Abstract
Coelomocytes are recognized as the main cellular component of the echinoderm immune system. They are the first line of defense and their number and type can vary dramatically during infections or following injury. Sea stars have been used as a model system to study the regeneration process after autotomy or predation. In the present study we examined the cellular and biochemical responses of coelomocytes from the European sea star Asterias rubens to traumatic stress using immunochemical and biochemical approaches. In terms of trauma and post-traumatic stress period, here we consider the experimental arm amputation and the repair phase involved in the first 24 hours post-amputation, which mimicked a natural predation event. Four cell morphotypes were distinguishable in the coelomic fluid of both control and post-traumatic-stressed animals (phagocytes, amoebocytes, vibratile cells, hemocytes), but phagocytes were the major components, accounting for about 95% of the total population. Thus, the effects measured relate to the overall population of coelomocytes. A modest increase in the total number of freely circulating coelomocytes was observed 6 hours post-amputation. Interestingly, a monoclonal antibody (McAb) to a sea urchin embryo adhesion protein (toposome) cross-reacted with isolated sea star coelomocytes and stained the coelomic epithelium of control animals with an increase in trauma-stressed arms. In addition, coelomocytes from trauma-stressed animals showed a time-dependent increase in Hsp70 levels, as detected by both immunocytochemistry and immunoblotting within 24 hours after arm tip amputation, with a peak at 6 hours after amputation. Our findings indicate a clear role for coelomocytes and classic stress molecules in the post-traumatic stress associated with the early repair phase of regeneration.
INTRODUCTION
The origins, cell lineage regulation, and fate of freely circulating coelomocytes (the immune cells of echinoderms), is receiving increased attention for several important reasons: (1) their relationship to vertebrate homologues (Smith and Davidson 1994; Hibino et al 2006), (2) access to new and tractable model systems (Candia-Carnevali 2005, 2006; Dupont and Thorndyke 2006), (3) as potential sources of stem cells in regeneration (Candia-Carnevali and Bonasoro 2001; Patruno et al 2001; Thorndyke et al 2001), and (4) the availability of genomic tools to analyze regulative functions (Matranga et al 2005; Hibino et al 2006; Sea Urchin Genome Sequencing Consortium 2006). Coelomocytes are found in the coelomic spaces of all echinoderms, including the perivisceral coelomic cavities, the water-vascular system, and the hemal system, as well as in the connective tissue and amongst tissues of various organs (Glinski and Jarosz 2000; see Muñoz-Chápuli et al 2005 for a review). They participate in functions similar to their immune system homologues in vertebrates, such as formation of cellular clots, phagocytosis, encapsulation and clearance of bacteria and other foreign materials, as well as oxygen transport (for a review see Matranga et al 2005). So far there is no common opinion about the origin of circulating coelomocytes. Most descriptive and experimental approaches point to the coelomic epithelium as the most probable progenitor tissue for these circulating cells (reviewed by Muñoz-Chápuli et al 2005), as shown by the delamination of mesothelial cells to form phagocytic cells seen in adult starfish when carbon particles are injected into the coelom (Bossche and Jangoux 1976). Other authors suggest that the echinoderm axial organ, a complex and elongated mass of tissue that represents the common junction of the circulatory system, could be the source of coelomocytes. This notion comes from older studies that described the release of coelomocytes from the axial organ after echinoid injury (Millott 1969). Thus, by analogy to the vertebrate system, the coelomic epithelium or the axial organ has been regarded as an ancestral primary lymphoid gland. Unfortunately, these ideas have received little recent attention. Some more recent studies have shown a rapid increase in the numbers of red amoebocytes, a minor group of sea urchin coelomocytes, accounting for 5% of the total population, in response to pollution or experimentally induced stress (Matranga et al 2000, 2002, 2005). This evidence can be explained by either the rapid division of circulating stem cells or by their recruitment from the coelothelium, axial organ, or other niches. However, these hypotheses have yet to be clearly demonstrated.
Many echinoderms, including asteroids, ophiuroids, and holothuroids, are known to possess remarkable regenerative capacities as well as the ability to reproduce by clonal division (Byrne 1985; Garcia-Arraras et al 1998; Candia-Carnevali 2006; Dupont and Thorndyke 2006). In those species examined, wound healing, growth, morphogenesis, and differentiation involved in tissue regenerative phenomena have been the main focus of study. In Asterias rubens, the aggregation of coelomocytes from adjacent tissues has been shown to contribute to wound healing. Loss of coelomic fluid can seriously affect many behavioral and physiological functions, making rapid repair of injured tissues essential (see Smith 1981 for a review; Moss et al 1998). In echinoderms, wound repair and encapsulation of invasive material requires the presence of adhesive activity in the coelomic fluid. In the past, a variety of different factors have been implicated in mediating this function (Matranga et al 1986; reviewed by Glinski and Jarosz 2000). In the sea urchin, both coelomic fluid and coelomocytes have been shown to contain the precursor of toposome (Cervello and Matranga 1989; Cervello et al 1994), an adhesive molecule previously identified in embryos, whose biological role in mediating cellular adhesion has been fully documented (Matranga et al 1986; Scaturro et al 1998). Sea urchin coelomocytes also have been shown to have increased Hsp70 levels in response to temperature stress, acidic pH, heavy metals, and other pollutants (Matranga et al 2000, 2002, 2005, 2006). The function of Hsp proteins in a number of intracellular processes, such as chaperone guidance (Becker and Craig 1994), protein folding (Buchner 1996), and protection against apoptosis (Parcellier et al 2003), explains their high evolutionary conservation. This, together with their upregulation during cellular stress, makes them an important part of both the defense and immune systems (Moseley 2000; Robert 2003). Interestingly, in addition to the increase in numbers of red amoebocytes, an increase in Hsp70 levels also was found in wounded sea urchins, suggesting its participation in traumatic events (Matranga et al 2000). Similarly, in armed echinoderms, Hsp70 has been shown to be involved in autotomy and wound repair, with a putative function in tissue remodelling and associated protein turnover during regeneration (Patruno et al 2001). The present study was designed to investigate the cellular and biochemical activities of coelomocytes from the common European sea star A. rubens in response to the post-traumatic stress following arm amputation, using immunochemical and biochemical approaches. The post-traumatic period considered was the repair phase between 0 and 24 hours post-amputation, a phase primarily characterized by wound closure and the initiation of clot formation. Modulation in the total number of circulating coelomocytes in response to post-traumatic stress was observed over time. An anti-toposome monoclonal antibody (McAb) was used to recognize coelomocytes in sections of paraformaldehyde (PFA)–fixed sea star arms from both control and amputated animals. Finally, coelomocytes from post-traumatic-stressed animals showed a time-dependent increase in Hsp70 levels, as detected by immunocytochemistry and immunoblotting within 24 hours of amputation.
