Abstract
Oxidative stress has been suggested to play a main role in the pathogenesis of type 2 diabetes mellitus and its complications. As a consequence of this increased oxidative status, a cellular-adaptive response occurs requiring functional chaperones, antioxidant production, and protein degradation. This study was designed to evaluate systemic oxidative stress and cellular stress response in patients suffering from type 2 diabetes–induced nephropathy and in age-matched healthy subjects. Systemic oxidative stress has been evaluated by measuring advanced glycation end-products (pentosidine), protein oxidation (protein carbonyls [DNPH]), and lipid oxidation (4-hydroxy-2-nonenal [HNE] and F2-isoprostanes) in plasma, lymphocytes, and urine, whereas the lymphocyte levels of the heat shock proteins (Hsps) heme oxygenase-1 (HO-1), Hsp70, and Hsp60 as well as thioredoxin reductase-1 (TrxR-1) have been measured to evaluate the systemic cellular stress response. We found increased levels of pentosidine (P < 0.01), DNPH (P < 0.05 and P < 0.01), HNE (P < 0.05 and P < 0.01), and F2-isoprostanes (P < 0.01) in all the samples from type 2 diabetic patients with nephropathy with respect to control group. This was paralleled by a significant induction of cellular HO-1, Hsp60, Hsp70, and TrxR-1 (P < 0.05 and P < 0.01). A significant upregulation of both HO-1 and Hsp70 has been detected also in lymphocytes from type 2 diabetic patients without uraemia. Significant positive correlations between DNPH and Hsp60, as well as between the degree of renal failure and HO-1 or Hsp70, also have been found in diabetic uremic subjects. In conclusion, patients affected by type 2 diabetes complicated with nephropathy are under condition of systemic oxidative stress, and the induction of Hsp and TrxR-1 is a maintained response in counteracting the intracellular pro-oxidant status.
INTRODUCTION
Oxidative stress, caused by a relative overload of oxidants and depletion of antioxidants, is implicated in the pathogenesis of several diseases including atherosclerosis, ischaemia/reperfusion injury, and rheumatoid arthritis (Droge 2002). As a consequence of oxidative stress, severe damage to DNA, proteins, and lipids occur (Droge 2002). Compelling evidence has been provided that both insulin-dependent and non–insulin-dependent diabetic patients are under conditions of oxidative stress and that the complications of diabetes mellitus (thereafter indicated as diabetes) could be partially mediated by oxidative stress (Davì et al 2005; Ceriello 2006). Several mechanisms seem to be involved in the genesis of oxidative stress in diabetic patients, including glucose autoxidation, protein glycation as well as the formation of advanced glycation end-products ([AGEs], Bonnefont-Rousselot et al 2000, Davì et al 2005). In particular, it has been shown that two subclasses of AGEs, such as N-ɛ(carboxymethyl)lysine and pentosidine, accumulate in expanded mesangial and nodular lesions in diabetic nephropathy (Tanji et al 2000). Numerous correlations have been shown between oxidative stress and AGEs. In fact, reactive oxygen species (ROS) accelerate the formation of AGEs, which in turn as glycated proteins, also are able to produce ROS via complex biochemical mechanisms (Mullarkey et al 1990). During these reactions, protein modifications take place and compounds with a carbonyl or dicarbonyl moieties are formed (Davì et al 2005).
Apart from AGEs and protein carbonyls, other products derived from lipid peroxidation have been identified in biological fluids and tissues of patients affected by diabetic nephropathy. 4-hydroxy-2-nonenal (HNE) is considered an important marker of lipid peroxidation, but at the meantime it is endowed with both cytotoxic and mutagenic activity. In fact, 4-HNE further reacts with protein residues, such as histidine, to generate stable Michael adducts (Toyokuni et al 2000). Interestingly, HNE-modified proteins have been identified in the serum of type 2 diabetic patients (Toyokuni et al 2000) and renal tissues of diabetic patients with nephropathy (Miyata et al 1999a). Isoprostanes derive mainly from the free radical–based oxidation of arachidonic acid (Morrow et al 1992; Patrono et al 2005) even if other authors propose that they also are formed through an alternative cyclooxygenase-dependent pathway in organs such as the brain (Pepicelli et al 2005). Increased levels of F2-isoprostanes can be found in the plasma or urine of patients affected by several diseases, including diabetes, and currently are used as in vivo indicators of lipid peroxidation (Davì et al 1999; Roberts and Morrow 2000; Basu 2004; Davì et al 2005).
To adapt to environmental changes and survive to different types of injuries, eukaryotic cells have evolved networks of different responses (cellular stress response) that detect and control different forms of stress. Important members of this adaptive response are the heat shock protein family (Calabrese et al 2003, 2004, 2006) as well as the thioredoxin system (Nakamura 2005). Together, they form a powerful system involved in many intracellular and extracellular processes, including cell proliferation, the redox regulation of gene expression and signal transduction, protection against oxidative stress, antiapoptotic functions, growth factor and cytokine-like effects, and the regulation of the redox state of the extracellular environment (Calabrese et al 2006).
The present study was designed to evaluate the presence of systemic oxidative stress by using several biochemical markers such as AGEs and protein and lipid oxidation products in plasma, lymphocytes, and urine of patients suffering from type 2 diabetes complicated by nephropathy. Furthermore, we demonstrated in diabetic patients with and without nephropathy the activation of the cellular stress response evaluated as the induction, at the circulating lymphocyte level, of the heat shock proteins (Hsp) heme oxygenase-1 (HO-1), Hsp60, and Hsp70 as well as the anti-oxidant enzyme thioredoxin reductase-1 (TrxR-1).
MATERIALS AND METHODS
Patients
All the procedures on human subjects have been conducted in accordance with the guidelines in the Declaration of Helsinki and Ethical Committee of the University of Catania formally approved the experimental protocol. All the patients gave also informed consent prior to undergo any procedure.
