Abstract
The protein kinase C (PKC) family is a major transducer of several intracellular pathways. In confirmation of this important role, PKCs exhibit high molecular heterogeneity, because they occur in at least 10 different isoforms differing in biochemical properties and sensitivity to activators. In this report we focused on the ability of different redox agents to induce modification of intracellular distribution of specific PKC isoforms in HeLa cells. To this end we utilized a panel of green fluorescent protein (GFP) chimeras and a high-speed digital imaging system. We observed a remarkable complexity of PKC signalling patterns occurring during redox stress with marked differences among PKC isoforms also belonging to the same subgroup. Moreover our results suggest that modifications of the intracellular redox state can modulate the responsiveness of specific PKC isoforms and, in turn, change the sensitivity of the different isoforms to cell stimulation.
INTRODUCTION
Protein kinase C (PKC) is a ubiquitous family of enzymes that consists of at least 10 structurally related serine/threonine kinases. The various PKC isoforms have been subdivided into 3 classes: classical isoforms (cPKC: α, βI, βII, and γ), which are activated by Ca2+ and diacylglycerol (DAG); novel isoforms (nPKC: δ, ɛ, η and θ), which are activated by DAG, but are Ca2+ independent; and atypical isoforms (aPKC: λ and ζ), which are insensitive to both Ca2+ and DAG. These PKC isoforms are unique, not only with respect to primary structure, but also on the basis of expression patterns, subcellular localization, activation in vitro, and responsiveness to extracellular signals. Inactive PKCs are present mainly in the cytosol, although activated PKCs are found associated with the plasma membrane, the nucleus, and other subcellular compartments (Mellor and Parker 1998; Toker 1998).
This communication focuses on the effects of redox stresses on the activation of classical and novel PKCs. Classical PKC isoforms are activated sequentially by Ca2+ and DAG signals. Although Ca2+ acts rapidly, DAG binding to PKC initially is prevented by a pseudosubstrate clamp, which keeps the DAG-binding site inaccessible and delays Ca2+ and DAG-mediated kinase activation. After termination of Ca2+ signals, bound DAG prolongs kinase activity (Oancea and Meyer 1998).
In contrast, novel PKC isoforms are activated directly by DAG without the Ca2+-priming because, in nPKCs, the DAG-dependent domain binds membranes with sufficiently high affinity to recruit nPKCs to membranes in the absence of any other targeting mechanism (Dries et al 2007). Many other intracellular mediators have been proposed to play a key role for PKC intracellular distribution, such as lipids (Nishizuka 1995), glucose (Gallo et al 2005), apoptotic mediators (Pinton et al 2001), and oxidative stress (Gopalakrishna and Jaken 2000).
PKCs are regulated by 2 distinct mechanisms: phosphorylation, which regulates the active site and subcellular localization of the enzyme, and second messengers, which promote PKC′s membrane association and resulting pseudosubstrate exposure. Regulation by 2 independent mechanisms provides exquisite fine-tuning for PKC (Dries et al 2007). Redox stresses change the phosphorylation state of PKC (Gopalakrishna and Jaken 2000) and in turn may regulate the activity of PKCs.
A large number of proteins are known that are phosphorylated by PKCs. However, the knowledge of proteins that are selectively phosphorylated by a distinct PKC isoform is extremely scarce. Once activated, PKCs regulate multiple physiological processes. In the cytosol, PKCs interact with several different proteins, including receptors for activated C kinase (RACKS), the product of the par-4 gene, zeta-interacting protein, lambda-interacting protein, and A kinase–anchoring protein. PKCs also are involved in remodeling the actin cytoskeleton, partly by phosphorylating specific PKC substrates such as the myristoylated alanine-rich PKC substrate (MARCKS) protein and pleckstrin. Thus, PKCs influence many distinct aspects of cell function (Toker 1998).
The PKC family has been implicated in a wide range of pathological processes ranging from cancer to degenerative disorders (Nishino et al 1989; O'Brian and Ward 1989; Pascale et al 1998). PKCs contain, both in the N-terminal regulatory domain and in the C-terminal catalytic domain, regions that are susceptible to redox modifications. Intracellular redox state is routinely subjected to modifications during cell life. The changes on redox state are due to the actions of reactive oxygen species and alkylating agents that are produced during cell life (Finkel 2003; Lindsay and Astley 2002). In several physio-pathological conditions, the cells undergo redox stress, ie, when the balance between oxidants, antioxidants, and alkylants is compromised. PKCs are part of cell-signaling proteins that are particularly sensitive to redox stress because modification of their redox-sensitive regions interferes with the activities of cellular PKCs (Gopalakrishna and Jaken 2000).