MATERIALS AND METHODS
Animals and post-traumatic stress conditions
Asterias rubens were collected from Gullmar Fjord, Swedish west coast (58.2°N, 11.3°E) and maintained at Kristineberg Marine Research Station (Fiskebäckskil, Sweden) in aquaria with running seawater (SW) at 12–14°C. Experimental post-traumatic stress was induced by removing 1 arm tip on each sea star using scissors. Amputated animals were kept in aquaria for a period of 24 hours (1 sea star per tank). Free coelomocytes were harvested for the detection of stress protein (Hsp70) by immunoblotting and immunocytochemistry (ICC) as described below. Sections of arms also were prepared in order to trace coelomocytes in tissues using ICC, according to the procedures described below. Amputated animals were returned to aquaria after each harvest of cells.
Coelomocytes preparation
Coelomocytes were collected, as a total cell population, by bleeding individual sea stars into an anticoagulant solution, coelomocyte culture medium (CCM), composed of 0.5 M NaCl, 5 mM MgCl2, 20 mM HEPES 1mM, ethyleneglycol-tetraacetic acid (EGTA) pH 7.9 (Henson et al 1992). Approximately 4 mL of coelomic fluid, harvested from each sea star after 0, 1, 3, 4.5, 6, and 24 hours post-amputation, was poured rapidly onto 4 mL of ice-cold 2× CCM. Cells were either counted, using a Burker chamber in order to monitor the number of circulating coelomocytes, or centrifuged and stored at −80°C for preparation of cell pellets.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting
Procedures for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-Page) and immunoblotting followed those previously reported (Matranga et al 2000, 2002, 2006) with some modification as follows. Briefly, cell pellets were homogenized in about 5 volumes of lysis buffer containing the 20 mM Tris, 2 mM ethylenediamine-tetraacetic acid (EDTA), 1% NP-40, 15% glycerol, and 2 mM dithiothreitol (DTT), supplemented with a cocktail of protease inhibitors: 2 μg/μL antipain and leupeptin, 1 μg/μL aprotinin and pepstatin, 1 mM benzamidine, and 0.1 mM phenylmethane-sulphonyfluoride (PMSF). Cell lysates were centrifuged at 10 000 rpm for 10 min at 4°C; supernatants were collected and protein concentration determined by a Bio-Rad Protein Assay (Bradford method). Equal amounts of protein extract, usually 15 μg of coelomocyte lysate for each sample, were separated by SDS-Page under reducing conditions according to Laemmli (1970) on a 7.5% gel to detect Hsp70 expression and on a 6% gel to detect anti-toposome expression. Proteins were transferred to nitrocellulose paper according to Towbin et al (1979). Immunoblottings were performed incubating for 1 hour at room temperature in anti-bovine brain 70 kDa heat shock protein McAb (Hsp70 McAb; Sigma Chemical Company, H-5147, St Louis, MO, USA) diluted 1 to 5000 or anti-toposome McAb (Noll et al 1985; Cervello and Matranga 1989) (BEVIB12b8, ascite fluid) diluted 1:800. As secondary antibody we used a peroxidase-conjugated anti-mouse IgG (Amersham) diluted 1 to 5000 for 1 hour at room temperature. Immunoreactivity was detected by chemiluminescence using a SuperSignal West Pico Chemiluminescent Substrate (Pierce) and Hyperfilm ECL films (Amersham). Densitometric analysis of bands intensities was performed by scanning with a Bio-Rad imaging densitometer (model Gel Doc 1000) equipped with an analysis program automatic integrator (MultiAnalyst, version 1.1).
Immunocytochemistry
Arm tissues
We utilized the technique previously reported with minor changes (Moss et al 1998). Briefly, animals were anesthetized in 3% MgCl2 in seawater (SW) for 30 minutes to immobilize tube feet pieces. Ten to 15 mm of amputated and control (nonamputated) arms were removed by a scalpel. Samples were fixed in 4% paraformaldehyde (PFA) for 4 hours at room temperature. Following fixation, tissues were decalcified by bathing for 48 hours in 0.5 M EDTA in filtered SW, pH 8. After dehydration, tissues were embedded in paraffin wax (Oxoid) and sectioned (6 μm) using standard methods. Sections were dewaxed, rehydrated through an alcohol series, rinsed with phosphate-buffered saline (PBS), and incubated for 1 hour in 5% (v/v) heat-inactivated normal goat serum (NGS) diluted in PBS. The specimens then were incubated overnight at 4°C with primary antibody: anti-toposome McAb (BEVIB12b8, ascite fluid) diluted 1:500 in 5% NGS in PBS. Slides were rinsed in PBS and incubated in the secondary antibody: goat anti-mouse IgG alkaline phosphatase conjugate (Sigma), diluted 1:1000 in 5% NGS in PBS for 1 hour at room temperature. Cross-reactivity as revealed by incubation with 5-bromo-4-chloro-3-indolyl phosphate/ nitroblue tetrazolium substrate (BCIP/NBT stock solution). Controls were run by omitting the primary antibody and showed no appreciable staining. Light microscopy (LM) was carried out using a Leica microscope, equipped with a digital camera.
Coelomocytes
After cutting a single arm tip with scissors and pouring coelomic fluid into a sterile tube, 100 μL were quickly diluted in 100 μL of 2× CCM, deposited onto 0.01% polylysine-coated (Sigma P8920) glass slides and left for 10 minutes to allow cells to settle. Following removal of excess fluid, adherent cells were fixed in 3% glutaraldehyde in SW for 1 hour at room temperature. Slides were then either directly used for photographic recording of morphology or processed for immunocytochemistry. Slides were washed twice (5 min) in SW, incubated for 45 minutes with anti-Hsp70 antibody (Sigma) diluted 1:500 in 3% bovine serum albumin (BSA) in SW or anti-toposome McAb (BEVIB12b8, ascite fluid) diluted 1:500 in 5% NGS in PBS. Slides then were washed 3 times (5 min each) in SW and incubated for 45 minutes in goat anti-mouse IgG alkaline phosphatase-conjugate (Sigma) diluted 1:1000 in 3% BSA in SW at room temperature. Cross-reactivity was revealed by incubation with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium substrate (BCIP/NBT Stock Solution). Controls performed omitting the primary antibody showed no appreciable staining. Photomicroscopy was carried out as above.