Twenty-one (17 males, 4 females; mean age 59 years) non-dialyzed and non-transplanted adults with renal insufficiency secondary to type 2 diabetes and followed on a regular basis in tertiary care Renal Units of the Catania metropolitan area joined this study. Patients were referred to the participating Renal Units by the primary care physicians or by one of the outpatient Diabetes Centers of the Catania metropolitan area. Subjects who, at the time of the participation, suffered from other causes of renal insufficiency or proteinuria were excluded. Inclusion criteria were: nephropathy secondary to type 2 diabetes, renal insufficiency in stages 1–3 according to National Kidney Foundation-kidney disease outcomes quality initiative (K/DOQI) clinical practice guidelines, and stable renal function for at least 60 days prior to their participation. Exclusion criteria were the following: ischemic heart disease or heart failure (stage 2 or above according to New York Heart Association (NYHA) classification), hepatic failure, endocrine disease other than diabetes mellitus, and any other clinical or laboratory evidence of major organ disease. In periods of 4 to 8 weeks, all patients underwent a run-in period designed to achieve a stabilization of blood pressure levels, blood glucose control, and renal function. Thirty-six patients underwent the initial evaluation; among these only twenty-one were eligible for the study. Their clinical parameters (mean ± standard error) were as follows: plasma creatinine 1.42 ± 0.59 mg/dl (0.8–2.45), proteinuria 1023 ± 168 mg/24 h (136–2800), glycated hemoglobin 6.8 ± 2.2% (4.3–9.4), body weight 79 ± 16 Kg, height 167 ± 4 cm, body mass index 29 ± 5.6 (23–36), systolic blood pressure 150 ± l0 mm Hg (165–140), diastolic blood pressure 82 ± 6 (70–90 mm Hg). Creatinine clearance (>20 mL/min) was without variation (±30%) within last 3 months.
The diabetic nonuremic group was joined by 10 patients (6 males, 4 females; mean age 61 years) with the following clinical parameters: plasma creatinine 1.01 ± 0.23 mg/dl (0.86–1.88), proteinuria 74 ± 18 mg/24 h (15– 127), glycated hemoglobin 6.4 ± 1.4% (4.4–9.0), creatinine clearance 70 ± 10 (40–100) mg/mL, body weight 81 ± 18 Kg (65–94), height 165 ± 10 cm (160–175), body mass index 27 ± 8 (20–38), systolic blood pressure 155 ± l0 mm Hg (130–170), diastolic blood pressure 85 ± 8 mm Hg (75–94).
The control group consisted of 16 healthy subjects (13 males, 3 females; mean age 59 years). All the biochemical parameters listed above were in the normal range.
All the subjects were not taking drugs such as antioxidants, ACE inhibitors, or sartans.
Samples
Blood (6 mL) was collected after an overnight fast by venopuncture from an antecubital vein in ethylenediamine-tetraacetic acid–coated tubes. Immediately after sampling, 2 mL of blood was centrifuged at 10 000 × g for 1 min at 4°C to separate plasma from red blood cells, and 4 mL were utilized for lymphocytes purification.
A 24-h urine was collected on the day before the blood sample and aliquots were taken. All samples were stored at −80°C until analysis.
Lymphocyte purification
Lymphocytes from peripheral blood were purified by using the Ficoll Paque System following the procedure provided by the manufacturer (GE Healthcare, Piscataway, NJ, USA).
Western blot analysis
Plasma samples were ready to use, although the lymphocyte pellet was homogenized and centrifuged at 10 000 × g for 10 min and the supernatant was used for analysis after dosage of proteins. The aliquot (40 μg) of protein extract was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred overnight to nitrocellulose membrane and the nonspecific binding of antibodies was blocked with 3% nonfat dry milk in phosphate-buffered saline. Membranes then were probed with a specific monoclonal mouse anti-Hsp70 antibody (SPA-810, Stressgen, Ann Arbor, MI, USA). Instead, immunodetection of HO-1, DNPH, HNE and TrxR-1 were performed using polyclonal rabbit antibodies: SPA-895 (Stressgen), V0401 (DAKO, Glostrup, Denmark), HNE11-S (Alpha Diagnostic International, San Antonio, TX, USA), and 07-613 (Upstate Biotechnology, Charlottesville, VA, USA), respectively. Immunodetection of Hsp60 was performed using a polyclonal goat antibody sc-1052 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). A goat polyclonal antibody specific for β-actin was used for loading control (1:1000). Blots then were incubated with secondary horseradish peroxidase (HRP)–conjugated goat anti-rabbit immunoglobulin (IgG) when probing DNPH, HNE, and TrxR-1, whereas an HRP-conjugated goat anti-mouse (IgG) was used in the case of Hsp70 and HRP-conjugated anti-goat (IgG) for detection of β-actin and Hsp60 followed by enhanced chemoluminescence (ECL) (Amersham, GE Healthcare, Piscataway, NJ, USA). Immunoreactive bands were quantified by scanning Western blot–imaged films with a laser densitometer (LKB-Ultrascan, XL model, Pharmacia, American Instruments, Haverhill, MA, USA).
Measurement of F2-isoprostanes and pentosidine by high-performance liquid chromatography
The high-performance liquid chromatography analysis of F2-isoprostanes was performed as described by Ritov et al (2002). Urinary creatinine was measured by Sigma diagnostic kit (St. Louis, MO, USA). Values are expressed as nM and nmol/mg creatinine for plasma and urinary F2-isoprostanes, respectively.
Urinary levels of pentosidine were assayed by fluorescence high-performance liquid chromatography as described by Tsukahara et al (2003), whereas plasma pentosidine was measured according to Miyata et al (1996). Values are expressed as pmol/mg protein and nmol/mg creatinine for plasma and urinary pentosidine, respectively.
