Abstract
Hsp90 is an abundant and highly conserved chaperone that functions at later stages of protein folding to maintain and regulate the activity of client proteins. Using a recently described in vitro system to fold a functional model kinase Chk1, we performed a side-by-side comparison of the Hsp90-dependent chaperoning of Chk1 to that of the progesterone receptor (PR) and show that these distinct types of clients have different chaperoning requirements. The less stable PR required more total chaperone protein(s) and p23, whereas Chk1 folding was critically dependent on Cdc37. When the 2 clients were reconstituted under identical conditions, each client folding was dose dependent for Hsp90 protein levels and was inhibited by geldanamycin. Using this tractable system, we found that Chk1 kinase folding was more effective if we used a type II Hsp40 cochaperone, whereas PR is chaperoned equally well with a type I or type II Hsp40. Additional dissection of Chk1-chaperone complexes and the resulting kinase activity suggests that kinase folding, like that previously shown for PR, is a dynamic, multistep process. Importantly, the cochaperones Hop and Cdc37 cooperate as the kinase transitions from immature Hsp70- to mature Hsp90-predominant complexes.
INTRODUCTION
Efficient folding of cellular proteins often requires the assistance of at least 1 family of molecular chaperones that function in complex, multiprotein machines (Hartl and Hayer-Hartl 2002). The molecular chaperone Hsp90 plays a critical role in the folding, maturation, and stability of an expanding list of proteins needed for cellular homeostasis as well as cellular responses to external signals or stress (Young et al 2001). Among these “client” proteins are the steroid receptors and many key regulatory kinases (Citri et al 2006). The molecular mechanisms for Hsp90-dependent chaperoning have been extensively studied for the progesterone (PR) and glucocorticoid (GR) receptors (Pratt and Toft 1997; Wegele et al 2004; Zhao and Houry 2005; Picard 2006). This chaperoning depends on Hsp90's association with a number of cochaperones and is intimately associated with and dependent on the folding activity of Hsp70 and its cofactors. A purified system consisting of 5 proteins (ie, Hsp70, Hsp40, Hop, Hsp90, and p23) has been an invaluable complement to genetic and structural studies to give us a clearer understanding of chaperone function (Kosano et al 1998).
Genome database studies suggest that about 1.7% of all human genes encode for protein kinases (Manning et al 2002). These kinases mediate and control many cellular processes, including transcription, cell cycle progression, apoptosis, and differentiation. In every subgroup of the kinome is situated 1 or more central members that require the molecular chaperone Hsp90 for their function (Citri et al 2006). Much of what we know about the folding of kinases has come from studies using yeast or cellular extracts. As a first step toward dissecting the steps involved in kinase folding, we recently described a purified system to reconstitute the activity of the checkpoint regulatory kinase Chk1 (Arlander et al 2006). Chk1 is an Hsp90 client that regulates cellular checkpoints in response to DNA damage (Arlander et al 2003), and inhibition of Hsp90 by the geldanamycin analog 17-AAG sensitizes cells to radiation-induced damage and cell cycle arrest. Unlike PR, which has a continuous need for chaperoning in the absence of hormone, Chk1 requires Hsp90 only during its synthesis and initial processing. The native Chk1 protein is independently stable (Arlander et al 2006). The purified model system for chaperoning Chk1 uses the core Hsp70-Hop-Hsp90 complex to reconstitute an active kinase, but this system differs from the established PR system in several important ways. The cochaperone p23 is dispensable for chaperoning Chk1, and Chk1 reconstitution requires the addition of the cochaperone Cdc37 and its phosphorylation by casein kinase II (CK2). In addition, our initial studies suggested that Chk1 and PR differ in the amount of Hsp90 protein necessary for folding in vitro.