RESULTS
Identification of circulating coelomocytes
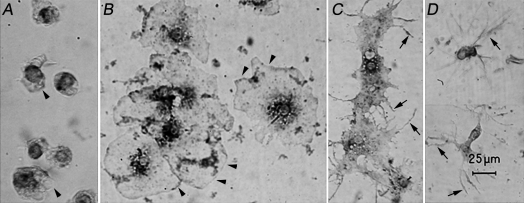
In order to characterize cell morphotypes circulating in sea star coelomic fluid under normal physiological conditions, coelomocytes were collected live by bleeding from the arm tip and observed without fixation or staining under the microscope. Phagocytes predominated, comprising about 95% of the total population present, as shown in Figure 1, which depicts a representative field of the whole phagocytic cell population. They showed a dendritic-like phenotype that underwent a striking morphological transition from petaloid to filopodial shape when collected live on slides. Petaloid phagocytes consisted of thin sheets of cytoplasm, the petals, organized around a central nuclear region (Fig 1A). The first phase of the transition process appeared as a rapid (between 1 and 2 min) increase in diameter from around 25 μm (Fig 1A) to about 50 μm (Fig 1B). The transition to the filopodial form occurred within 5–8 minutes in fresh preparations on glass slides and was similar for suspended cells (not shown). This transition began with the formation of several microspikes at the edge of each petal (Fig 1B,C). The retraction of cytoplasm occurred quickly while the filopodia began to elongate; the average length of each filopodium was about 20 μm, with some filopodia extending up to 30 μm (Fig 1D).
Fig 1.
Sea star phagocytes: morphological transition from petaloid to filopodial forms. Cells were attached to polylysine-coated glass slides (see text for more details) and observed after (A) 1, (B) 2, (C) 4, and (D) 8 minutes. Arrowheads and arrows point to petaloid or filopodial cytoplasmic protrusions, respectively. Interference contrast, 63× objective. Bar = 25 μm
On the basis of their features and behavior at light microscopy 3 other cell types were recognizable in the coelomic fluid: amoebocytes, vibratile cells, and hemocytes. White and red amoebocytes (Figs 3B, 4D), slow-moving cells with an irregular shape, as well as vibratile cells, have been described previously in various species of sea urchins (Bertheussen and Seljelid 1978; Smith and Davidson 1992; Matranga et al 2006). We found that sea star amoebocytes underwent rapid cytoplasmic fragmentation when illuminated under the microscope (not shown); conversely, vibratile cells had a very fast helicoidal movement (not shown). Hemocytes, another morphotype often found in holothurians and ophiuroids (Smith 1981), were rarely observed in this study. The term hemocyte (Smiley 1994) has been used to indicate those cells carrying compartmentalized highly pigmented regions of the cytoplasm (usually blue color), very dissimilar from red amoebocytes that carry natural red pigments uniformly dispersed within the cytoplasm. Together, amoebocytes, vibratile cells, and hemocytes comprised about 5% of the total population of cells circulating in A. rubens. The effects measured in the following are related to the whole population of coelomocytes.
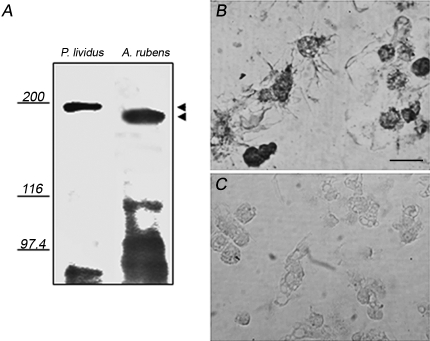
Fig 3.
McAb to sea urchin toposome recognizes sea star coelomocytes by immunoblotting and immunocytochemistry. (A) Coelomocyte lysates from Paracentrotus lividus (left lane), or Asterias rubens (right lane) were run on 6% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-Page), electro-blotted, and treated with antitopome McAb (see text for more details). Indicated on the left are the molecular weight markers: myoglobin 200 kDa, β-galactosidase 116 kDa, and phosphorylase b 97.4 kDa. Arrowheads indicate position of cross-reacting bands. (B) Free-circulating coelomocytes from untreated sea stars were fixed in 3% glutaraldehyde and labelled by anti-toposome McAb (see text for more details). (C) Negative control without primary antibody
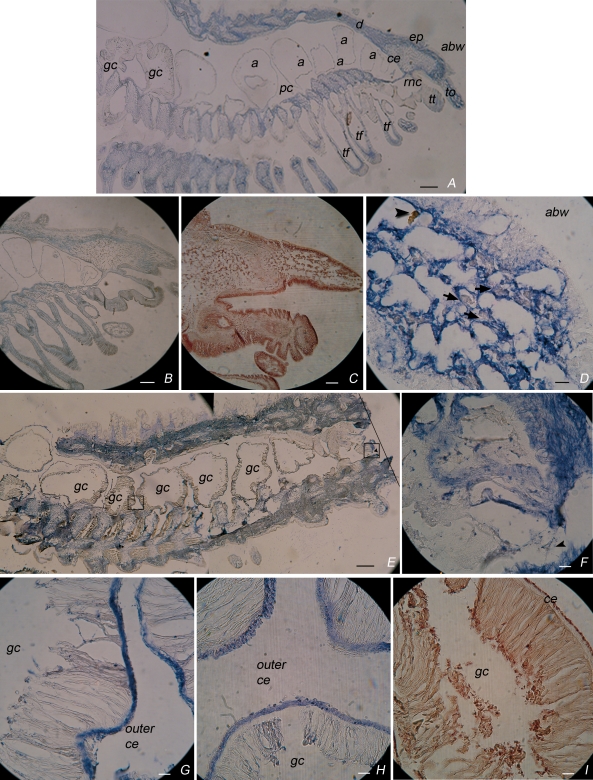
Fig 4.