Given that redox stress may be involved in the pathogenesis of several diseases (Cross et al 1987; Li 2001; Emerit et al 2004), the PKC family is a logical candidate to mediate the pathological transduction of redox stress in diseases. Recently, several lines of evidence demonstrated a crucial role of PKC activity in several pathologies (Nagpala et al 1996; Koya and King 1998; Nishikawa et al 2000; Ways and Sheetz 2000). Interestingly, in relation to redox stress, recent evidence suggests that PKC activation also plays a key role in glucose-induced oxidative stress inside the endothelial cell (Gallo et al 2005) and in oxidative-dependent mitochondrial dysfunction (Pinton et al 2007). In this scenario, dissecting the signalling pathway in which PKCs are involved is a difficult task, given the overlapping substrate specificity and the complexity of the molecular machinery that regulates the specific sorting of the various isoforms (Dekker and Parker 1994; Parekh et al 2000). Partial and sometimes contrasting information is available on differential recruitment and/or function of the various isoforms of PKC in the transduction of redox signals.
For all PKCs, translocation to the cell membrane is a key step in the activation process. Therefore, an efficient way to monitor the activation of PKC isoforms is to follow their intracellular distribution. Traditionally, this was achieved by subcellular fractionation and measurement of protein sorting to the membrane fraction or in fixed cells by immunofluorence. With the advent of GFP and the development of imaging systems with high spatial and temporal resolution, it is now possible to visualize the translocation process in living cells. In order to obtain a deeper insight into the involvement of the PKCs during redox stress, we utilized a panel of GFP chimeras, allowing us to dynamically monitor the recruitment of the various PKCs. In particular, the present study investigated the redox-induced translocation of two classes of PKC, cPKC (PKCα and PKCβ), and nPKC (PKCδ and PKCɛ) in HeLa cells.
RESULTS AND DISCUSSION
Oxidative and reducing stress have different effects on cPKCs in HeLa cells
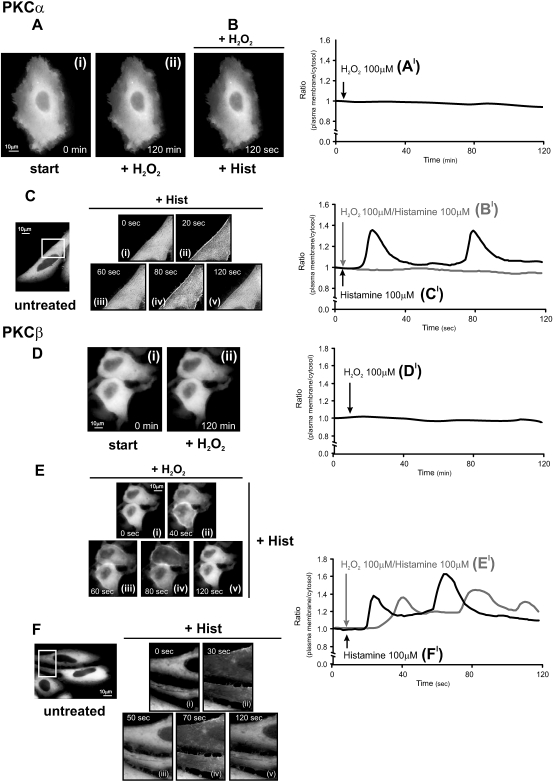
In order to analyze the effect of redox stresses on PKCs, a panel of PKC-GFP chimeras was transfected in HeLa cells and analyzed by imaging experiments (Rizzuto et al 1998). When expressed, each PKC-GFP chimera was brightly fluorescent throughout the cytoplasm, including the nucleoplasm in the transfected PKCδ-GFP cells (see Figs 1–3), as previously reported by us and others (Sakai et al 1997; Oancea and Meyer 1998; Ohmori et al 1998; Chiesa et al 2001; Pinton et al 2002, 2007; Takahashi and Namiki 2007). To induce oxidative stress we utilized 100 μM H2O2, a concentration that induced in HeLa cells only 5 ± 2% of cell death (after 30 minutes of treatment). The reducing stress was obtained using 2 mM dithiothreitol ([DTT], less than 3% cell death after a treatment of 30 minutes). Under those redox stress conditions, the GFP fluorescence was practically unmodified (not shown). We first examined the distribution of cPKC during H2O2 treatment. In Figure 1 the cellular distribution of PKCα and β isoform is shown. In resting conditions both kinases were localized uniformly in the cytosolic compartment, as expected for inactive PKCs. Several images were taken at different times after the addition of H2O2. It is apparent that no change in the distribution of kinases is induced by the oxidative agent (compare Fig 1Ai with 1Aii and Fig 1Di with 1Dii). Interestingly, the treatment with H2O2 rendered PKCα insensitive to histamine stimulation, an agonist coupled to the generation of DAG and inositol 1,4,5-trisphosphate (and thus the release of Ca2+ from intracellular stores with consequent increase of cytosolic Ca2+ concentration, [Ca2+]c; Fig 1B).
