samples from cotransfections of K-bZIP mutants lacking either the BR or LZ, plus D6 as negative control, and wt K-Rta. (F) BR or LZ regions of K-bZIP can interact with K-Rta. FLAG Lysate: protein samples from transfections reacted with anti-FLAG antibody, HA Lysate: protein samples from transfections reacted with anit-HA antibody, FLAG IP HA West: cotransfections were immunoprecipitated with anti-FLAG antibody and western blots were reacted with anti-HA antibody, FLAG IP FLAG West: cotransfections were immunoprecipitated with anti-FLAG antibody and western blots were reacted with anti-FLAG antibody. (G) Mutations in the LZ and BR abrogate the K-bZIP-K-Rta interaction. Western blot of co-immunoprecipitated protein samples from cotransfection of various K-bZIP BR-LZ mutants and wt K-Rta. FLAG Lysate: protein samples from transfections reacted with anti-FLAG antibody, HA Lysate: protein samples from transfections reacted with anit-HA antibody, FLAG IP HA West: cotransfections were immunoprecipitated with anti-FLAG antibody and western blots were reacted with anti-HA antibody, FLAG IP FLAG West: cotransfections were immunoprecipitated with anti-FLAG antibody and western blots were reacted with anti-FLAG antibody.
The next step was to determine which specific K-bZIP domain(s) were responsible for interacting with K-Rta. It was previously reported that the BR of K-bZIP alone could interact with K-Rta (Izumiya et al., 2003a). However, another report found that only the K-bZIP leucine zipper motif was the point of interaction between the two proteins (Liao et al., 2003). Both of these studies used peptide fragments of K-bZIP protein corresponding to the LZ or BR regions to analyze these interactions. We were interested in the binding characteristics of K-bZIP with K-Rta in the context of the complete protein coding region minus the BR, LZ or other protein domains. As stated earlier, the BR region of K-bZIP consists of six amino acids just upstream of the LZ motif (Fig. 1A). We used site-directed mutagenesis to generate 9 K-bZIP expression plasmids with multiple mutations in the BR region. Each of these expression plasmids was used in the cotransfection co-immunoprecipitation assay with a K-Rta expression plasmid. All BR mutated K-bZIP proteins species were able to interact with K-Rta (Fig. 3D). We also performed K-bZIP-K-Rta co-immunoprecipitations using the K-bZIP leucine zipper mutants, two of which, LZ-1 and LZ-2, failed to interact with wt K-bZIP. Again all LZ mutants were capable of interacting with K-Rta (Fig. 3E).
Since all of the K-bZIP proteins with amino acid residue changes in either the BR or LZ domains were capable of interacting with K-Rta we next decided to evaluate the ability of LZ or BR K-bZIP deletion mutants to interact with K-Rta in the co-immunoprecipitation assay. We generated expression plasmids where either the entire BR or LZ regions were deleted (Fig. 3A). These plasmids were co-transfected with a K-Rta expression plasmid and immunoprecipitations were preformed. Surprisingly, both of these K-bZIP mutant proteins could interact with K-Rta (Fig. 3F, lanes ΔBR and ΔLZ). The control plasmid, where the entire BR/LZ region was removed from the K-bZIP protein, failed to interact with K-Rta. This result indicated that either the LZ or the BR domains could interact with K-Rta. In order to confirm this finding we used plasmid clones that had either the LZ or BR domain deleted and then introduced several point mutations into the remaining LZ or BR region. These expression plasmids were cotransfected along with a wt K-Rta expression plasmid and co-immunoprecipitations were performed. An expression plasmid encoding a K-bZIP protein species that lacked the entire BR and had leucine residues 198 and 205 mutated interacted weakly with K-Rta (Fig. 3G). A weak interaction was also observed when using a plasmid clone that expressed a K-bZIP species that lacked the entire LZ region and included mutations in amino acid residues 170/171/181/185 (Fig. 3G). No interaction was detected in cotransfected cells using K-bZIP expression plasmids that lacked the entire LZ domain and contained mutations in residues 171/174/181/185 or 174/177/181/185 (Fig. 3G). These experiments showed that both the BR and LZ of K-bZIP can interact with K-Rta and that several amino acid residues within the BR domain contribute to binding. Residues within the LZ region that confer homodimerization also contribute to heterodimerization.
The basic region, as well as leucine zipper region, contributes to suppression of K-Rta mediated transactivation
K-bZIP suppresses the transactivation function mediated by K-Rta. This suppression appears to be promoter specific in that two early promoters, those regulating the expression of K-bZIP and ORF57, are down regulated in the presence of K-bZIP (and K-Rta), whereas the promoter responsible for the expression of PAN is unaffected by K-bZIP. We wanted to evaluate the ability of some of our LZ or BR mutants to suppress K-Rta mediated transactivation. Vero cells were transfected with a K-bZIP promoter luciferase reporter plasmid (gift from H-S Kung, UC Davis) along with plasmids expressing either wt K-bZIP or LZ or BR mutants, and a K-Rta expression plasmid. Cells were harvested 24 h post transfection and assayed for luciferase activity. All luciferase activity comparisons were made against basal K-bZIP promoter activity. As expected, wt K-bZIP efficiently suppressed K-Rta-mediated transactivation of the K-bZIP promoter (Fig. 4A, wt-K-bZIP). In addition, complete removal of the LZ and BR domains resulted in a loss of repressive function by K-bZIP (Fig. 4A). Some of the K-bZIP BR mutants failed to suppress K-Rta mediated activation of the K-bZIP promoter (Fig. 4A, BR 171/181/185 and BR 170/171/181/185). The K-bZIP recombinant with mutations in amino acid residues 170/181/185 retained its ability to suppress K-Rta mediated transactivation similar to wt K-bZIP (Fig. 4A). However, when the LZ domain was removed, this species lost its ability to repress (Fig. 4A, BR 170/181/185 LZ). This data demonstrated that even in the presence of a protein-protein interaction between K-bZIP and K-Rta, suppression of transcriptional activation was dependent upon the retention of at least some basic amino acid residues within the BR of the K-bZIP protein.