Tracing coelomocytes in tissues of normal and amputated sea star arms. Longitudinal sections of (A–D) normal or (E–I) amputated arms, labelled with (A, D, E–H) anti-toposome McAb, (B) Domangk dye, or (C, I) hematoxylin dyes. The main recognizable anatomical features are: abw, aboral body wall; a, ampulla; ce, coeleomic epithelium, d, dermis; ep, epidermis; gc, gastric caecae; pc, perivisceral coelom; tf, tube feet; to, terminal ossicle; tt, terminal tentacle; rnc, radial nerve cord. On the right side (E) a line indicates the amputation plan. (D) Enlargement of the terminal ossicle shown in (A); arrowhead: red amoebocyte; arrows: white amoebocytes dispersed in the connective stroma of the skeletal plate. (F,G) Enlargements of the truncated stump and coelomic epithelium respectively, shown at lower magnification (E). Bar = 25 μm
Effects of post-traumatic stress on circulating coelomocytes
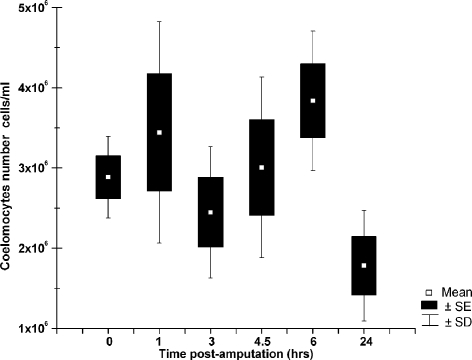
In our previous studies on sea urchin coelomocytes, we showed an increase in the number of red amoebocytes in response to exposure to pollutants, experimentally induced temperature stress, or natural injury/mutilations caused by predation (Matranga et al 2000), the latter being a stress comparable to that experimentally induced in the present study. This finding was explained either by the rapid division of circulating stem cells or the recruitment from tissue reservoirs. The post-traumatic stress of sea stars provided a very useful experimental model to test our previous hypotheses. Coelomocytes were collected (in anticoagulant solution) by cutting 1 arm tip from each of 8 replicate sea stars, at time intervals between 1 and 24 hours. This experimental procedure was designed because it is known that no clot formation is observed before 24 hours, because bleeding probably is stopped initially by muscle contraction at the arm tip (Moss et al 1998). In addition, we observed that all sea stars, once returned to the aquaria, for some time maintained their amputated arm in an upright position, presumably a mechanism for reducing loss of fluid from bleeding. Analysis of morphotype composition showed that removing the arm tip did not influence its normal distribution, with no appreciable variation in the relative number of phagocytes, amoebocytes, vibratile cells, or hemocytes (not shown). This is in contrast with what was shown for coelomocytes in sea urchins exposed to post-traumatic stress (Matranga et al 2000). Conversely, we found time-dependent variations in the circulating coelomocyte concentration of 8 independent animals in response to post-traumatic stress (Fig 2). Specifically, we found that, after the first hour post-amputation, the number of cells/mL in 6 different animals increased (4 out of 6), almost doubling in 1 case (from 2.9 to 5.8 × 106 cells per mL). In 1 animal, the number was similar to that recorded at time 0, ie, between 1.8 and 2 × 106 cells per mL, and in another it was lower than the number at time 0. Three hours post-amputation all animals showed a decrease in cell concentration. Then, a progressive increase in cell concentration was observed between 4.5 and 6 hours post-amputation in all cases scored (8 of 8) reaching 6 × 106 cells per mL. This was followed by a decrease at 24 hours post-amputation in all samples, when cell numbers were similar to those at time 0.
Fig 2.
Time-dependent modulation of coelomocyte cell density in amputated sea stars. Graphic representation of cell density numbers of 8 independent replicate amputated sea stars from which cells were harvested 0, 1, 3, 4.5, 6, and 24 hours post-amputation. SE, standard error; SD, standard deviation
Coelomocytes in normal and post-traumatic stressed animals
Because we found an overall increase in the number of freely circulating coelomocytes after post-traumatic stress, it was important to determine whether they originated from a specific tissue or from reservoir sites, as suggested for other echinoderms (see for a review Candia-Carnevali 2006). First, it was essential to have a specific cell marker able to identify sea star coelomocytes. We have previously shown that a monoclonal antibody (BEVIB12b8) produced against toposome, a 22S glycoprotein complex from Paracentrotus lividus embryos (Matranga et al 1986), whose precursor is found in the unfertilized egg (also known as major yolk protein [MYP]) label sea urchin coelomocytes (Cervello et al 1994). In order to validate the anti-toposome antibody as a potential coelomocyte marker in sea stars, we probed A. rubens coelomocytes by immunoblotting and immunocytochemistry (Fig 3). As expected, the antibody recognized a band of about 200 kDa in the P. lividus coelomocyte lysate used as positive control (Fig 3A, left lane). Interestingly, in the A. rubens lysate a single sharp band cross-reacted with the anti-toposome antibody with an apparent molecular size of 180 kDa (Fig 3A, right lane). The apparent discrepancy in the molecular mass probably can be explained on the basis of possible different-sized homologues present in 2 phylogenetically distant species. Low molecular weight smears observed at the bottom of the immunoblotting were interpreted as degradation products present in both lysates. In order to confirm the result obtained by immunoblotting we performed immunocytochemistry studies on isolated cells. We found that coelomocytes were strongly positive for the anti-toposome antibody (Fig 3B) as compared to control cells where the incubation with the primary antibody was omitted (Fig 3C).
Thus, we were confident to use the anti-toposome McAb to investigate by immunocytochemistry the specific location of labelled coelomocytes in the tissues. We used serial longitudinal sections of PFA-fixed sea star arms from controls and amputated animals 6 hours post-amputation, at which time we found the maximum increase in the number of freely circulating cells (see Fig 2). We found that immunoreactivity was restricted to the connective tissues in control sea stars (Fig 4A–D), as confirmed by the Domangk connective-specific staining of the same regions (compare Fig 4A to B). In particular, the labelling was observed in the body wall, within the dermal connective tissue including the skeletal ossicles (Fig 4A); in the enlargement of the terminal ossicle (Fig 4D) red and white amoebocytes are visible, dispersed in the connective stroma of the skeletal plate. In addition, anti-toposome staining was clearly detectable in cells of the coelomic epithelium (coelothelium) lining the coelomic canals of the stump. In contrast, no staining was found in the epidermis (see for comparison the nucleus-specific hematoxylin staining, Fig 4C). When we analyzed sections from post-traumatic-stressed sea stars fixed 6 hours post-amputation (Fig 4E–I), an increase in the staining of the body wall was observed (Fig 4E), as well as within cells (plausibly phagocytes) along the wound edge (Fig 4F, arrowhead). An increase in staining also was observed at the level of the gastric caeca, particularly in the outer coelothelium covering their external wall (Fig 4G,H). It should be mentioned here that tissue identification in the histological section (Fig 4E,F) was difficult because of the distortion caused by muscle contraction, which contributes to the wound closure. Single coelomocytes within the outer coelomic epithelium were also strongly labelled (Fig 4H), as confirmed by comparison with the nucleus-specific hematoxylin staining (Fig 4I).