Fig 1.
Effects of oxidative stress on protein kinase C (PKC)α-GFP and PKCβ-GFP in HeLa cells. (A, D) Effects of 100 μM H2O2; (B, E) histamine 100 μM in H2O2-pretreated cells; (C, F) effects of histamine 100 μM in untreated cells. HeLa cells were grown in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal calf serum (FCS), in 75-cm2 flasks. The cells were seeded before transfection onto 24-mm glass coverslips and allowed to grow to 50% confluence. At this stage, transfection with 8 μg of the appropriate PKC plasmid DNA was carried out by Calcium phosphate technique as described previously (Chiesa et al 2001). Microscope analysis (and cell stimulation) were performed 36 h after transfection. To this end the medium was changed from DMEM + 10% FCS to KRB (125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM Na2HPO4, 5.5 mM glucose, 20 mM NaHCO3, 2 mM L-glutamine, 1 mM CaCl2, and 20 mM HEPES; pH 7.4). All the stimuli (H2O2, dithiothreitol [DTT], histamine, PMA, ascorbic acid) were added to the KRB at the time indicated in the figures. Images were recorded using a digital imaging system based on a Zeiss Axiovert 200 fluorescence microscope equipped with a back-illuminated charge-coupled device (CCD) camera (Roper Scientific, Trenton, NJ, USA). Rapid focussing in the z plane was guaranteed by excitation and emission filterwheels (Sutter Instrument Company, Novato, CA, USA) and piezoelectric motoring of the z stage (Physik Instrumente, GmbH & Co., Karlsruhe, Germany). The data were acquired and processed using the MetaMorph analyzing program (Universal Imaging Corporation, Downington, PA, USA). This allows the direct monitoring of fluorescence intensity. A high-resolution, 3D reconstruction of the distribution of a GFP chimera can be obtained with the technique of digital image restoration, also called deconvolution or deblurring. In brief, a series of 20 optical section images, spaced at 0.5-μm intervals through the depth of the cell, was acquired. Each optical section image was acquired in less than 1 second, and the entire through-focus series in less than 20 seconds. The image series then was deblurred using a constrained, iterative restoration algorithm that incorporates an empirically determined optical point spread function. The deblurred images then were used to visualize the 3D intracellular patterns. A more exhaustive description of this approach is presented in (Carrington et al 1995). In Figure 1Ci–v and Fi–ii, a larger magnification of the images is presented in the insets to allow a better appreciation of PKC translocation. The graphs (A′–F′) indicate the time course of plasma membrane translocation of PKC-GFP expressed as the increase in fluorescence ratio with respect to time zero (calculated as ratio of plasma membrane: average intracellular fluorescence). In all experiments, the images and traces are representative of at least 10 from 3 independent experiments, which gave similar results
On the contrary, in untreated cells, as expected for a classical PKC, histamine-induced PKCα oscillations (ie, different translocation to the plasma membrane and retranslocation to the cytosol; Fig 1Ci–v). This inhibition was specific for PKCα and not for all classical PKCs. In fact, H2O2 pretreatment did not modify the sensitivity to histamine of PKCβ. In this case, histamine induced the typical translocation of part of the PKCβ pool to the plasma membrane (Fig 1Ei–v), as observed without H2O2 pretreatment (Fig 1Fi–v; Chiesa et al 2001).