Figure 4. Mutations introduced into the BR or LZ regions of K-bZIP affect suppression of K-Rta mediated transcriptional activation.
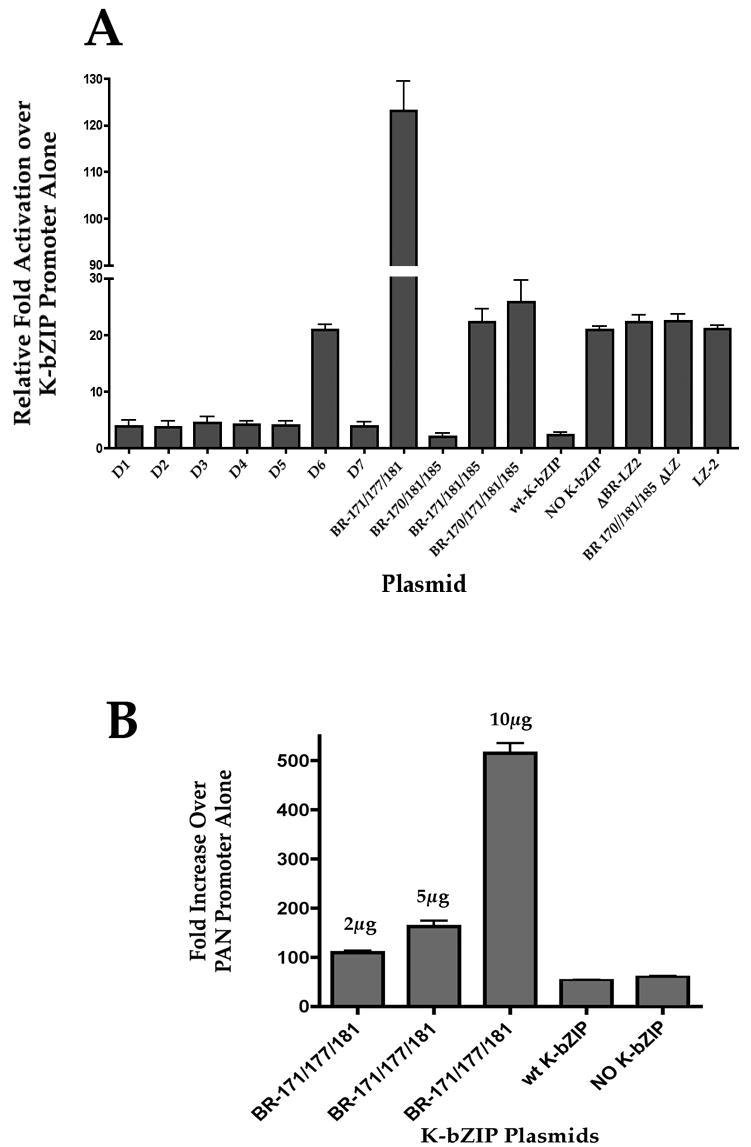
(A) Vero cells were cotransfected with either a wt K-bZIP expression plasmid or various BR-or LZ-mutated K-bZIP expression plasmids, a K-Rta expression plasmid and a luciferase reporter plasmid that has the K-bZIP promoter driving the expression of the luciferase gene. Data is shown as relative fold activation over luciferase activity measured in cells transfected with only the K-bZIP luciferase reporter plasmid. Total DNA for all transfection was equal and maintained by using pGEM as filler DNA, except where noted. (B) K-bZIP BR-171/177/181 activates the PAN promoter. BR-171/177/181 was cotransfected with a PAN promoter luciferase reporter plasmid along with a K-Rta expression plasmid. Increasing amounts (2μg, 5μg and 10μg) of the BR-171/177/181 plasmid were added to the transfection mixture.
Interestingly, one BR mutant, BR-171/177/181, increased the transactivation activity of K-Rta on the K-bZIP promoter (Fig. 4A). This increased activity was over 5-fold higher than that observed for K-Rta alone and over 100-fold higher than the suppression mediated by wt K-bZIP (Fig. 4A). This positive affect on K-Rta transactivation was also observed when using a promoter (PAN) not normally regulated by K-bZIP (Izumiya et al., 2003a). Nevertheless, when the K-bZIP mutant BR-171/177/181 was cotransfected along with a K-Rta expression plasmid and a PAN luciferase reporter plasmid, the activity of the PAN promoter was shown to increase over 500-fold when compared to PAN promoter alone and over 10-fold higher than when K-Rta was present, with or without a wt K-bZIP expression plasmid (Fig. 4B). As expected, the addition of a wt K-bZIP expression plasmid had no effect on luciferase activity from the PAN promoter (Fig. 4B).