Protein measurements
Samples protein concentrations were determined by the bicinchoninic acid method (Smith et al 1985).
Statistical analysis
All results are expressed as means ± standard error of mean. Each experiment was performed, unless otherwise specified, in triplicate. Data were analyzed by one-way analysis of variance, followed by inspection of all differences by Duncan's new multiple-range test. Differences were considered significant at P < 0.05.
RESULTS
As previously reported, AGEs, such as pentosidine, are reliable markers of oxidative stress in diabetes and diabetic nephropathy, but they can trigger per se further inflammation, thus creating a self-sustained vicious circle responsible for tissue damage (Thomas et al 2005). In accordance with this theory, we demonstrated a significant increase (P < 0.01) in both urinary and plasma levels (Fig 1) of pentosidine in patients suffering from type 2 diabetes complicated by nephropathy respect to control subjects. The next step was to evaluate the effects of this systemic pro-oxidant condition on both protein and lipids. Protein oxidation has been evaluated by measuring the amount of protein carbonyls (DNPH), whereas lipid peroxidation was estimated by measuring both protein-bound HNE and circulating F2 isoprostanes. As shown in Figure 2, DNPH levels resulted significantly higher (P < 0.05 and 0.01) in plasma and lymphocytes from patients with nephropathy secondary to type 2 diabetes than in control subjects. In these same samples, a significant elevation (P < 0.05) of protein-bound HNE has been shown (Fig 2). With regard to isoprostanes, a significant increase in both urinary and plasma levels of total F2-isoprostanes has been found in type 2 diabetic nephropatic patients (P < 0.01) with respect to controls (Fig 3).
Fig 1.
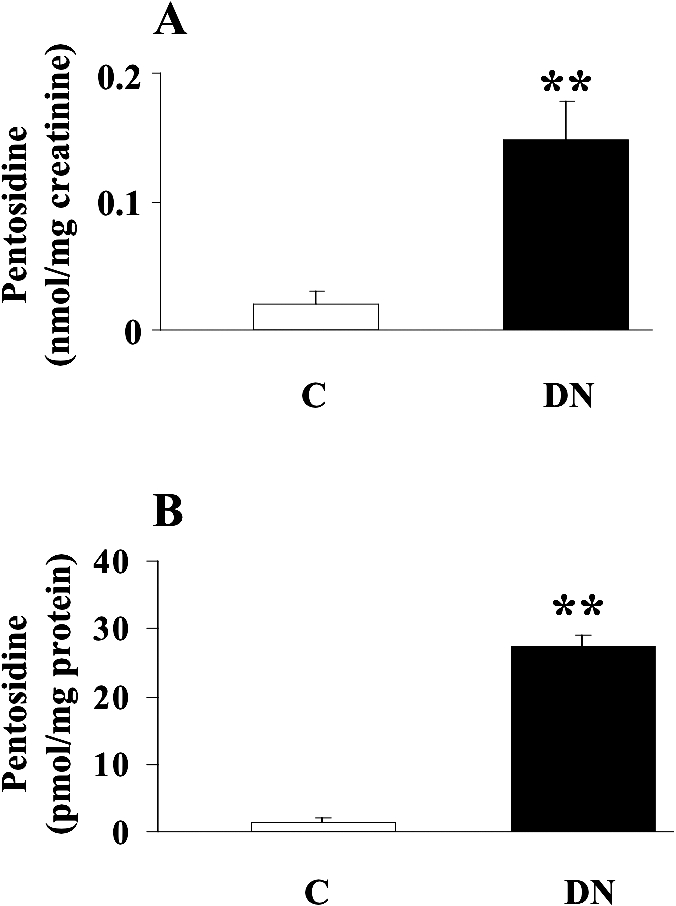
Pentosidine levels in urine and plasma from diabetic patients. (A) Urine and (B) plasma samples from patients with nephropathy secondary to type 2 diabetes and age-matched controls were assayed for pentosidine by high-performance liquid chromatography, as described in Materials and Methods. Data are expressed as mean ± standard error of mean of 3 independent analyses on 16– 21 patients per group. **P < 0.01 vs controls; C, controls; DN, type 2 diabetic patients with nephropathy
Fig 2.
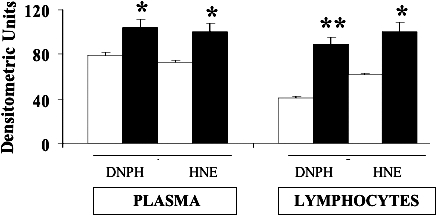
Protein carbonyls and 4-hydroxy-2-nonenals levels in plasma and lymphocytes from diabetic patients. Plasma and lymphocytes samples from type 2 diabetic patients with nephropathy (black columns) and age-matched controls (white columns) were assayed for protein carbonyls (DNPH) and 4-hydroxy-2-nonenals (HNE) by Western blot, as described in Materials and Methods. Values are expressed as mean ± standard error of mean of 3 independent analyses on 16–21 patients per group. *P < 0.05 and **P < 0.01 vs controls
Fig 3.