The ability to manipulate the in vitro system presented us with the opportunity to characterize, in more detail, key steps in Hsp90-dependent kinase folding. By altering the composition of chaperones in a head-to-head study of PR and Chk1, we tested whether the intrinsic stability of a client correlated with the amount of various chaperones needed to reconstitute its activity in vitro and whether the requirement for Hsp90 directly determined its sensitivity to the drug geldanamycin. We also documented the differences in Hsp40 requirements for PR and Chk1 folding. Order-of-addition and chaperone dropout experiments suggest that the chaperoning of kinase clients by Hsp90 shares an overall strategy previously shown for steroid receptors in that it is (1) dynamic, (2) a stepwise process, and one that (3) requires presentation of the kinase to Hsp90 after it has been chaperoned by Hsp70 and its cochaperone Hsp40. These data expand our knowledge of kinase folding and predict the requirement for intermediate steps in which both Cdc37 and Hop modulate Hsp90 chaperoning. We present a model to help understand these processes and focus future studies.
MATERIALS AND METHODS
Proteins and antibodies
All protein preparations used in these studies have been described previously. Chicken PR was expressed in Sf9 cells that had been coinfected with p23 expression virus (Cintron and Toft 2006). Human Chk11–265 was expressed in Escherichia coli as a GST fusion (Arlander et al 2006). The expression and purification of human Hsp90β, Hop, Hsp70, Hsp40 (Ydj1, DJB1, DJA1), p23, and Cdc37 have been described previously (Kosano et al 1998; Hernandez et al 2002b; Arlander et al 2006). Casein Kinase II (CK2) was obtained from Sigma (C-3460). The monoclonal antibodies PR22, BB70, and H9010 have been described previously (Sullivan et al 1986; Smith et al. 1993). Antibodies to DJB1 (SPA450, Stressgen) and Cdc37 (MA3-029, Affinity Bioreagents) were from commercial sources.
Chaperoning assays (in vitro reconstitutions)
PR from Sf9 cell lysate was immobilized using PR22 antibody bound to protein A-Sepharose and stripped of associated cellular proteins similar to what has been previously described (Cintron and Toft 2006). Sf9 lysate (0.7 mL) was diluted into a final volume of 3.5 mL stripping buffer (20 mM Tris pH 7.4, 500 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol (DTT), 0.1% Triton X-100, 5 mM adenosine triphosphate [ATP]) and incubated on ice for 30 minutes. PR22 resin, enough for 8–12 reactions, was added to the lysate, and the mixture was rocked in the cold and on ice for 1.5 hours. After a brief centrifugation (∼1 minute at 1000 × g), a second round of stripping (30 minutes in 1 mL of buffer) was used to facilitate full dissociation of endogenous chaperones from the PR. The resin-bound PR was washed 3 times with 1 mL of cold reaction buffer (see below) without detergent. Reconstitution was accomplished by the addition of purified chaperone proteins and ATP and incubation at 30°C as previously described (Kosano et al 1998). Further details regarding the amount of proteins used are shown in Figure 1. The reconstitution reaction mixtures were then placed on ice and incubated with [3H]Progesterone for 1.5 hours. After washing with cold reaction buffer (20 mM Tris pH 7.4, 50 mM KCl, 5 mM MgCl2, 1 mM DTT, 0.01% Triton X-100), bound hormone was measured by scintillation counting a portion of each sample. Protein complexes of reconstituted PR were verified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Coomassie Blue.