These observations provide evidence that oxidation of the regulatory sites in PKCα causes a preconditioning with a consequent lack of activation. Moreover, they highlight a different behavior to oxidative stress between PKCα and PKCβ suggesting that each PKC isoform has a spatially and temporally different targeting mechanism that depends on the extracellular and intracellular signals, contributing to the isoforms-specific functions of PKC.
In order to quantify the occurrence of PKC translocation, we calculated the increase in fluorescence ratio with respect to time zero calculated as ratio of plasma membrane: average intracellular fluorescence (Figs 1A′–F′, 2A′–C′ and 3A′–3C′). Similar results were obtained using higher H2O2 concentration (1 mM).
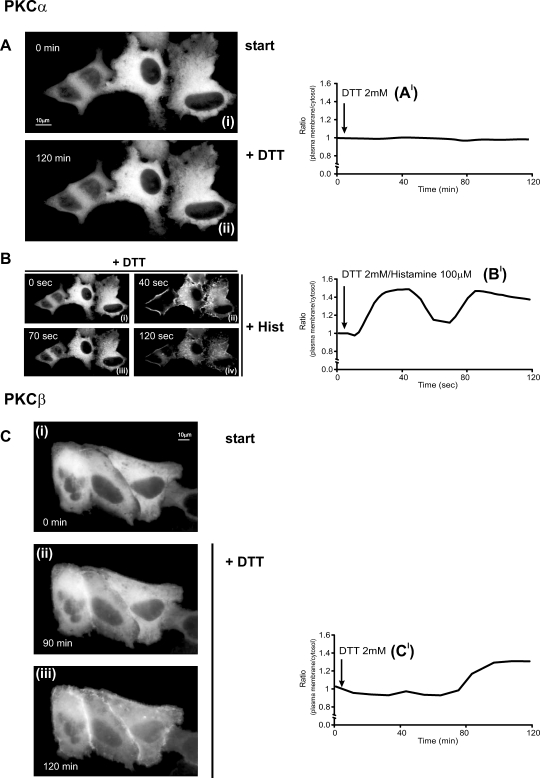
Fig 2.
Effects of reducing stress on classical protein kinase C (cPKC)–GFP (α, β) in HeLa cells. (A, C) Effects of 2 mM dithiothreitol (DTT). (B) Histamine 100 μM stimulation in PKCα-GFP DTT-pretreated cells. All conditions as in Figure 1
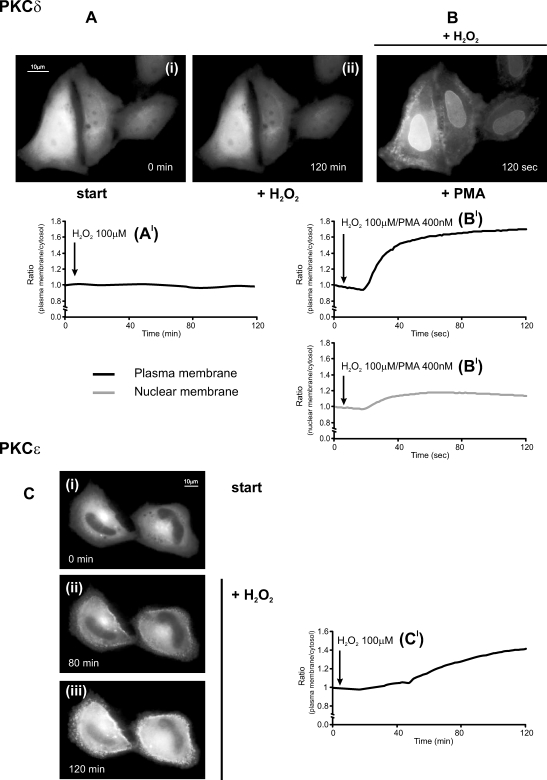
Fig 3.