Leucine zipper mutant LZ-2 also failed to suppress activation of the K-bZIP promoter (Fig. 4A). This species contained mutations in leucine residues 198 and 205. This result could suggest that this region of the protein is important for binding with K-Rta or may indicate that a K-bZIP-K-bZIP interaction is a prerequisite for suppression of K-Rta mediated activation. This set of experiments showed that the BR of K-bZIP could serve to regulate the activity of K-Rta in a negative, or in one case, a positive manner.
The leucine zipper domain contributes to K-bZIP replication function
Since it was previously demonstrated that K-bZIP supplied an essential function in DNA replication, we next wanted to identify important domains within the protein that contributed to its function. It is known that mutations introduced into two of the leucine residues within the putative leucine zipper abolished the DNA replication function of K-bZIP (AuCoin et al., 2004b), hence we sought to investigate the physical parameters behind this observation. We next wanted to evaluate the ability of K-bZIP mutants to complement oriLyt-dependent DNA replication. We previously demonstrated that the core replication proteins plus K-Rta and K-bZIP were required for efficient amplification of HHV8 oriLyt in the transient replication assay (AuCoin et al., 2004a). We cotransfected core replication proteins plus K-Rta, oriLyt and the various K-bZIP mutants and examined the ability of several mutants to complement oriLyt amplification. Since it was demonstrated that K-bZIP was one of the non-core proteins required for oriLyt-dependent DNA replication, we wanted to investigate the role of the specific protein domains of K-bZIP with respect to oriLyt-dependent DNA replication. As mentioned earlier, it was previously reported that K-bZIP is capable of forming both a homodimer and heterodimer with K-Rta (Al Mehairi, Cerasoli, and Sinclair, 2005; Izumiya et al., 2003a; Liao et al., 2003). It was shown here and previously that the LZ domain of K-bZIP is required for interaction with K-Rta and a mutant lacking the LZ domain no longer repressed K-Rta mediated transactivation (Liao et al., 2003). Further, it was also demonstrated that multimerization of K-bZIP is also conferred through the LZ domain (Al Mehairi, Cerasoli, and Sinclair, 2005). In addition, we have demonstrated that 2 key leucine residues contribute to homodimerization and mutations of leucine residues within the LZ region of K-bZIP inactivated the DNA replication activity of the protein in the transient cotransfection replication assay (AuCoin et al., 2004a). Our goals now were to narrow down which leucine residues directly contributed to DNA replication and determine if multimerization of K-bZIP is necessary to confer the DNA replication activity of the protein.
Plasmids encoding mutated K-bZIP proteins were used in the cotransfection-replication assay in place of wt K-bZIP. All of the other required core replication proteins as well as K-Rta were included in the mixture along with HHV-8 oriLyt. As a control for demonstrating authentic amplification of oriLyt, we treated all samples with either DpnI (and EcoRI), which cleaves unmethylated/replicated DNA, or MboI, which has the same base sequence recognition as DpnI but cleaves both methylated and unmethylated DNA. Therefore any amplified oriLyt plasmid will be cleaved when treated with MboI ensuring that amplification of oriLyt occurred. Also, we ran duplicate gels of cotransfected DNA that was cleaved with EcoRI alone and performed Southern blots on these samples to show that an equal amount of input oriLyt was in the transfection mixture and in gel samples (Fig. 5B). K-bZIP species that lacked the entire LZ or the BR region failed to complement oriLyt-dependent DNA replication (Fig. 5A, lanes 3, 3* and 4, 4*). Also, the K-bZIP LZ-1 and LZ-2 mutants failed to complement oriLyt amplification indicating that these leucine residues are required for the replication function and suggests that homodimerization is essential (Fig. 5A, lanes 5, 5* and 6, 6*).
Figure 5. Amino acid changes within the BR and the LZ motif affect the ability of K-bZIP to complement oriLyt-dependent DNA replication.
Southern blot of DNA from cotransfection-replication assay using Vero cells transfected with various K-bZIP mutants. All cotransfections included the core replication proteins, a K-Rta expression plasmid, cloned oriLyt and wt K-bZIP or various mutant K-bZIP expression plasmids as indicated. (A) Cells were cotransfected with K-bZIP expression plasmids that produced mutated protein with amino acid changes in the BR. (* lanes were treated with MboI) Lanes: 1, wt K-bZIP; 2, K-bZIP D6; 3, K-bZIP ΔBR; 4, K-bZIP ΔLZ; 5, K-bZIP LZ-1; 6, K-bZIP LZ-2; 7, wt K-bZIP; 8, BR 171/177/181; 9, BR 170/171/181/185; 10, BR 170/181/185; 11, BR 171/181/185; 12, No K-bZIP. (B) OriLyt input control. Southern of total cell DNA extracted from cotransfected Vero cells cleaved with EcoRI only and hybridized with a probe specific for HHV8 oriLyt sequences.