Expression of Hsp70 in circulating coelomocytes after post-traumatic stress
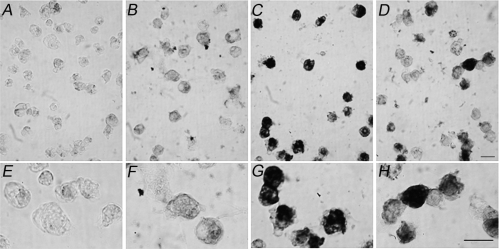
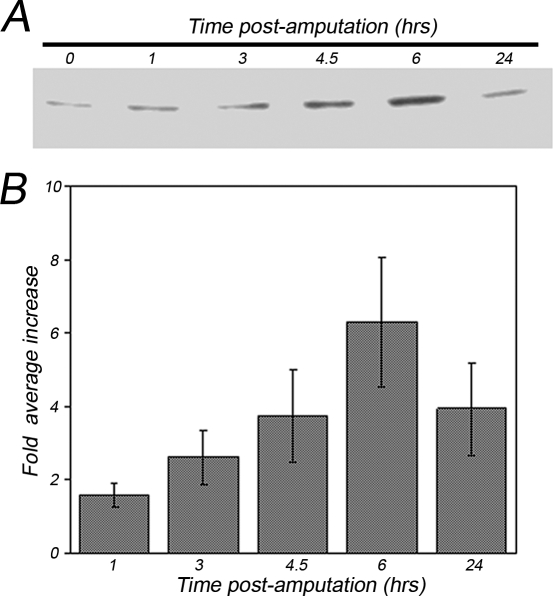
By analogy with other systems where Hsps have been described as good indicators of stress in response to various stimuli (Rylander et al 2005), it was of interest to determine if, during the first 24 hours following amputation, there was an activation of the 70 kDa heat shock protein (Hsp70). An immunocytochemical analysis was performed, using a commercially available anti-Hsp70 antibody, on glutaraldehyde-fixed coelomocytes collected from animals at 0, 6, and 24 hours post-amputation. Results of a representative experiment are shown in Figure 5. Because the antibody used recognizes both the constitutive and inducible Hsp70 isoforms, the detection of some basal levels of the protein was expected. Thus, in order to enhance the putative differences in Hsp70 immunostaining, we simultaneously stopped the staining reactions of all samples when time 0 controls were just above the background (compare Fig 5A,E with Fig 5B,F). Between 0 and 6 hours post-amputation we found a variability in the stress response elicited, in agreement with previous reports on other echinoderms (Matranga et al 2000). However, in all the experiments performed, the Hsp70 levels were clearly upregulated 6 hours post-amputation (Fig 5C,G) as compared to time 0 controls (Fig 5B,F). In coelomocytes collected 24 hours post-amputation, Hsp70 expression levels were always higher than time 0 control (Fig 5D,H) but less than those observed at 6 hours. In order to confirm and quantify the Hsp70 stress response elicited in sea stars after post-traumatic stress, Western blots were performed using coelomocyte lysates. Figure 6A shows results of a representative experiment on cells collected at 1, 3, 4.5, 6, and 24 hours post-amputation. Results from 6 independent experiments are shown in Figure 6B. Fold increases were calculated from the ratios between band intensity values at different times (from 1 to 24 hours) and those found at time 0 (assumed as basal Hsp70 level). The histogram reports the mean values obtained ± standard error (SE). We found a modest increase in the levels of the Hsp70 protein already after 1 hour; for longer periods (3 and 4.5 hours) a considerable increase in Hsp70 levels was observed, peaking at 6 hours, with a 6.2-fold average increase. After 24 hours, the Hsp70 levels decreased, although they remained about 4 times higher than time 0 controls. It should be noted that, as described for the immunocytochemistry experiments (see Fig 5) and as already reported for other systems (Matranga et al 2000), basal Hsp70 levels measured reflected a high variability between individual sea stars.
Fig 5.
Time-dependent increase in Hsp70 crossreactivity in coelomocyte preparations from amputated sea stars. Cells were collected from amputated arms at times: (A, E, B, F) 0, (C, G) 6, and (D, H) 24 hours, fixed in 3% glutaraldehyde and treated directly with the secondary antibody (A, E) as negative control or with anti-Hsp70 antibody (B–D, F–H). Bar = 25 μm
Fig 6.
Sea star coelomocytes respond to post-traumatic stress by a time-dependent increase in Hsp70 levels. (A) Time course of the Hsp70 expression analyzed by immunoblotting in coelomocytes collected 0, 1, 3, 4.5, 6, and 24 hours post-amputation. Equal amounts (15 μg) of lysates were separated by 7.5% sodium dodecyl sulfate– polyacrylamide gel electrophoresis (SDS-PAGE), electro-blotted, and treated with anti-Hsp70 antibody. (B) Quantitative analysis of Hsp70 expression measured by densitometric scanning of filters obtained from 6 independent experiments. Histogram represents the fold average increase calculated from the ratios between band intensity values at different times (from 1 to 24 h) and those found at time 0 assumed as 1 (basal Hsp70 level); obtained mean values ± standard error (SE)
DISCUSSION
The study of regenerative capacity in adult armed echinoderms, including asteroids, crinoids, and ophiuroids, is attracting increased attention because of the obvious implications for stem cell biology and research (Candia-Carnevali 2006). The reconstruction of a new organ after the original has been removed is an integral part of their adaptive system (Candia-Carnevali and Bonasoro 2001; Dupont and Thorndyke 2006) and starts after a first fast repair phase (Candia-Carnevali et al 1998; Thorndyke et al 2001). Previous reports indicated an initial stress response followed by massive intracellular protein turnover during regeneration in armed echinoderms (Patruno et al 2001). Other studies described the capacity of sea urchin coelomocytes to respond to a variety of experimentally induced stresses, including traumatic injuries; in all these cases a critical component activated upon stress and associated with the defense mechanism against injury was the Hsp70 protein (Matranga et al 2000, 2002, 2005, 2006).
In this report we described the cellular and biochemical responses of coelomocytes from the common European sea star A. rubens, subjected to a traumatic stress induced in the laboratory by arm amputation mimicking predation. The crucial time period analyzed was the repair phase following the first 24 hours post-amputation. As a prerequisite for the analysis of stress response at the cellular level, the characterization of all cell types present first was taken into consideration. We identified by light microscopy 4 different cell types contained in the coelomic fluid: phagocytes, white and red amoebocytes, vibratile cells, and hemocytes. It has been known for some time that echinoderm coelomocytes fall into different categories but there is considerable discrepancy in the use of a common classification. Some reports describe the same or similar cell types present in different species or classes using different nomenclature (see Matranga et al 2006). However, the final morphological characterization can be provided only after careful and detailed analyses. A complete characterization of coelomocytes by transmission electron microscopy (TEM) and scanning electron microscopy (SEM) is provided for crinoids (Candia-Carnevali and Bonasoro 2001).
We found that, among the freely circulating cells, phagocytes represent the most abundant population, in agreement with reports on the occurrence of phagocytes in a few sea urchin species (Bertheussen and Seljelid 1978; Smith et al 1992; Matranga and Bonaventura 2002; Matranga et al 2006). All the other cell types present constitute only about 5% of the total, in contrast with data from sea urchins where, depending on the species, white and red amoebocytes and vibratile cells (hemocytes are not present in sea urchins), constitute 20% to 34% of the total population (Smith et al 1992; Matranga et al 2006). Because phagocytes constitute about 95% of the total A. rubens population, they are likely to account for nearly all of the biochemical processes described in this study. However, the contribution of other cell types cannot be excluded. On the basis of present results we confirmed that the concentration of circulating coelomocytes is comprised between 2 and 5 × 106 cells per mL, in good agreement with the only other recent report where coelomocyte concentration was found to be between 3 and 9 × 106 cells per mL (Coteur et al 2004). Thus, this range of values can be used as an index of viability in most studies using A. rubens. Interestingly, we found that the number of circulating cells underwent a mild time-dependent modulation in amputated sea stars. The progressive increase in cell number, observed between 4.5 and 6 hours post-amputation, can be explained by either the rapid division of circulating stem cells or by their recruitment from specific niches in the epithelial tissue (or other areas). Both hypotheses are interesting, although experimental confirmation of the relative importance of either phenomenon is awaited.