Effects of oxidative stress on novel protein kinase C (nPKC)–GFP (δ, ɛ) in HeLa cells. (A, C) Effects of 100 μM H2O2. (B) PMA 400 nM in PKCδ-GFP H2O2-pretreated cells. All conditions as in Figure 1. The graph presented in Figure 3B′ reports both the time course of plasma (upper panel) and nuclear membrane (lower panel) translocation
Afterwards, we analyzed the behavior of cPKC in HeLa cells upon reducing stress induced by DTT (Fig 2). As described above, HeLa cells were transfected with the appropriate cPKC-GFP chimera and analyzed by digital imaging microscopy. As for the H2O2-dependent oxidative stress, the distribution of PKCα was unaffected by incubation with DTT (Figs 2A,A′). However, in this case, the PKCα maintained the ability to pulse between cytosol and plasma membrane, after application of histamine (Fig 2Bi–iv,B′).
PKCβ showed a different response to DTT. As shown in Figure 2C, a clear translocation of the kinase to the plasma membrane was observed after the application of the reducing agent (Fig 2Ci–iii,C′), emphasizing an unexpected role of PKCβ during reducing stress and suggesting its involvement in signal transduction associated to DTT treatment.
In summary, it is apparent that 2 isozymes belonging to the same class and exhibiting comparable molecular structure (ie, with the same regulatory C1 and C2 domains [Mellor and Parker 1998]) and biochemical properties (ie, with the same activators such as phosphatidylserine, Ca2+, and DAG) show a completely different pattern of responsiveness to changes in intracellular redox state.
Effects of oxidative and reducing stresses on nPKCs
The distribution of PKC chimeras of the novel class was investigated (Fig 3); 36 hours after transfection with the appropriate chimera, the cells were analyzed on the microscope and the effects of H2O2 and DTT were assessed. At rest, the nPKC analyzed are localized in the cytosol, as for the cPKCs, but in this case also in the perinuclear region corresponding to the Golgi apparatus (Fig 3A, C) as previously reported (Lehel et al 1995; Chiesa et al 2001; Pinton et al 2002; Dries et al 2007). Although histamine stimulation induces in HeLa cells DAG generation, a classical activator of nPKC, under these conditions there was no translocation of PKCδ in control cells. Conversely, PKCɛ rapidly pulsed from the cytosol to the plasma membrane after histamine stimulation (data not shown). The different behavior during histamine stimulation between nPKCs suggests a greater sensitivity of PKCɛ compared to that of PKCδ. On the contrary, the stimulation with phorbol ester phorbol 12-myristate 13-acetate (PMA) (a Ca2+-independent DAG-like agonist) induces a drastic translocation both of PKCδ and PKCɛ (Chiesa et al 2001).
As observed above for the cPKCs, it is apparent that, upon stimulation with H2O2, also for nPKCs, 2 isozymes belonging to the same class show a distinct pattern of subcellular distribution. The cells expressing PKCδ-GFP showed the same localization of the protein before and after H2O2 addition (Fig 3Ai–ii,A′). Conversely, part of the PKCɛ-GFP pool, after addition of H2O2, translocated and remained associated to the plasma membrane (Fig 3Ci– iii,C′). No retranslocation to the cytosol was observed even after 2 hours, suggesting an irreversible H2O2 effect. Thus, the different behavior during oxidative stress between nPKCs confirms a greater sensitivity of PKCɛ compared to that of PKCδ. However, oxidative stress did not block the typical PKCδ-translocation to the plasma and nuclear membrane triggered by PMA (Fig 3B,B′; Ohmori et al 1998; Chiesa et al 2001; Pinton et al 2002), suggesting insensitivity of this isoform to H2O2-challenge.
Finally, similar behavior has been observed between PKCδ and PKCɛ during DTT-reducing stress. Under those conditions, no modifications in the distribution of nPKC are present, and the sensitivity to PMA stimulation is the same as the control cells. Moreover, the analysis of PKC involvement during redox stress was carried out, verifying the effect of an antioxidant agent, such as ascorbic acid, on intracellular localization of the cPKCs and nPKC. In all cases, treatment for 2 hours with ascorbic acid had no effects on PKC distribution and on the activity of agents inducing PKC redistribution such as histamine and PMA (data not shown).