Repression of K-Rta mediated transcriptional activation by K-bZIP is not necessary for complementation of oriLyt-mediated DNA replication
We also used the replication assay to evaluate K-bZIP protein species with mutations in the BR domain. The K-bZIP protein species lacking the entire BR failed to complement oriLyt amplification in the transient cotransfection replication assay (Fig. 5A, lane 3 and 3*). However, those K-bZIP mutants with mutations in the BR domain were still capable of complementing oriLyt amplification, even those mutants that failed to repress K-Rta-mediated transactivation (Fig. 5A, lanes 8–11 and 8*–11*). This result strongly suggests that the repressive function of K-bZIP does not participate in DNA synthesis. Interestingly, the K-bZIP mutant that conferred an increase in K-Rta transcriptional activation also showed an increase in oriLyt amplification indicating that increased activity of K-Rta can lead to a higher accumulation of replicated oriLyt. Both replication controls (MboI cleavage) and input control show that the replicated product is authentic and similar amounts of input oriLyt DNA were transfected.
Previously we demonstrated that the leucine zipper region of K-bZIP was essential for conferring a replication function to the protein (AuCoin et al., 2004a). We have now extended those previous findings by showing that mutations introduced into key leucine residues within the zipper motif profoundly affect the activity of K-bZIP in the origin-dependent replication assay. The results presented here show that leucine residue 205, along with residue 198 from the previous studies, are key amino acids with respect to DNA replication and homodimerization. One ramification of mutations within the leucine zipper region is that these mutations no longer allow for a K-bZIP-K-bZIP interaction. This suggests that dimerization of K-bZIP is essential for complementation of DNA replication.
Dimerization appears not to be necessary for the interaction of K-bZIP with K-Rta. K-bZIP mutant LZ-2 still interacted with K-Rta although it no longer complemented oriLyt-dependent DNA replication. This result can be interpreted two ways: i) it could be that K-bZIP interacts with K-Rta as a monomer or ii) it is essential that K-bZIP be in a dimer or multimer state when binding to K-Rta and it is this structure that contributes to activity. In a previous report, the BR of K-bZIP was implicated as the domain that interacts with K-Rta (Izumiya et al., 2003a). It also appears that the regulatory function of K-bZIP is distinct from the replication function. This is exemplified by the fact that a K-bZIP BR mutant was still capable of complementing oriLyt-dependent DNA replication but was not able to suppress K-Rta-mediated transactivation.
Our results show that both the LZ and BR domains can interact with K-Rta. This may explain the apparent conflicting results from studies seeking to identify the K-bZIP domain that interacts with K-Rta (Izumiya et al., 2003a; Liao et al., 2003). It is clear from the results shown in this report that both structures contribute to the interaction of K-bZIP with itself, to the suppression of K-Rta, and to DNA replication.
Mutations introduced into the BR did not affect K-bZIP homodimerization but this domain still may be required for an as yet unidentified DNA-protein interaction. Indeed the complete removal of either the BR or LZ regions resulted in a loss of replication function. Interestingly, even though efficient binding was observed in co-immunoprecipitation experiments, mutations in the BR locus still affected the ability of K-bZIP to suppress the transactivation activity of K-Rta. This strongly suggests that inhibition of transactivation is not exclusively due to a protein-protein interaction. While others have investigated the role of specific elements in the repression of transcriptional activation by K-Rta, ours is the first study to evaluate the effects of specific amino acid residue changes within K-bZIP, particularly in the BR. Recently, it was shown that SUMO modification was partly responsible for transcriptional repression activity of K-bZIP (Izumiya et al., 2005). Sumoylation was shown to occur at lysine residue 158, a residue that was not altered in our studies. In addition, phosphorylation also plays a role in the repressive activity of K-bZIP on K-Rta mediated transactivation (Izumiya et al., 2007). These data and the results presented here suggest that regulation of repression activity of K-bZIP is controlled by multiple elements and mechanisms. It is worth noting that another splice variant of K-bZIP exists in cells harboring viral DNA. This splice variant, K8β, contains the upstream basic region but not the C-terminal leucine zipper domain (Yamanegi et al., 2005). The K8β variant apparently serves to antagonize the activity of K-bZIP. The studies presented here involving the leucine zipper domain of K-bZIP would not affect the antagonistic function of K8β.
One BR mutant, BR-171/177/181, surprisingly increased the activity of K-Rta with respect to transcriptional activation of both the K-bZIP and PAN promoters. This result may be due to a change in the mode in which K-bZIP interacts with K-Rta, which may subsequently alter the binding of K-Rta, directly or indirectly, to K-Rta-binding sites within these promoters, leading to increased activity. This effect was also confirmed in the cotransfection replication assay where the addition of this BR mutant in the cotransfection mixture led to an increased intensity of amplified oriLyt. HHV8 oriLyt contains an ORF50 Response Element (RE) that is an almost exact copy of the PAN promoter ORF50RE. The apparent reason for an increased amplification signal for oriLyt is probably due to an up-regulation of transcriptional activation, from K-Rta, within oriLyt similar to what was observed with the K-bZIP promoter. Recently, it was postulated that the ORF50REs in K-Rta-responsive promoters fall into two groups (Chang et al., 2005). The interaction of K-Rta with the K-bZIP and ORF57 promoter may be indirect, in that cellular transcription factors may be involved and may indicate an increased affinity for RBP-Jκ or increase the binding of the K-Rta complex for DNA.