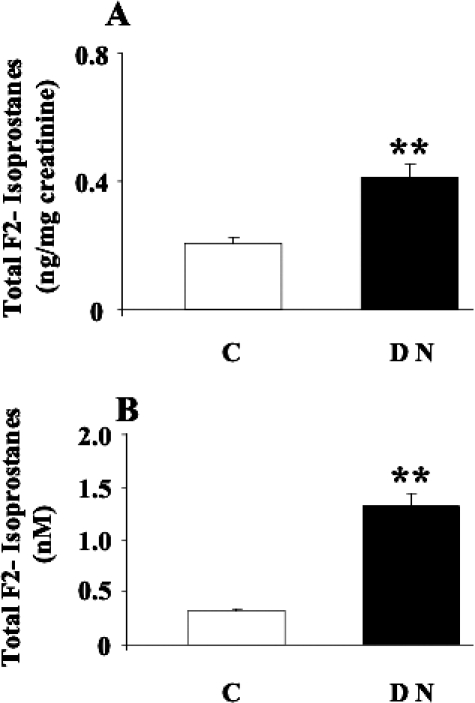
Total F2-isoprostanes levels in urine and plasma from diabetic patients. (A) Urine and (B) plasma samples from patients with nephropathy secondary to type 2 diabetes and age-matched controls were assayed for F2-isoprostanes by high-performance liquid chromatography as described in Materials and Methods. Data are expressed as mean ± standard error of mean of 3 independent analyses on 16–21 patients per group. **P < 0.01 vs controls; C, controls; DN, type 2 diabetic patients with nephropathy
A common way for the body to counteract oxidative stress is to induce protective genes such as those belonging to the HSP family as well as thioredoxin reductase (Calabrese et al 2003, 2004, 2006; Nakamura 2005). As shown in Figures 4–7, HO-1, Hsp60, Hsp70, and TrxR-1 have been significantly (P < 0.05 and 0.01) induced in lymphocytes of patients suffering from type 2 diabetic nephropathy with respect to controls. Interestingly, both HO-1 and Hsp70 were upregulated in lymphocytes from type 2 diabetic patients without uraemia (P < 0.05; Fig 8), according to previous data in circulating mononuclear cells (Yabunaka et al 1995).
Fig 4.
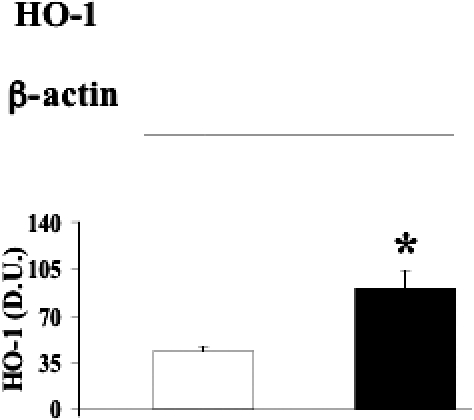
Heme oxygenase-1 levels in lymphocytes from diabetic nephropathic patients. Lymphocyte samples from patients with nephropathy secondary to type 2 diabetes (black columns) and age-matched controls (white columns) were assayed for heme oxygenase-1 (HO-1) by Western blot as described in Materials and Methods. A representative immunoblot is shown. β-actin has been used as loading control. The bar graph shows the densitometric evaluation and values are expressed as mean ± standard error of mean of 3 independent analyses on 16–21 patients per group. *P < 0.05 vs control; D.U., densitometric units
Fig 8.
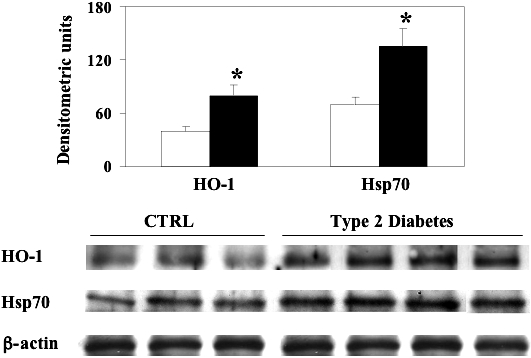
Heme oxygenase-1 and heat shock protein 70 (Hsp70) levels in lymphocytes from type 2 diabetic patients without nephropathy. Lymphocyte samples from patients with type 2 diabetes (dashed columns) and age-matched controls (white columns) were assayed for heme oxygenase-1 (HO-1) and Hsp70 by Western blot as described in Materials and Methods. A representative immunoblot containing samples from 3 control subjects and 4 patients with type 2 diabetes is shown. β-actin has been used as loading control. The bar graphs show the densitometric evaluation; values are expressed as mean ± standard error of mean of 3 independent analyses on 10–21 patients per group. P < 0.05 vs control; CTRL, control
A significant positive linear correlation between DNPH and Hsp60 expression levels in lymphocytes was found thus corroborating the importance of the heat shock response in the oxidative stress–induced protein oxidation in type 2 diabetic nephropathic patients (Fig 9). Finally, the heat shock response in lymphocytes has been found to correlate with the degree of renal insufficiency. As shown in Figure 10A, a significant positive correlation between HO-1 expression levels in lymphocytes and the 24-h proteinuria, this latter used as a clinical index of renal insufficiency, has been found. Similar results have been obtained by plotting the Hsp70 lymphocyte levels against the urinary protein output (Fig 10B). No relationship has been found between lymphocyte HSP and blood parameters related to the glycemic control (glycaemia, glycated hemoglobin) in accordance with previous data (Yabunaka et al 1995).
Fig 9.
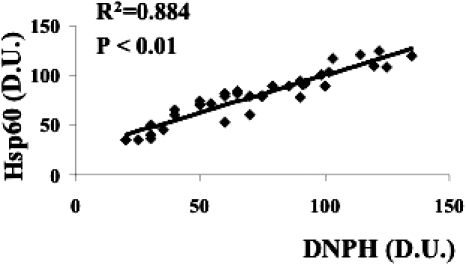
Relationship between protein carbonyls and heat shock protein 60 (Hsp60) in lymphocytes from diabetic nephropathic patients. Lymphocyte samples from 21 patients with nephropathy secondary to type 2 diabetes and 16 age-matched controls were assayed for both protein carbonyls (DNPH) and Hsp60 as described in Figures 2 and 5, respectively. A significant linear positive correlation has been found. Data are expressed as densitometric units (D.U.). Control subjects had a DNPH level ranging from 20 to 70 D.U
Fig 10.