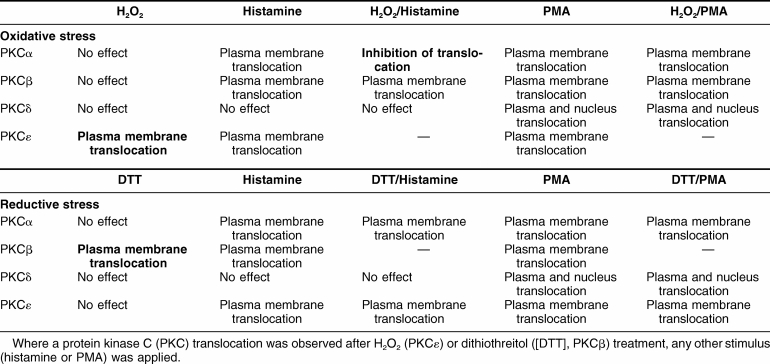
These results (summarized in Table 1) may be not applicable to all cell types. In endothelial cells (Gallo et al 2005) and in mouse embryonic fibroblasts (Pinton et al 2007) the PKCβ isoform translocates to the plasma membrane after H2O2 treatment. Herein they clearly indicate a remarkable complexity of PKCs signalling patterns occurring during redox stress. Moreover, they suggest that the intracellular redox state could control the selectivity of PKC isoform activation and thus change the sensitivity of the different isoforms to cell stimulation, as observed in the case of PKCα during agonist stimulation after H2O2 pretreatment. These data thus could explain why stimuli that in principle could activate a broad number of PKC isoforms can become selective only for a specific isoform by delivering either a reductive or an oxidative stress.
Table 1.
Schematic summary of the results presented
Acknowledgments
This work was supported by grants from the Italian University Ministry, Telethon—Italy (grant GGP05284), the Italian Association for Cancer Research, the Italian Space Agency, European Union (EU) (fondi strutturali Obiettivo 2), National Institutes of Health (NIH) (grant name, A mitochondrial longevity pathway: p66shc mechanisms), and the Programma Regionale per la Ricerca Industriale L'Innovazione ed il Trasferimento Tecnologico (PRRIITT) program of the Emilia Romagna Region.
REFERENCES
- Carrington WA, Lynch RM, Moore ED, Isenberg G, Fogarty KE, Fay FS. Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science. 1995;268:1483–1487. doi: 10.1126/science.7770772.0193-4511(1995)268[1483:STIOFI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chiesa A, Rapizzi E, Tosello V, Pinton P, de Virgilio M, Fogarty KE, Rizzuto R. Recombinant aequorin and green fluorescent protein as valuable tools in the study of cell signalling. Biochem J. 2001;355:1–12. doi: 10.1042/0264-6021:3550001.0264-6021(2001)355[0001:RAAGFP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987;107:526–545. doi: 10.7326/0003-4819-107-4-526.0003-4819(1987)107[0526:ORAHD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dekker LV, Parker PJ. Protein kinase C—a question of specificity. Trends Biochem Sci. 1994;19:73–77. doi: 10.1016/0968-0004(94)90038-8.0376-5067(1994)019[0073:PKCQOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dries DR, Gallegos LL, Newton AC. A single residue in the C1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. J Biol Chem. 2007;282:826–830. doi: 10.1074/jbc.C600268200.0021-9258(2007)282[0826:ASRITC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004.0753-3322(2004)058[0039:NDAOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4.0955-0674(2003)015[0247:OSAOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gallo A, Ceolotto G, and Pinton P. et al. 2005 Metformin prevents glucose-induced protein kinase C–beta2 activation in human umbilical vein endothelial cells through an antioxidant mechanism. Diabetes. 54:1123–1131. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5.0891-5849(2000)028[1349:PKCSAO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859.0012-1797(1998)047[0859:PKCAAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lehel C, Olah Z, Jakab G, Anderson WB. Protein kinase C epsilon is localized to the Golgi via its zinc-finger domain and modulates Golgi function. Proc Natl Acad Sci U S A. 1995;92:1406–1410. doi: 10.1073/pnas.92.5.1406.1091-6490(1995)092[1406:PKCEIL]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. Molecular epidemiology of pancreatic cancer. Cancer J. 2001;7:259–265.0765-7846(2001)007[0259:MEOPC]2.0.CO;2 [PubMed] [Google Scholar]