A recent study suggested that K-bZIP may serve to recruit the core DNA replication enzymes and K-Rta to oriLyt (Wang et al., 2006). This recruitment could be in conjunction with transcription factors such as C/EBPα and the viral transactivator K-Rta. It is clear from the data presented here that the leucine zipper and basic regions contribute to the activity of K-bZIP and K-bZIP may aid in the function of K-Rta within oriLyt. Another scenario is that, in the context of the viral genome, K-bZIP may serve a dual role: i) as a negative regulator of DNA replication by controlling the effects of K-Rta, and ii) supplying an as yet undefined enzymatic function to lytic DNA replication, independent of its observed suppression activity.
Materials and Methods
Plasmids
The set of plasmids expressing the HHV8 core replication genes, K-Rta and K-bZIP was previously described (AuCoin et al., 2004a).
K-bZIP site-directed mutagenesis
A parent K-bZIP expression plasmid was generated by subcloning the K-bZIP ORF into pCMV Xi (Genlantis) such that either an in frame HA or FLAG tag was added. Site-directed point mutations were introduced into the K-bZIP ORF using QuickChange mutagenesis kit (Stratagene) according to manufacture’s instructions. For the introduction of several different base pair changes we subjected plasmids to multiple rounds of mutagenesis. Primers were used such that amino acids were changed to alanine residues in the basic regions and to valine residues in the leucine zipper region. The individual plasmid names within the figures indicate which amino acid changes were made; all plasmids were sequenced and the entire ORF was examined to ensure that the proper mutation was made. For leucine zipper mutants: LZ-1, primer 5′-GATGCACAAGTATGTTTCGTAGCGGCGAGATTGGAGGCACATAAG-3, was used and the resulting mutant was used with primer, 5′-AGGAACAGATTATTTTCCGTCGCGACATGCTGATGCGAATGTGCCAGC-3′; LZ-2, primer 5′-GAAAAGGATGCACAAGTATGTTTCCTAGCGGCGAGAGTGGAGGCACATAAGGAACA G-3′ was used; for LZ-3, primer 5′-AAGGATGCACAAGTATGTTTCGTAGCGGCGAGATTGGAGGCACATAAG-3′ was used. Primers used to generate BR mutants and other mutants are listed in Table 1.
Table 1.
Primers used to generate site-directed K-bZ IP mutants.
| Mutant | Oligonucleotide Sequence | |
|---|---|---|
| BR-A171/174/185 | F | BR-A171/174 Template + Primer for BR-A185 |
| RC | BR-A171/174 Template + Primer for BR-A185 | |
| BR-A170/177/181 | F | BR-A177/181 Template + Primer for BR-A170 |
| RC | BR-A177/181 Template + Primer for BR-A170 | |
| BR-A171/177/181 | F | BR-A177/181 Template + Primer for BR-A171 |
| RC | BR-A177/181 Template + Primer for BR-A171 | |
| BR-A170/171/177/181 | F | BR-A177/181 Template + Primer for BR-A170/171 |
| RC | BR-A177/181 Template + Primer for BR-A170/171 | |
| BR-A171/174/185 | F | BR-A181/185 Template + Primer for BR-A170 |
| RC | BR-A181/185 Template + Primer for BR-A170 | |
| BR-A171/181/185 | F | BR-A181/185 Template + Primer for BR-A171 |
| RC | BR-A181/185 Template + Primer for BR-A171 | |
| BR-A174/181/185 | F | BR-A181/185 Template + Primer for BR-A174 |
| RC | BR-A181/185 Template + Primer for BR-A174 | |
| BR-A170/171/181/185 | F | BR-A181/185 Template + Primer for BR-A170/171 |
| RC | BR-A181/185 Template + Primer for BR-A170/171 | |
| BR-A171/174/181/185 | F | BR-A181/185 Template + Primer for BR-A171/174 |
| RC | BR-A181/185 Template + Primer for BR-A171/174 | |
| BR-A174/177/181/185 (BR-A174/177 Template) | F | 5′-GGCGCCGTGTCATCGGCAGCATACACAGCACAGCTGCAGCAG-3′ |
| RC | 5′-GCCTGCTGCAGCTGTGCTGTGTATGCTGCCGATGACACGGCGC-3′ | |