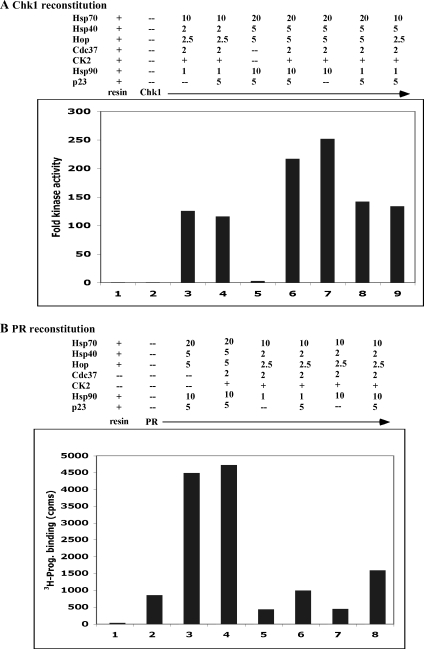
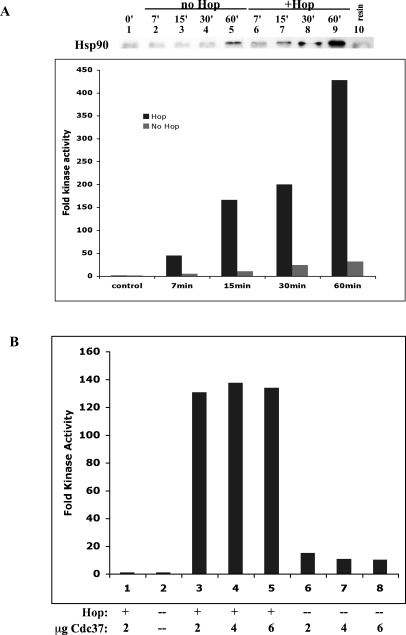
Fig 1.
Chaperone composition and amounts required are different for in vitro reconstitution of Chk1 and PR. (A) Kinase activity of purified GST-Chk11–265 was reconstituted using conditions developed previously (control conditions, lane 3 = Hsp70, Hsp40, Hop, Hsp90, Cdc37, CK2 [0.07 units]) and compared to reconstitution using conditions used for PR (typical 5p, lane 5 = Hsp70, Hsp40, Hop, Hsp90, p23). All reconstitutions were conducted for 1 hour at 30°C. Different combinations or different amounts (in micrograms) of various chaperones were as indicated. (B) Sf9-expressed PR was stripped of cellular proteins. Hormone-binding activity was reconstituted under PR control conditions (lane 3), Chk1 conditions (lane 5), or with variations as indicated. Chk1 activity is expressed as the fold increase in phosphorylation of the substrate Cdc25C by the chaperoned kinase compared to that of the unchaperoned control. PR activity is expressed as counts per minute (cpms) of [3H]-progesterone bound
Bacterially expressed GST-Chk11–265 was shown previously to reconstitute the same as full-length kinase (Arlander et al 2006) yet was more stable to storage and thus was utilized for all the Chk1 reconstitutions described here. GST-Chk11–265 was immobilized onto glutathione-agarose beads (Glutathione-Uniflow resin, Clontech) by incubating the protein and resin for 1 hour at 4°C in GST buffer (20 mM Tris pH 7.4, 0.27 M sucrose, 1% Triton X-100, 1 mM ethylenediamine tetraacetic acid, 1 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N;prN;pr-tetraacetic acid (EGTA), 5 mM sodium pyrophosphate, 5 mM NaF, and freshly added 1 mM sodium orthovanadate, 20 mM β-glycerophosphate, and 0.1% 2-mercaptoethanol) plus 0.5 M NaCl with constant rotation. The resin-bound client was washed twice with the same buffer and 3 times with cold PR reaction buffer, without detergent, prior to use. After reconstitution with various chaperones and ATP, the resin-bound complexes were washed with cold wash buffer (GST buffer plus 1 M NaCl and 0.025% SDS) and then cold kinase buffer (50 mM Tris pH 7.4, 10 mM MgCl2, 1 mM DTT). Kinase activity was measured by incubation with Cdc25C substrate and [γ-32P]ATP for 10 minutes at 30°C. The kinase reactions were stopped by the addition of SDS sample buffer. The final samples, containing Chk1, remaining chaperones, and substrate, were resolved by SDS-PAGE and transferred to Immobilon-P membrane. Membrane-bound radiolabeled substrate protein was detected and quantitated using a Storm 840 PhosphorImager. Fold increase in kinase activity was determined by dividing the activity of the reconstituted sample by the activity in the unchaperoned kinase sample. Bound chaperones were detected by sequential immunoblotting.