In this study we found no difference in the normal distribution of cell types, during the early post-traumatic phases. This result is quite incompatible with that reported for sea urchins, where 1 minor cell group (red amoebocytes) showed a great expansion in animals collected from polluted seawater or subjected to “accidental” injury (Matranga et al 2000, 2002, 2005, 2006). We do not have an explanation for this discrepancy, other than perhaps different mechanisms operating in the 2 phylogenetically distant echinoderm species with morphologically divergent body shapes (armed asteroids as opposed to globose, nonarmed echinoderms, not subjected to autotomy of relevant body appendices, apart from spines and pedicellariae) and thus provided with different defense strategies and mechanisms against predation and trauma. The post-traumatic arm of sea stars provided a good model for analyzing the origin of coelomocytes in the attempt to reinforce the notion that coelomocytes are produced in the coelomic epithelium, as reported by earlier studies (Bossche and Jangoux 1976). Our assumption was that a stressful event would have caused either self-replication of free cells or proliferation in the so-called cytopoietic (hematopoietic) organs or tissues. To facilitate this analysis we employed a previously characterized anti-toposome McAb, which is a coelomocyte-specific marker (Cervello et al 1994). Immunoreactivity was restricted to cells of the coelomic epithelium lining the coelomic canals of the stump, thus confirming older reports. It is noteworthy that all the above-mentioned areas were found more intensely labelled 6 hours post-amputation (when an increase in freely circulating coelomocytes is apparent), reinforcing the notion of the coelothelial origin of coelomocytes. This is in agreement with what was observed in other echinoderms: in fact, a massive cell proliferation occurring during the first 24 hours post-amputation has been demonstrated in the crinoid Antedon mediterranea by BrdU Incorporation at the level of the cicatricial layer along the length of the brachial nerve and the coelomic wall, as well as in free cells in the coelomic canals (Candia-Carnevali et al 1995) and this recruitment of coelomocytes from the coelothelial wall gives a continuous contribution through the overall regeneration period (Candia-Carnevali and Bonasoro 2001). In response to metabolic disturbances and injuries, all cells from any organism mount a stress response with the induction of a variety of proteins with the 70-kDa heat shock protein notable as being perhaps one of the most extensively studied. The elevation of Hsp70 levels in coelomocytes from other echinoderms in response to external insults has been reported previously (Matranga et al 2000; reviewed in Matranga et al 2005). As far as the sea star is concerned, the high levels of Hsp70 measured in coelomocytes from amputated animals could be interpreted as a prerequisite for subsequent regenerative capability. Indeed, molecular approaches to the analysis of echinoderm regeneration have inferred the involvement of Hsp70 as well as growth factors (Patruno et al 2001). In agreement with this, recent studies on limb regeneration in vertebrates (axolotl) demonstrated an upregulation of the RNA transcript for Hsp70 as early as 24 hours following amputation (Levesque et al 2005), confirming earlier reports describing an increased synthesis and accumulation of a 73-kDa protein (Carlone et al 1993). Other studies showed a coordinated expression of Hsp40 and Hsp70 as part of a stress response system operating after injury in zebrafish (Tawk et al 2000). A recent accumulation of literature suggests the use of sea stars as sentinel organisms for the assessment of environmental pollution. In such studies the effects of contaminants such as heavy metals (cadmium, lead, zinc, copper) and PCBs have been studied both in vivo (laboratory experiments) and in situ (field studies in contaminated areas) and correlated to innate immune responses, as measured by the elevation of (1) reactive oxygen species (ROS) (Coteur et al 2003), (2) cytochrome P450 (Danis et al 2006), and (3) Hsp70 (Matranga et al, in preparation). The machinery for sensing stress is thus activated as proven by the expression of stress markers (ROS, P450, Hsp70, others) and therefore it is suggested that sea star coelomocytes can be used profitably as a model system for ecotoxicological studies. Here we used sea star coelomocytes to study the stress response in the laboratory and propose Hsp70 as an ideal biomarker for this phenomenon in starfish. Recent findings postulate a role for inhibitors of apoptotic pathways for Hsp70, thus increasing the chances of survival of cells (Sreedhar and Csermely 2004). On the other hand, as already shown in other systems, stem cells are presumed to survive various stresses and to be recruited later to areas of tissue damage and regeneration, where inflammatory cytokines and cytotoxic cells may result in severe cell injury. Despite the elevated Hsp70 levels found in cells in response to traumatic events, at the moment we have no evidence that Hsp70 is actively released in the surrounding medium to induce a “bystander effect” on other cells. It has been reported that, in addition to serving as molecular chaperones, heat shock proteins (particularly Hsp60, Hsp70, Hsp90, and gp96) may be potent activators of the innate immune system capable of inducing the production of proinflammatory cytokines by the monocyte-macrophage system (Tsan and Gao 2004). The recent survey of the sea urchin genome for genes associated with immunity (Hibino et al 2006; Sea Urchin Genome Consortium 2006), together with data obtained from in vivo and in vitro experiments on coelomocytes (this paper; Matranga et al 2000, 2002, 2005, 2006), supports the requirement (existence) of an autocrine loop for the regulation of cell replacement, growth, and death. Thus, Hsp70, after being overexpressed in response to external stimuli (such as trauma, variation in body temperature, exposure to pollutants or UV-B radiation), could be released in the extracellular medium (A. Pinsino, unpublished data). A similar event has been demonstrated for the toposome precursor in sea urchin coelomocytes subjected to a centrifugal stress (Cervello et al 1994). Increasing evidence comes from the literature on the role played by Hsp70 in eliciting cell division in other systems (Levesque et al 2005). In favor of this hypothesis are earlier reports on the stimulation of coelomocytes by IL-1 (Burke and Walkins 1991). Although no homologue of IL-1 was identified in the sea urchin genome (Hibino et al 2006), the presence of IL-1 receptor–like and Sp-ICE-like genes are consistent with the occurrence of an IL-1–like gene. Recent findings demonstrated tumor necrosis factor (TNF) alpha overexpression in sea urchin coelomocytes exposed to physical (temperature or UV-B radiation) stress (Madarazs et al, in preparation), in accord with sequence models predicting at least 4 TNF ligands and 8 TNF receptors in the echinoid genome (Robertson et al 2006). Now that genomic tools are available, a major revision of the traumatic stress and regeneration processes is possible and should make a significant contribution to our understanding of the operating mechanisms.
Acknowledgments
The authors wish to express their gratitude to the anonymous reviewers who improved the quality of the manuscript by their relevant suggestions. This research has been partially supported by a visiting program of the European Network of Excellence “Marine Genomics Europe” (GOCE-CT-2004-505403). One of us (A.P.) has been supported by a scholarship from the University of Palermo. V.M. acknowledges partial support from the EU Project RedCod (contract B4-3070/2003/368585/SUB/D.3) and the Italian Space Agency Project MoMa (contract 1/ 014/06/0). M.C.T. acknowledges support of the Swedish Research Council, Wallenberg Foundation, Broms Fund, Crafoord Fund, and Royal Swedish Academy of Sciences. Authors are indebted to K. Holm and B. Hernroth for helpful discussion and comments, as well as advice with experimental techniques.