| Deletion 1 | F | 5′ – AGCTCAAGCTTCGAATTCATGTTTTTTACGGACAATACTG – 3′ |
| RC | 5′ – CAGTATTGTCCGTAAAAAACATGAATTCGAAGCTTGAGCT – 3′ | |
| Deletion 2 | F | 5′ – AGCCTCAACGGGCAACCAGAAACGGTCATTGACCTTACAG – 3′ |
| RC | 5′ – CTGTAAGGTCAATGACCGTTTCTGGTTGCCCGTTGAGGCT – 3′ | |
| Deletion 3 | F | 5′ – CCCGGCCATACCGGTCTGTCCTTCCTGGACGCTCTCTC – 3′ |
| RC | 5′ – GAGAGAGCGTCCAGGAAGGACAGACCGGTATGGCCGGG – 3′ | |
| Deletion 4 | F | 5′ – TAAATTCCACATCCCCGATGAACGCTTATGCACTAAGG – 3′ |
| RC | 5′ – CCTTAGTGCATAAGCGTTCATCGGGGATGTGGAATTTA – 3′ | |
| Deletion 5 | F | 5′ – ACATAGAAAGTTTGAAGAGCAAGGCAGATACGGCCGCG – 3′ |
| RC | 5′ – CGCGGCCGTATCTGCCTTGCTCTTCAAACTTTCTATGT – 3′ | |
| Deletion 6 | F | 5′ – GTCACATTCTCCCACGCGAAAGGAGGCACATAAGGAACAGA – 3′ |
| RC | 5′ – TCTGTTCCTTATGTGCCTCCTTTCGCGTGGGAGAATGTGAC – 3′ | |
| Deletion 7 | F | 5′ – CCTAGCGGCGAGATTGGATTACAAGGATGACGACGATAAGTG – 3′ |
| RC | 5′ – CACTTATCGTCGTCATCCTTGTAATCCAATCTCGCCGCTAGGA – 3′ | |
| NO BR | F | 5′ – GTATGTGATCAGTCACATTCTCCCCAGCTGCAGCAGGCATTA – 3′ |
| RC | 5′ – TAATGCCTGCTGCAGCTGGGGAGAATGTGACTGATCACATAC - 3′ | |
| NO LZ | F | 5′ – AAGACAGCTGCAGCAGGCAATGCGAATGTGCCAGCAGCCAG – 3′ |
| RC | 5′ – CTGGCTGCTGGCACATTCGCATTGCCTGCTGCAGCTGTCTTGT – 3′ | |
| LZ-1 (LZ-3 Template) | F | 5′ – CATAAGGAACAGATTATTTTCGTTCGCGACATGCTGATGCGA – 3′ |
| RC | 5′ – TCGCATCAGCATGTCGCGAACGAAAATAATCTGTTCCTTATG – 3′ | |
| LZ-2 | F | 5′ – GATGCACAAGTATGTTTCCTAGCGGCGAGAGTGGAGGCACAT – 3′ |
| RC | 5′ – ATGTGCCTCCACTCTCGCCGCTAGGAAACATACTTGTGCATC – 3′ | |
| LZ-3 | F | 5′ – GAAAAGGATGCACAAGTATGTTTCGTAGCGGCGAGATTGGAG – 3′ |
| RC | 5′ – CTCCAATCTCGCCGCTACGAAACATACTTGTGCATCCTTTTC – 3′ | |
| NO BR –V198/205 | F | LZ-2 Template + Primer for NO BR |
| RC | LZ-2 Template + Primer for NO BR | |
| NO LZ – A170/171/181/185 | F | BR-A170/171/181/185 Template + Primer for NO LZ |
| RC | BR-A170/171/181/185 Template + Primer for NO LZ | |
| NO LZ – A171/174/181/185 | F | BR-A171/174/181/185 Template + Primer for NO LZ |
| RC | BR-A171/174/181/185 Template + Primer for NO LZ | |
| NO LZ – A174/177/181/185 | F | BR-A174/177/181/185 Template + Primer for NO LZ |
| RC | BR-A174/177/181/185 Template + Primer for NO LZ |
F= Forward Primer RC=Reverse Complement Primer
Cotransfection replication assay
Vero cells were transfected with the complete set of core replication proteins and wild type or mutated K-bZIP expression plasmids. A K-Rta expression plasmid was included in the transfection mixture when using wild type oriLyt but was omitted when using oriLyt plasmids containing the HCMV MIEP. Assays were performed as previously described (AuCoin et al., 2004a). To test K-bZIP BR or LZ mutants, the compete set of replication plasmids, along with a K-Rta expression plasmid and pDA15 were included in the transfection mixture. Mutant K-bZIP-encoding plasmids were substituted for a wt K-bZIP expression plasmid.
Antibodies
Mouse monoclonal anti-FLAG antibody (Sigma, St. Louis, MO) and mouse monoclonal anti-HA antibody (Sigma) were used as primary antibodies for immunoprecipitation and immunoblotting analysis; horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA) were used as secondary antibodies. M2 monoclonal mouse anti-FLAG-conjugated agarose beads (Sigma, St. Louis, MO) were used for the immunoprecipitation of FLAG-tagged K-bZIP proteins and complexes. A K-Rta antibody (gift from D. Ganem, UC San Francisco) was also used in co-immunoprecipitations. The anti-K-bZIP antibody was generated at the University of Nevada Monoclonal Antibody Facility using antigen purified from mammalian (Vero) cells.