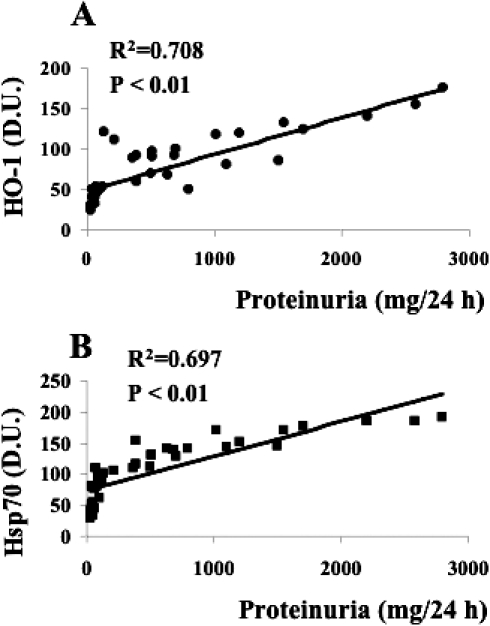
Relationship between the degree of renal failure and the lymphocyte heat shock response in diabetic nephropathic patients. Lymphocyte samples from 21 patients with nephropathy secondary to type 2 diabetes and 16 age-matched controls were assayed for heme oxygenase-1 (HO-1, panel A) and heat shock protein70 (Hsp70, panel B) as described in Figures 4 and 6, respectively. Heme oxygenase-1 and Hsp70 levels then were plotted against the 24-h proteinuria values of each subject. Proteinuria is a reliable clinical index of renal failure. Significant linear positive correlations have been found. Heme oxygenase-1 and Hsp70 levels are expressed as densitometric units (D.U.), whereas urinary protein is in mg/24 h. Control subjects had urinary proteins ranging from 30 to 120 mg/24 h
DISCUSSION
Diabetes is responsible for 30–40% of all end-stage renal disease cases in the United States. Although both type 1 (insulin-dependent) and type 2 (non–insulin-dependent) diabetes lead to end-stage renal disease, the great majority of patients are those suffering from type 2 diabetes.
As mentioned above, chronic hyperglycemia along with oxidative stress result in the formation and accumulation of AGEs in diabetes (Bierhaus et al 1998). AGEs have a wide range of chemical, cellular, and tissue effects that contribute to the development of microvascular complications. In particular, it has been demonstrated that AGEs play a key role in the development of diabetic nephropathy (Wolf 2004; Thomas et al 2005). The importance of AGEs as downstream mediators of tissue injury in diabetic kidney disease has been demonstrated by animal studies in which the inhibition of the advanced glycation reaction delayed the development of nephropathy without any direct effect on the glycemic control (Thomas et al 2005). Furthermore, AGE-modified proteins undergo physico-chemical changes that alter charge, solubility, and conformation, resulting in an altered protein (Thomas et al 2005). Finally, the effects of AGEs, together with hyperglycaemia and ROS, induce growth factors and cytokines causing renal hypertrophy and accumulation of extracellular matrix components (Wolf 2004). Our results showing an elevation in urine and plasma of AGEs in patients with diabetic nephropathy compared with healthy subjects is in accordance with current literature (Dolhofer-Bliesener et al 1995; Schleicher et al 1997; Daimon et al 1999) and contribute to put in a common frame hyperglycaemia, oxidative stress, as well as protein and lipid oxidation.
Reactive carbonyl compounds, which are known precursors of carbonyl stress, can be generated during the AGE-mediated free radical formation (Miyata et al 1999b). Our evidence of an increased levels of carbonyls both in plasma and lymphocytes from type 2 diabetic nephropathic patients are in line with clinical and experimental data showing an increased generation of ROS in diabetes (Rösen et al 2001; Davì et al 2005; Oksala et al 2007), and underlie the importance of the protein conformational changes in the pathogenesis of diabetic nephropathy. Furthermore, the linear correlation between protein carbonyls and the Hsp60 levels in lymphocytes (Fig 9) underlies the close relationship between the degree of ROS-mediated protein damage and the systemic heat shock response. 4-hydroxy-2-nonenal are formed from arachidonic acid or other unsaturated fatty acids following free radical attack and bind, by Michael addition, to proteins particularly at cysteine, hystidine, or lysine residues (Butterfield et al 1997). Lipid oxidation, measured by protein-bound HNE, also can occur in different organs under conditions of oxidative stress (Miyata et al 1999b; Lauderback et al 2001) including diabetes and diabetic nephropathy (Miyata et al 1999a; Toyokuni et al 2000; Oksala et al 2007). On the other hand, isoprostanes, produced by the free radical–catalyzed peroxidation of arachidonic acid apparently are formed in situ on phospholipids and subsequently released, possibly by a phospholipase. They circulate in the blood stream and finally are excreted in urine (Morrow et al 1992). Isoprostanes, in contrast to lipid hydroperoxides, are chemically stable end products of lipid peroxidation, and the measurement of their levels in plasma or urine may permit a sensitive and specific method for detection of lipid oxidative damage in vivo (Roberts and Morrow 2000). Although a significant increase in the F2-isoprostane levels in type 2 diabetic patients has been reported extensively (Gopaul et al 1995; Davì et al 1999; Mori et al 1999), few studies demonstrated such an increase also in the case of diabetes complications. The results shown in this study demonstrated an increased level of F2 isoprostanes also in type 2 diabetic patients with nephropathy, according with a recent study by Beisswenger et al (2005). During the last years, it was disputed whether urinary isoprostanes derive from local production in the kidney, from the circulation, or a combination of both (Morrow and Roberts 1996). Our results show a significant increase in both plasma and urinary levels of F2-isoprostanes and provide further evidence about a condition of systemic rather than local pro-oxidant status.