REFERENCES
- Becker J, Craig EA. Heat shock proteins as molecular chaperones. Eur J Biochem. 1994;219:11–23. doi: 10.1007/978-3-642-79502-2_2.0014-2956(1994)219[0011:HSPAMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bertheussen K, Seljelid R. Echinoid phagocytes in vitro. Exp Cell Res. 1978;111:401–412. doi: 10.1016/0014-4827(78)90185-4.0014-4827(1978)111[0401:EPIV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bossche JP, Jangoux M. Epithelial origin of starfish coelomocytes. Nature. 1976;261:227–228. doi: 10.1038/261227a0.1476-4687(1976)261[0227:EOOSC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Buchner J. Supervising the fold: functional principles of molecular chaperones. FASEB J. 1996;10:9–10.0892-6638(1996)010[0009:STFFPO]2.0.CO;2 [PubMed] [Google Scholar]
- Burke RD, Watkins RF. Stimulation of starfish coelomocytes by interleukin-1. Biochem Biophys Res Comm. 1991;180:579–584. doi: 10.1016/s0006-291x(05)81104-0.0006-291X(1991)180[0579:SOSCBI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Byrne M. The mechanical properties of the autotomy tissues of the holothurian Eupentacta quinquesemita and the effects of certain physico-chemical agents. J Exp Biol. 1985;117:69–86.0022-0949(1985)117[0069:TMPOTA]2.0.CO;2 [Google Scholar]
- Candia-Carnevali MD. Regenerative response and endocrine disrupters in crinoid echinoderms: an old experimental model, a new ecotoxicological test. Prog Mol Subcell Biol. 2005;39:167–200. doi: 10.1007/3-540-27683-1_8.0079-6484(2005)039[0167:RRAEDI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Candia-Carnevali MD. Regeneration in Echinoderms: repair, regrowth, cloning. ISJ. 2006;3:64–76.1824-307X(2006)003[0064:RIERRC]2.0.CO;2 [Google Scholar]
- Candia-Carnevali MD, Bonasoro F, Lucca E, Thorndyke MC. Pattern of cell proliferation in the early stages of arm regeneration in the feather star Antedon mediterranea. The Journal of Experimental Zoology. 1995;272:464–474.1097-010X(1995)272[0464:POCPIT]2.0.CO;2 [Google Scholar]
- Candia-Carnevali MD, Bonasoro F, Patruno M, Thorndyke MC. Cellular and molecular mechanisms of arm regeneration in crinoid echinoderms: the potential of arm explants. Dev Genes Evol. 1998;208:421–430. doi: 10.1007/s004270050199.0949-944X(1998)208[0421:CAMMOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Candia-Carnevali MD, Bonasoro F. A microscopic overview of crinoid regeneration. Microsc Res Tech. 2001;55:403–426. doi: 10.1002/jemt.1187.1059-910X(2001)055[0403:AMOOCR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Carlone RL, Boulianne RP, Vijh K, Karn H, Fraser GA. Retinoic acid stimulates the synthesis of a novel heat shock protein in the regenerating forelimb of the newt. Biochem Cell Biol. 1993;71:43–50. doi: 10.1139/o93-007.1208-6002(1993)071[0043:RASTSO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cervello M, Matranga V. Evidence of a precursor-product relationship between vitellogenin and toposome, a glycoprotein complex–mediating cell adhesion. Cell Differ Dev. 1989;26:67–76. doi: 10.1016/0922-3371(89)90784-3. [DOI] [PubMed] [Google Scholar]
- Cervello M, Arizza V, Lattuca G, Parrinello N, Matranga V. Detection of vitellogenin in a subpopulation of sea urchin coelomocytes. Europ J Cell Biol. 1994;64:314–319.0171-9335(1994)064[0314:DOVIAS]2.0.CO;2 [PubMed] [Google Scholar]
- Coteur G, Gillan D, Joly G, Pernet P, Dubois P. Field contamination of the starfish Asterias rubens by metals. Part 2: Effects on cellular immunity. Environ Toxicol Chem. 2003;22:2145–2151. doi: 10.1897/02-490.1552-8618(2003)022[2145:FCOTSA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Coteur G, Corriere N, Dubois P. Environmental factors influencing the immune responses of the common European starfish (Asterias rubens) Fish Shellfish Immunol. 2004;16:51–63. doi: 10.1016/s1050-4648(03)00030-5.1050-4648(2004)016[0051:EFITIR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Danis B, Wantier P, Flammang R, Pernet P, Chambost-Manciet Y, Coteur G, Warnau M, Dubois P. Bioaccumulation and effects of PCBs and heavy metals in sea stars (Asterias rubens, L.) from the North Sea: a small-scale perspective. Sci Total Environ. 2006;356:275–289. doi: 10.1016/j.scitotenv.2005.05.029.0048-9697(2006)356[0275:BAEOPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dupont S, Thorndyke MC. Growth or differentiation? Adaptive regeneration in the brittlestar Amphiura filiformis. J Exp Biol. 2006;209:3873–3881. doi: 10.1242/jeb.02445.0022-0949(2006)209[3873:GODARI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Garcia-Arraras JE, Estrada-Rodgers L, Santiago R, Torres II, Diaz-Miranda L, Torres-Avillan I. Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea:Echinodermata) J Exp Zool. 1998;28:288–304. doi: 10.1002/(sici)1097-010x(19980701)281:4<288::aid-jez5>3.0.co;2-k.1097-010X(1998)028[0288:CMOIRI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Glinski Z, Jarosz J. Immune phenomena in echinoderms. Archivum Immunologiae et Therapiae Experimentalis. 2000;48:189–193.0004-069X(2000)048[0189:IPIE]2.0.CO;2 [PubMed] [Google Scholar]
- Henson JH, Nesbitt D, Wright BD, Scholey JS. Immunolocalization of kinesin in sea urchin coelomocytes: association of kinesis with intracellular organelles. J Cell Sci. 1992;103:309–320. doi: 10.1242/jcs.103.2.309.0021-9533(1992)103[0309:IOKISU]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hibino T, Loza-Coll M, and Messier C. et al. 2006 The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol. 300:349–365. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0.1476-4687(1970)227[0680:COSPDT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Levesque M, Guimond JC, Pilote M, Leclerc S, Moldovan F, Roy S. Expression of heat shock protein 70 during limb development and regeneration in the axolotl. Dev Dyn. 2005;233:1525–1534. doi: 10.1002/dvdy.20458.1058-8388(2005)233[1525:EOHSPD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Matranga V, Kuwasaki B, Noll H. Functional characterization of toposomes from sea urchin blastula embryos by a morphogenetic cell aggregation assay. EMBO J. 1986;5:3125–3132. doi: 10.1002/j.1460-2075.1986.tb04619.x.1460-2075(1986)005[3125:FCOTFS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matranga V, Toia G, Bonaventura R, Muller WEG. Cellular and biochemical responses to environmental and experimentally induced stress in sea urchin coelomocytes. Cell Stress & Chaperones. 2000;5:158–165. doi: 10.1379/1466-1268(2000)005<0113:cabrte>2.0.co;2.1466-1268(2000)005[0158:CABRTE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matranga V, Bonaventura R 2002 Sea urchin coelomocytes, the progenitors of vertebrate immune effectors, as bioindicators of stress and pollution. In: The Sea Urchin: From Basic Biology to Aquaculture, ed Yokota Y, Matranga V, Smolenicka Z. Swets and Zeitlinger, Lisse, The Netherlands, 161–176. [Google Scholar]