- Lindsay DG, Astley SB. European research on the functional effects of dietary antioxidants—EUROFEDA. Mol Aspects Med. 2002;23:1–38. doi: 10.1016/s0098-2997(02)00005-5.0098-2997(2002)023[0001:EROTFE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332(2):281–292. doi: 10.1042/bj3320281.0264-6021(1998)332[0281:TEPKCS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpala PG, Malik AB, Vuong PT, Lum H. Protein kinase C beta 1 overexpression augments phorbol ester–induced increase in endothelial permeability. J Cell Physiol. 1996;166:249–255. doi: 10.1002/(SICI)1097-4652(199602)166:2<249::AID-JCP2>3.0.CO;2-P.0021-9541(1996)166[0249:PKCBOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, and Du XL. et al. 2000 Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 404:787–790. [DOI] [PubMed] [Google Scholar]
- Nishino N, Kitamura N, Nakai T, Hashimoto T, Tanaka C. Phorbol ester binding sites in human brain: characterization, regional distribution, age-correlation, and alterations in Parkinson's disease. J Mol Neurosci. 1989;1:19–26. doi: 10.1007/BF02896852.0895-8696(1989)001[0019:PEBSIH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496.0892-6638(1995)009[0484:PKCALS]2.0.CO;2 [PubMed] [Google Scholar]
- O'Brian CA, Ward NE. Biology of the protein kinase C family. Cancer Metastasis Rev. 1989;8:199–214. doi: 10.1007/BF00047337.0167-7659(1989)008[0199:BOTPKC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95:307–318. doi: 10.1016/s0092-8674(00)81763-8.0092-8674(1998)095[0307:PKCAAM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ohmori S, Shirai Y, Sakai N, Fujii M, Konishi H, Kikkawa U, Saito N. Three distinct mechanisms for translocation and activation of the delta subspecies of protein kinase C. Mol Cell Biol. 1998;18:5263–5271. doi: 10.1128/mcb.18.9.5263.1098-5549(1998)018[5263:TDMFTA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496.1460-2075(2000)019[0496:MPCPKC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale A, Govoni S, Battaini F. Age-related alteration of PKC, a key enzyme in memory processes: physiological and pathological examples. Mol Neurobiol. 1998;16:49–62. doi: 10.1007/BF02740602.0893-7648(1998)016[0049:AAOPAK]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Di Virgilio F, Pozzan T, Rizzuto R. Molecular machinery and signalling events in apoptosis. Drug Development Research. 2001;52:558–570.0272-4391(2001)052[0558:MMASEI]2.0.CO;2 [Google Scholar]
- Pinton P, Rimessi A, and Marchi S. et al. 2007 Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 315:659–663. [DOI] [PubMed] [Google Scholar]
- Pinton P, Tsuboi T, Ainscow EK, Pozzan T, Rizzuto R, Rutter GA. Dynamics of glucose-induced membrane recruitment of protein kinase C beta II in living pancreatic islet beta cells. J Biol Chem. 2002;277:37702–37710. doi: 10.1074/jbc.M204478200.0021-9258(2002)277[37702:DOGMRO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Carrington W, Tuft RA. Digital imaging microscopy of living cells. Trends Cell Biol. 1998;8:288–292. doi: 10.1016/s0962-8924(98)01301-4.0962-8924(1998)008[0288:DIMOLC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sakai N, Sasaki K, Ikegaki N, Shirai Y, Ono Y, Saito N. Direct visualization of the translocation of the gamma-subspecies of protein kinase C in living cells using fusion proteins with green fluorescent protein. J Cell Biol. 1997;139:1465–1476. doi: 10.1083/jcb.139.6.1465.0021-9525(1997)139[1465:DVOTTO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Namiki H 2007 Mechanism of membrane redistribution of protein kinase C by its ATP-competitive inhibitors. Biochem J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. Signaling through protein kinase C. Front Biosci. 1998;3:D1134–D1147. doi: 10.2741/a350.1093-4715(1998)003[D1134:STPKC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ways DK, Sheetz MJ. The role of protein kinase C in the development of the complications of diabetes. Vitam Horm. 2000;60:149–193. doi: 10.1016/s0083-6729(00)60019-5.0083-6729(2000)060[0149:TROPKC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]