Co-immunoprecipitation and Western analysis
Ten-centimeter dishes of 293FT cells were transfected with 5 μg of wild-type HA-tagged K-bZIP expression plasmid and 5 μg of FLAG-tagged K-bZIP mutant expression plasmid using the TransIt LT1 Transfection Reagent (Mirus, Madison, WI). Protein was harvested from the transfected 293FT cells 24–48 hours after transfection. The transfected 293FT cells were rinsed twice with ice-cold PBS (phosphate-buffered saline solution, 10 mM NaPO4, 137 mM NaCl, 2.5 mM KCl, pH 7.5) and lysed in lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Triton X, 0.1% Tween 20, pH 7.5) with 10μl/mL protease inhibitor cocktail for mammalian tissues (Sigma, St. Louis, MO). DNA was sheared by passing the lysate three times through a 22-gauge needle, and cell debris was cleared by centrifugation at 12 000xg for 10 min at 4°C. The K-bZIP-K-bZIP immunocomplex was captured by addition of 40 μl of anti-FLAG M2 mouse monoclonal antibody-conjugated agarose beads (Sigma, St. Louis, MO) and incubation overnight at 4°C with gentle rotation. The beads were washed three times for 5 min each in TBS (50 mM Tris-HCl, 150 mM NaCl, pH 7.4) and then boiled for 5 min in 70 μl of Laemli sample buffer-5% β-mercaptoethanol (Bio-Rad, Hercules, CA) to denature the anti-FLAG antibodies and elute the K-bZIP-K-bZIP immunocomplex. Protein samples from the cell lysate and the immunoprecipitated protein were separated by 10%-SDS polyacrylamide gel electrophoresis and then transferred onto 22 μm polyvinyldene difluoride membrane (Bio-Rad, Hercules, CA) using a semidry transfer apparatus (Bio-Rad, Hercules, CA). After blocking non-specific antigen binding for 15 min at room temperature in TBST (20 mM Tris-HCl, 137 mM NaCl, 0.05% Tween 20, pH 7.6)-5% skim milk, the membranes were incubated with primary antibodies for 1hr at room temperature or overnight at 4°C with gentle rotation. Protein species within each immunoprecipitate were detected using anti-HA antibody and separately using anti-FLAG antibody, to detect the presence of both wild-type (HA-tagged) and mutant (FLAG-tagged) K-bZIP. The final dilution of the M2 mouse monoclonal anti-FLAG primary antibody used was 1:5000. After incubation with primary antibody the membranes were washed with TBST for 15 min at room temperature, with rotation. The membranes were then incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG secondary antibody (1:5000 dilution) for 30 min at room temperature, then washed with TBST for 15 min at room temperature. The membranes were exposed for 5 min using a chemiluminescent HRP substrate (SuperSignal West Femto Maximum Sensitivity Substrate, Pierce, Madison, WI), and visualized using a Bio-Rad Chemi Doc.
Luciferase Reporter Assay
Vero cells were cotransfected with pGL3-K-bZIP promoter (provided by H-S Kung, UC Davis), a K-Rta expression plasmid (AuCoin et al., 2004a) and plasmids expressing K-bZIP BR or LZ mutants. Cells were lysed and standard luciferase detection was performed (Promega). Each assay was measured in triplicate and each experiment was repeated three times. A representative experiment is shown.
Acknowledgments
This work was supported by PHS grant CA115279. We thank H-S. Kung for generously providing luciferase reporter plasmids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Mehairi S, Cerasoli E, Sinclair AJ. Investigation of the multimerization region of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) protein K-bZIP: the proposed leucine zipper region encodes a multimerization domain with an unusual structure. J Virol. 2005;79(12):7905–10. doi: 10.1128/JVI.79.12.7905-7910.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AuCoin DP, Colletti KS, Cei SA, Papouskova I, Tarrant M, Pari GS. Amplification of the Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 lytic origin of DNA replication is dependent upon a cis-acting AT-rich region and an ORF50 response element and the trans-acting factors ORF50 (K-Rta) and K8 (K-bZIP) Virology. 2004a;318(2):542–55. doi: 10.1016/j.virol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- AuCoin DP, Colletti KS, Cei SA, Papouskova I, Tarrant M, Pari GS. Amplification of the Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 lytic origin of DNA replication is dependent upon a cis-acting AT-rich region and an ORF50 response element and the trans-acting factors ORF50 (K-Rta) and K8 (K-bZIP) Virology. 2004b;318(2):542–555. doi: 10.1016/j.virol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Byun H, Gwack Y, Hwang S, Choe J. Kaposi’s sarcoma-associated herpesvirus open reading frame (ORF) 50 transactivates K8 and ORF57 promoters via heterogeneous response elements. Mol Cells. 2002;14(2):185–91. [PubMed] [Google Scholar]
- Chang PJ, Miller G. Autoregulation of DNA binding and protein stability of Kaposi’s sarcoma-associated herpesvirus ORF50 protein. J Virol. 2004;78(19):10657–73. doi: 10.1128/JVI.78.19.10657-10673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PJ, Shedd D, Miller G. Two subclasses of Kaposi’s sarcoma-associated herpesvirus lytic cycle promoters distinguished by open reading frame 50 mutant proteins that are deficient in binding to DNA. J Virol. 2005;79(14):8750–63. doi: 10.1128/JVI.79.14.8750-8763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Young A, Sun R. Auto-activation of the rta gene of human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus. J Gen Virol. 2000;81(Pt 12):3043–8. doi: 10.1099/0022-1317-81-12-3043. [DOI] [PubMed] [Google Scholar]