- Matranga V, Bonaventura R, Di Bella G. Hsp70 as a stress marker of sea urchin coelomocytes in short term cultures. Cell Mol Biol. 2002;48:345–359.0145-5680(2002)048[0345:HAASMO]2.0.CO;2 [PubMed] [Google Scholar]
- Matranga V, Pinsino A, Celi M, Natoli A, Bonaventura R, Schröder HC, and Müller WEG 2005 Monitoring chemical and physical stress using sea urchin immune cells. In: Echinodermata, ed Matranga V. Springer, Heidelberg, 85–110. [DOI] [PubMed] [Google Scholar]
- Matranga V, Pinsino A, Celi M, Di Bella G, Natoli A. Impacts of UV-B radiation on short-term cultures of sea urchin coelomocytes. Marine Biology. 2006;149:25–34.0025-3162(2006)149[0025:IOUROS]2.0.CO;2 [Google Scholar]
- Millot N. Injury and the axial organ of echinoids. Experientia. 1969;25:756–757.0014-4754(1969)025[0756:IATAOO]2.0.CO;2 [Google Scholar]
- Moseley P. Stress proteins and the immune response. Immunopharmacology. 2000;48:299–302. doi: 10.1016/s0162-3109(00)00227-7.0162-3109(2000)048[0299:SPATIR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Moss C, Hunter J, Thorndyke MC. Pattern of bromodeoxyuridine incorporation and neuropeptide immunoreactivity during arm regeneration in the starfish Asterias rubens. Phil Trans R Soc London B. 1998;353:421–436.0261-0523(1998)353[0421:POBIAN]2.0.CO;2 [Google Scholar]
- Muñoz-Chápuli R, Carmona R, Guadix JA, Macías D, Pérez-Pomares JM. The origin of the endothelial cells: an evo-devo approach for the invertebrate/vertebrate transition of the circulatory system. Evolution & Development. 2005;7:351–358. doi: 10.1111/j.1525-142X.2005.05040.x.1525-142X(2005)007[0351:TOOTEC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Noll H, Matranga V, Cervello M, Humphreys T, Kuwasaki B, Adelson D. Characterization of toposomes from sea urchin blastula cells: a cell organelle mediating cell adhesion and expressing positional information. Proc Natl Acad Sci U S A. 1985;82:8062–8066. doi: 10.1073/pnas.82.23.8062.1091-6490(1985)082[8062:COTFSU]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun. 2003;304:505–512. doi: 10.1016/s0006-291x(03)00623-5.0006-291X(2003)304[0505:HSPCCT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Patruno M, Thorndyke MC, Candia Carnevali MD, Bonasoro F, Beesley PW. Growth factors, heat shock proteins, and regeneration in Echinoderms. J Exp Biol. 2001;204:843–848. doi: 10.1242/jeb.204.5.843.0022-0949(2001)204[0843:GFHSPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Robert J. Evolution of heat shock protein and immunity. Dev Comp Immunol. 2003;27:449–464. doi: 10.1016/s0145-305x(02)00160-x.0145-305X(2003)027[0449:EOHSPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Robertson AJ, Croce J, Carbonneau S, Voronina E, Miranda E, McClay DR, Coffman JA. The genomic underpinnings of apoptosis in Strongylocentrotus purpuratus. Dev Biol. 2006;300:321–334. doi: 10.1016/j.ydbio.2006.08.053.1095-564X(2006)300[0321:TGUOAI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rylander MN, Feng Y, Bass J, Diller KR. Thermally induced injury and heat shock protein expression in cells and tissues. Ann N Y Acad Sci. 2005;1066:222–242. doi: 10.1196/annals.1363.009.0077-8923(2005)1066[0222:TIIAHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Scaturro G, Zito F, Matranga V. The oligomeric integrity of toposome is essential for its morphogenetic function. Cell Biology International. 1998;22:321–326. doi: 10.1006/cbir.1998.0260.1065-6995(1998)022[0321:TOIOTI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sea Urchin Genome Sequencing Consortium. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–952. doi: 10.1126/science.1133609.0193-4511(2006)314[0941:TGOTSU]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley S 1994 Holothurioidea. In: Microscopic Anatomy of Invertebrate, ed Harrison FW, Chia FS. vol. 14: Echinodermata. Wiley-Liss, New York, 401–471. [Google Scholar]
- Smith LC, Davidson EH. The echinoid immune system and the phylogenetic occurrence of immune mechanisms in deuterostomes. Immunol Today. 1992;13:356–362. doi: 10.1016/0167-5699(92)90172-4.0167-5699(1992)013[0356:TEISAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith LC, Davidson EH. The echinoderm immune system: characters shared with vertebrate immune systems and characters arising later in deuterostome phylogeny. Ann NY Acad Sci. 1994;712:213–226. doi: 10.1111/j.1749-6632.1994.tb33575.x.0077-8923(1994)712[0213:TEISCS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith VJ 1981 The echinoderm. In: Invertebrate Blood Cells, ed Ratcliffe NA, Rowley AF. Academic Press, London, 513–562. [Google Scholar]
- Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy. A comprehensive review. Pharmacology & Therapeutics. 2004;101:227–257. doi: 10.1016/j.pharmthera.2003.11.004.0163-7258(2004)101[0227:HSPITR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tawk M, Joulie C, Vriz S. Zebrafish Hsp40 and Hsc70 genes are both induced during caudal fin regeneration. Mech Dev. 2000;99:183–186. doi: 10.1016/s0925-4773(00)00478-0.1872-6356(2000)099[0183:ZHAHGA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thorndyke MC, Chen WC, Beesley PW, Patruno M. Molecular approach to echinoderm regeneration. Microsc Res Tech. 2001;55:474–485. doi: 10.1002/jemt.1192.1059-910X(2001)055[0474:MATER]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350.1091-6490(1979)076[4350:ETOPFP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Heat shock protein and innate immunity. Cellular & Molecular Immunology. 2004;1:274–279. [PubMed] [Google Scholar]