A novel approach to counteract oxidative stress-related disease is the manipulation, mainly via nutritional antioxidants, of cell chaperones. Chaperones (stress proteins) are essential proteins to help the formation and maintenance of the proper conformation of other proteins and to promote cell survival after a large variety of environmental stresses. Therefore, normal chaperone function is a key factor for endogenous stress adaptation of several tissues, whereas altered chaperone function has been associated with the development of several diseases (Calabrese et al 2006; Mancuso et al 2007). Our data provide the first evidence to our knowledge about an increased expression of HO-1, Hsp60, and Hsp70 as well as of TrxR-1 in pheripheral lymphocytes of patients suffering from nephropathy secondary to type 2 diabetes. The induction of Hsp70 has been proved to be an efficient help in the recovery from a large number of diseases, such as ischemic heart disease and neurodegeneration (Calabrese et al 2003, 2004, 2006; Mancuso et al 2007). Interestingly, a significant increase in Hsp70 protein has been found in the kidney tissue of diabetic rats as well as in mononuclear cells from type 2 diabetic patients (Yabunaka et al 1995; Oksala et al 2007).
Much more intriguing is the physiological importance of HO-1 protein expression in human lymphocytes. In fact, HO-1 is one of the early genes to be induced during oxidative stress and it exerts cytoprotective functions by metabolizing pro-oxidant heme and producing both the vasoactive molecule carbon monoxide and biliverdin, this latter being the precursor of the powerful antioxidant and antinitrosative molecule bilirubin (Calabrese et al 2006; Mancuso et al 2006, 2007). Furthermore, HO-1 has been shown to be overexpressed in the kidney of streptozotocin-treated rats (Oksala et al 2007). The HO-1 induction observed in the lymphocytes of our patients with type 2 diabetes complicated by nephropathy suggests that, in these patients, the immune system is trying to react to an oxidant insult by inducing an early gene and this is much more interesting considering a chronic disease such as diabetes. The thioredoxin system (thioredoxin, thioredoxin reductase, and reduced nicotinamide adenine dinucleotide phosphate (NADPH)) regulates cellular redox balance through the reversible oxidization of its redox-active cysteine residues (-Cys-Gly-Pro-Cys-) to form a disulfide bond that in turn is reduced by thioredoxin reductase and NADPH (Holmgren 1985; Nakamura 2005). Thioredoxin plays an essential role in cell function by limiting oxidative stress directly via antioxidant effects and indirectly by protein–protein interactions with key signaling molecules such as the thioredoxin-interacting protein (Yamawaki and Berk 2005). In this study we have shown an increased TrxR protein expression in lymphocytes, which is in accordance with the well-known effects induced by oxidative stress (Chen et al 2005). In fact, as part of the cellular stress response, cells overexpress thioredoxin reductase under condition of oxidative stress.
It is noteworthy to point out that HO-1 and Hsp70 have been found upregulated also in lymphocytes from type 2 diabetic patients without uraemia. At this time we cannot rule out whether the heat shock response was related to diabetes, uraemia, or a combination of both. However, the positive correlation found between the degree of renal failure and the HO-1 and Hsp70 induction (Fig 10) compared with the lack of correlation between HbA1c and HSP (data not shown), seem to underlie the important role played by nephropathy in the induction of the cellular stress response in lymphocytes. Accordingly, previous data from type 2 diabetic patients and diabetic rats have shown a significant decrease in Hsp70, HO-1 and Hsp60 gene expression and protein in muscle and heart samples (Kurucz et al 2002; Bruce et al 2003; Oksala et al 2006). On this basis, the significant increase in Hsp level in human lymphocytes from diabetic patients with or without nephropathy should not be considered as a mere epiphenomenon of a generalized tissue response to local oxidative stress conditions, but conversely as a systemic antioxidant response activated by the immune system to counteract oxidative and glycoxidative stress-induced pro-oxidant status.
Based on these findings, it is plausible to hypothesize that alternative therapies that inhibit the formation of toxic products such as AGEs or remove established AGE-promoted modifications will form an important component part of future prophylaxis and therapy in patients with diabetes, acting in concert with conventional approaches to prevent diabetic renal injury.
Fig 5.
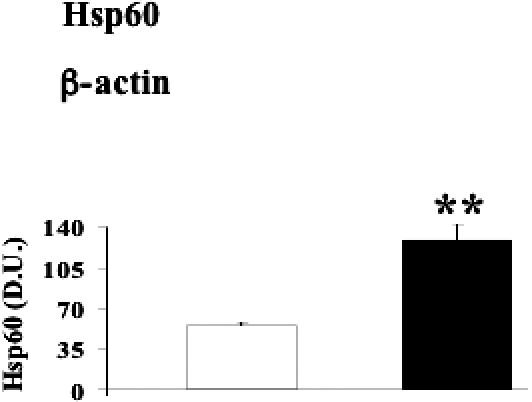
Heat shock protein 60 (Hsp60) levels in lymphocytes from diabetic nephropathic patients. Lymphocyte samples from patients with nephropathy secondary to type 2 diabetes (black columns) and age-matched controls (white columns) were assayed for Hsp60 by Western blot as described in Materials and Methods. A representative immunoblot is shown. β-actin has been used as loading control. The bar graph shows the densitometric evaluation and values are expressed as mean ± standard error of mean of 3 independent analyses on 16–21 patients per group. **P < 0.01 vs control; D.U., densitometric units.
Fig 6.
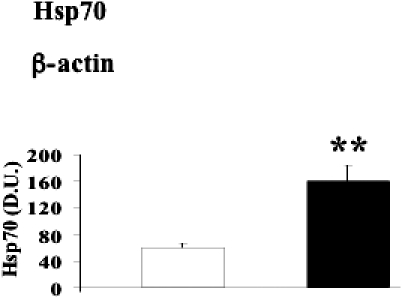
Heat shock protein 70 (Hsp70) levels in lymphocytes from diabetic nephropathic patients. Lymphocyte samples from patients with nephropathy secondary to type 2 diabetes (black columns) and age-matched controls (white columns) were assayed for Hsp70 by Western blot as described in Materials and Methods. A representative immunoblot is shown. β-actin has been used as loading control. The bar graph shows the densitometric evaluation and values are expressed as mean ± standard error of mean of 3 independent analyses on 16–21 patients per group. **P < 0.01 vs control; D.U., densitometric units.