- Fixman ED, Hayward GS, Hayward SD. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J Virol. 1992;66(8):5030–9. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradoville L, Gerlach J, Grogan E, Shedd D, Nikiforow S, Metroka C, Miller G. Kaposi’s sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J Virol. 2000;74(13):6207–12. doi: 10.1128/jvi.74.13.6207-6212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W, Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988;55:427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Izumiya Y, Ellison TJ, Yeh ET, Jung JU, Luciw PA, Kung HJ. Kaposi’s sarcoma-associated herpesvirus K-bZIP represses gene transcription via SUMO modification. J Virol. 2005;79(15):9912–25. doi: 10.1128/JVI.79.15.9912-9925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Izumiya C, Van Geelen A, Wang DH, Lam KS, Luciw PA, Kung HJ. Kaposi’s sarcoma-associated herpesvirus-encoded protein kinase and its interaction with K-bZIP. J Virol. 2007;81(3):1072–82. doi: 10.1128/JVI.01473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Lin SF, Ellison T, Chen LY, Izumiya C, Luciw P, Kung HJ. Kaposi’s Sarcoma-Associated Herpesvirus K-bZIP Is a Coregulator of K-Rta: Physical Association and Promoter-Dependent Transcriptional Repression. J Virol. 2003a;77(2):1441–51. doi: 10.1128/JVI.77.2.1441-1451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Lin SF, Ellison TJ, Levy AM, Mayeur GL, Izumiya C, Kung HJ. Cell Cycle Regulation by Kaposi’s Sarcoma-Associated Herpesvirus K-bZIP: Direct Interaction with Cyclin-CDK2 and Induction of G(1) Growth Arrest. J Virol. 2003b;77(17):9652–61. doi: 10.1128/JVI.77.17.9652-9661.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Tang Y, Lin SF, Kung HJ, Giam CZ. K-bZIP of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) binds KSHV/HHV-8 Rta and represses Rta-mediated transactivation. J Virol. 2003;77(6):3809–15. doi: 10.1128/JVI.77.6.3809-3815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Garibyan L, Kirshner JR, Palmeri D, Ganem D. DNA binding by Kaposi’s sarcoma-associated herpesvirus lytic switch protein is necessary for transcriptional activation of two viral delayed early promoters. J Virol. 2001;75(15):6786–99. doi: 10.1128/JVI.75.15.6786-6799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Kirshner JR, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi’s sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol. 1999;73(11):9348–61. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarisky RT, Gao Z, Lieberman PM, Fixman ED, Hayward GS, Hayward SD. A replication function associated with the activation domain of the Epstein-Barr virus Zta transactivator. J Virol. 1996;70(12):8340–7. doi: 10.1128/jvi.70.12.8340-8347.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers A, Pich D, Hammerschmidt W. Activation of oriLyt, the lytic origin of DNA replication of Epstein- Barr virus, by BZLF1. Virology. 1996;220(2):367–76. doi: 10.1006/viro.1996.0325. [DOI] [PubMed] [Google Scholar]
- Schepers AD, Pich D, Mankertz J, Hammerschmidt W. Cis-acting elements in the lytic origin of DNA replication of Epstein-Barr virus. J Virol. 1993;67:4237–4245. doi: 10.1128/jvi.67.7.4237-4245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Liu S, Wu M, Geng Y, Wood C. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8 ORF50 gene product contains a potent C-terminal activation domain which activates gene expression via a specific target sequence. Arch Virol. 2001;146(7):1415–26. doi: 10.1007/s007050170102. [DOI] [PubMed] [Google Scholar]
- Wang SE, Wu FY, Fujimuro M, Zong J, Hayward SD, Hayward GS. Role of CCAAT/Enhancer-Binding Protein Alpha (C/EBPalpha) in Activation of the Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Lytic-Cycle Replication-Associated Protein (RAP) Promoter in Cooperation with the KSHV Replication and Transcription Activator (RTA) and RAP. J Virol. 2003a;77(1):600–23. doi: 10.1128/JVI.77.1.600-623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SE, Wu FY, Yu Y, Hayward GS. CCAAT/Enhancer-Binding Protein-alpha Is Induced during the Early Stages of Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Lytic Cycle Reactivation and Together with the KSHV Replication and Transcription Activator (RTA) Cooperatively Stimulates the Viral RTA, MTA, and PAN Promoters. J Virol. 2003b;77(17):9590–612. doi: 10.1128/JVI.77.17.9590-9612.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tang Q, Maul GG, Yuan Y. Kaposi’s sarcoma-associated herpesvirus ori-Lyt-dependent DNA replication: dual role of replication and transcription activator. J Virol. 2006;80(24):12171–86. doi: 10.1128/JVI.00990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, AuCoin DP, Huete AR, Cei SA, Hanson LJ, Pari GS. A Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 ORF50 deletion mutant is defective for reactivation of latent virus and DNA replication. J Virol. 2005;79(6):3479–87. doi: 10.1128/JVI.79.6.3479-3487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanegi K, Tang S, Zheng ZM. Kaposi’s sarcoma-associated herpesvirus K8beta is derived from a spliced intermediate of K8 pre-mRNA and antagonizes K8alpha (K-bZIP) to induce p21 and p53 and blocks K8alpha-CDK2 interaction. J Virol. 2005;79(22):14207–21. doi: 10.1128/JVI.79.22.14207-14221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]