Fig 7.
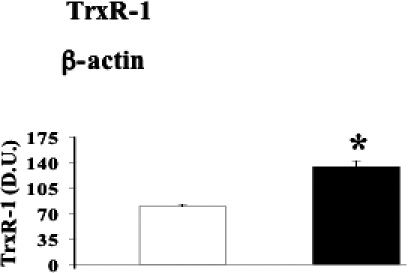
Thioredoxin reductase-1 levels in lymphocytes from diabetic nephropathic patients. Lymphocyte samples from patients with nephropathy secondary to type 2 diabetes (black columns) and age-matched controls (white columns) were assayed for thioredoxin reductase-1 (TrxR-1) by Western blot as described in Materials and Methods. A representative immunoblot is shown. β-actin has been used as loading control. The bar graphs show the densitometric evaluation and values are expressed as mean ± standard error of mean of 3 independent analyses on 16–21 patients per group. *P < 0.05 vs control; D.U., densitometric units
Acknowledgments
This work was supported by grants by Italian Cofin 2000 (FIRB RBNE01ZK8F).
REFERENCES
- Basu S. Isoprostanes: novel bioactive products of lipid peroxidation. Free Radic Res. 2004;38:105–122. doi: 10.1080/10715760310001646895.1071-5762(2004)038[0105:INBPOL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes. 2005;54:3274–3281. doi: 10.2337/diabetes.54.11.3274.0012-1797(2005)054[3274:STDNIR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus I. The AGE concept. Cardiovasc Res. 1998;37:586–600. doi: 10.1016/s0008-6363(97)00233-2.0008-6363(1998)037[0586:AATIWA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 2000;26:163–176.0338-1684(2000)026[0163:COTDSO]2.0.CO;2 [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52:2338–2345. doi: 10.2337/diabetes.52.9.2338.0012-1797(2003)052[2338:IHSPAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Howard BJ, Yatin S, Allen KL, Carney JM. Free radical oxidation of brain proteins in accelerated senescence and its modulation by N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci USA. 1997;94:674–678. doi: 10.1073/pnas.94.2.674.1091-6490(1997)094[0674:FROOBP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Butterfield DA, Scapagnini G, Stella AM, Maines MD. Redox regulation of heat shock protein expression by signaling involving nitric oxide and carbon monoxide: relevance to brain aging, neurodegenerative disorders, and longevity. Antioxid Redox Signal. 2006;8:444–477. doi: 10.1089/ars.2006.8.444.1523-0864(2006)008[0444:RROHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Colombrita C, Ravagna A, Pennisi G, Giuffrida Stella AM, Galli F, Butterfield DA. Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach. Amino Acids. 2003;25:437–444. doi: 10.1007/s00726-003-0048-2.0939-4451(2003)025[0437:RROHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Colombrita C, Spadaro F, Butterfield DA, Giuffrida Stella AM. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech Ageing Dev. 2004;125:325–335. doi: 10.1016/j.mad.2004.01.003.0047-6374(2004)125[0325:IEOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ceriello A. Oxidative stress and diabetes-associated complications. Endocr Pract. 2006;12:60–62. doi: 10.4158/EP.12.S1.60.1530-891X(2006)012[0060:OSADC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200.0021-9258(2005)280[41921:HIARAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Daimon M, Ono Y, and Saito T. et al. 1999 Increased serum levels of pentosidine, but not carboxymethyl lysine, in type 2 diabetes without obvious diabetic nephropathy. Diabetes Care. 22:877–878. [DOI] [PubMed] [Google Scholar]
- Davì G, Ciabattoni G, and Consoli A. et al. 1999 In vivo formation of 8-iso prostaglandin F2 alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 99:224–229. [DOI] [PubMed] [Google Scholar]
- Davì G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7:256–268. doi: 10.1089/ars.2005.7.256.1523-0864(2005)007[0256:LPIDM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dolhofer-Bliesener R, Lechner B, Deppisch R, Ritz E, Gerbitz KD. Immunological determination of advanced glycosylation end-products in human blood and urine. Nephrol Dial Transplant. 1995;10:657–664.1460-2385(1995)010[0657:IDOAGE]2.0.CO;2 [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001.0031-9333(2002)082[0047:FRITPC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gopaul NK, Anggard EE, Mallet AI, Betteridge DJ, Wolff SP, Nourooz-Zadeh J. Plasma 8-epi-PGF 2 alpha levels are elevated in individuals with non–insulin-dependent diabetes mellitus. FEBS Lett. 1995;368:225–229. doi: 10.1016/0014-5793(95)00649-t.0014-5793(1995)368[0225:PEALAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321.0066-4154(1985)054[0237:T]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–1109. doi: 10.2337/diabetes.51.4.1102.0012-1797(2002)051[1102:DEOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1-42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x.0022-3042(2001)078[0413:TGGTGI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mancuso C, Pani G, Calabrese V. Bilirubin: an endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep. 2006;11:207–213. doi: 10.1179/135100006X154978.1351-0002(2006)011[0207:BAESON]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mancuso C, Scapagnini G, Currò D, Giuffrida Stella AM, De Marco C, Butterfield DA, Calabrese V. Mitochondrial dysfunction, free radical generation, and cellular stress response in neurodegenerative disorders. Front Biosci. 2007;12:1107–1123. doi: 10.2741/2130.1093-4715(2007)012[1107:MDFRGA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Miyata T, Sugiyama S, Suzuki D, Inagi R, Kurokawa K. Increased carbonyl modification by lipids and carbohydrates in diabetic nephropathy. Kidney Int Suppl. 1999a;71:S54–S56. doi: 10.1046/j.1523-1755.1999.07114.x.0085-2538(1999)071[S54:ICMBLA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Miyata T, Taneda S, Kawai R, Ueda Y, Horiuchi S, Hara M, Maeda K, Monnier VM. Identification of pentosidine as a native structure for advanced glycation end products in beta-2-microglobulin–containing amyloid fibrils in patients with dialysis-related amyloidosis. Proc Natl Acad Sci USA. 1996;93:2353–2358. doi: 10.1073/pnas.93.6.2353.1091-6490(1996)093[2353:IOPAAN]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Van Ypersele De Strihou C, Kurokawa K, Baynes JW. Alterations in nonenzymatic biochemistry in uremia: origin and significance of carbonyl stress in long-term uremic complications. Kidney Int. 1999b;55:389–399. doi: 10.1046/j.1523-1755.1999.00302.x.0085-2538(1999)055[0389:AINBIU]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mori TA, Dunstan DW, Burke V, Croft KD, Rivera JH, Beilin LJ, Puddey IB. Effect of dietary fish and exercise training on urinary F2-isoprostanes excretion in non–insulin-dependent diabetic patients. Metabolism. 1999;48:1402–1408. doi: 10.1016/s0026-0495(99)90150-6.0026-0495(1999)048[1402:EODFAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ. Non–cyclo-oxygenase–derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci USA. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721.1091-6490(1992)089[10721:NPFAFI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ II. The isoprostanes. Current knowledge and directions for future research. Biochem Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1.0006-2952(1996)051[0001:TICKAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mullarkey CJ, Edelstein D, Brownlee M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990;173:932–939. doi: 10.1016/s0006-291x(05)80875-7.0006-291X(1990)173[0932:FRGBEG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nakamura H. Thioredoxin and its related molecules: update 2005. Antioxid Redox Signal. 2005;7:823–828. doi: 10.1089/ars.2005.7.823.1523-0864(2005)007[0823:TAIRMU]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Oksala NK, Laaksonen DE, Lappalainen J, Khanna S, Nakao C, Hanninen O, Sen CK, Atalay M. Heat shock protein 60 response to exercise in diabetes: effects of alpha-lipoic acid supplementation. J Diabetes Complications. 2006;20:257–261. doi: 10.1016/j.jdiacomp.2005.07.008.1056-8727(2006)020[0257:HSPRTE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Oksala NK, Lappalainen J, Laaksonen DE, Khanna S, Kaarniranta K, Sen CK, Atalay M. Alpha-lipoic acid modulates heat shock factor-1 expression in streptozotocin-induced diabetic rat kidney. Antioxid Redox Signal. 2007;9:497–506. doi: 10.1089/ars.2006.1450.1523-0864(2007)009[0497:AAMHSF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Patrono C, Falco A, Davi G. Isoprostane formation and inhibition in atherothrombosis. Curr Opin Pharmacol. 2005;5:198–203. doi: 10.1016/j.coph.2004.11.003.1471-4892(2005)005[0198:IFAIIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pepicelli O, Fedele E, Berardi M, Raiteri M, Levi G, Greco A, Ajmone-Cat MA, Minghetti L. Cyclo-oxygenase-1 and -2 differently contribute to prostaglandin E2 synthesis and lipid peroxidation after in vivo activation of N-methyl-D-aspartate receptors in rat hippocampus. J Neurochem. 2005;93:1561–1567. doi: 10.1111/j.1471-4159.2005.03150.x.0022-3042(2005)093[1561:CADCTP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ritov VB, Kelley DE, Kagan VE. Derivatization of F2-isoprostanes with 1-pyrenyldiazomethane and their subsequent determination by fluorescence high-performance liquid chromatography. Anal Biochem. 2002;311:10–18. doi: 10.1016/s0003-2697(02)00392-5.0003-2697(2002)311[0010:DOFWPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roberts LJ, Morrow JD. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6.0891-5849(2000)028[0505:MOFAAI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rösen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product Nɛ-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;99:457–468. doi: 10.1172/JCI119180.0021-9738(1997)099[0457:IAOTGP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, and Hermanson GT. et al. 1985 Measurement of protein using bicinchoninic acid. Anal Biochem. 150:76–85.1985. [DOI] [PubMed] [Google Scholar]
- Tanji N, Markowitz GS, and Fu C. et al. 2000 Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 119:1656–1666. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Forbes JM, Cooper ME. Advanced glycation end products and diabetic nephropathy. Am J Ther. 2005;12:562–572. doi: 10.1097/01.mjt.0000178769.52610.69. [DOI] [PubMed] [Google Scholar]
- Toyokuni S, Yamada S, and Kashima M. et al. 2000 Serum 4-hydroxy-2-nonenal–modified albumin is elevated in patients with type 2 diabetes mellitus. Antioxid Redox Signal. 2:681–685. [DOI] [PubMed] [Google Scholar]
- Tsukahara H, Sekine K, and Uchiyama M. et al. 2003 Formation of advanced glycosylation end products and oxidative stress in young patients with type 1 diabetes. Pediatr Res. 54:419–424. [DOI] [PubMed] [Google Scholar]
- Wolf G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur J Clin Invest. 2004;34:785–796. doi: 10.1111/j.1365-2362.2004.01429.x.0014-2972(2004)034[0785:NIITPO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yabunaka N, Ohtsuka Y, Watanabe I, Noro H, Fujisawa H, Agishi Y. Elevated levels of heat shock protein 70 (HSP70) in the mononuclear cells of patients with non–insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;30:143–147. doi: 10.1016/0168-8227(95)01151-x.0168-8227(1995)030[0143:ELOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Berk BC. Thioredoxin: a multifunctional antioxidant enzyme in kidney, heart, and vessels. Curr Opin Nephrol Hypertens. 2005;14:149–153. doi: 10.1097/00041552-200503000-00010.1062-4821(2005)014[0149:TAMAEI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]