RESULTS
Hsp90 cochaperone differences for reconstituting the client proteins Chk1 (a model kinase) and PR (a model steroid receptor)
Arlander and coworkers (2006) established that a mixture of Hsp90, Hop, Hsp70, Ydj1 (yeast Hsp40), Cdc37, and CK2 was sufficient to reconstitute Chk1 kinase activity to Chk1-GST bound to glutathione-agarose. Kinase activity, once restored by this mixture of chaperones, was stable to very stringent washing. This degree of stability to washing had not been our experience with PR reconstitution, which we routinely perform using higher amounts of Hsp90 and the addition of the cochaperone p23. We tested whether reconstituting PR using the newer conditions for Chk1 would better stabilize PR to stringent washing but found that PR was still quite labile (not shown). We then more rigorously tested whether the receptor and kinase model clients differed in their net requirements for chaperoning by asking how well each client could be reconstituted under the conditions developed for the other client. The reconstitutions were for 1 hour at 30°C, and thus the success of folding each client was tested as the chaperoning was near completion.
In Figure 1A, Chk1 reconstitution is shown for its control conditions (lane 3) or a number of conditions that approach PR-reconstituting conditions (lanes 4–9). The typical PR-reconstituting conditions that do not contain Cdc37 and CK2 were not effective for reconstituting Chk1 kinase activity (lane 5). The addition of Cdc37 and CK2 to this condition profoundly increased kinase reconstitution (lanes 6–9). Kinase reconstitution was unaffected by the addition of p23 to either Chk1 control conditions (lane 4) or PR conditions containing Cdc37/CK2 (lane 6, compared to 7). The standard conditions for Chk1 (1 μg, lane 3) could be improved up to 2-fold by the use of 10 μg Hsp90 protein (lanes 6 and 7 compared to lanes 8 and 9). In contrast, additional Hsp70, Hsp40, or Hop was not necessary (lanes 8 and 9 compared to lane 4).
In Figure 1B, PR reconstitution is shown under our “5 protein” PR control conditions (lane 3) or under Chk1-like reconstituting conditions (lanes 4–8). The addition of Cdc37 and CK2 had neither a positive nor a negative effect on PR reconstitution (lane 4). The typical Chk1 conditions were not sufficient to reconstitute PR hormone binding (lane 5). The addition of p23 to these conditions resulted in only a modest increase in PR activity (lane 6) unless more Hsp90 was also added (lane 8). These results reinforce the model that p23 is a cochaperone for Hsp90 and is particularly important for the chaperoning of steroid receptors (Grad et al 2006). PR reconstitution was much better when the amounts of Hsp70, Hsp40, and Hop also were higher than what was needed to reconstitute Chk1 (lane 8 compared to lane 4).
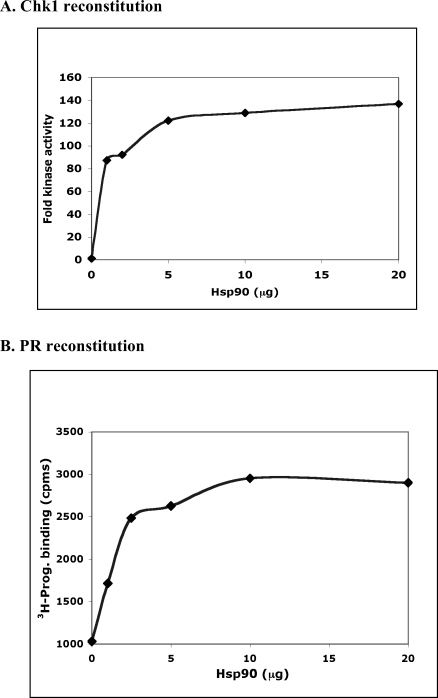
Chk1 is an example of an Hsp90 client that requires Hsp90 but is not continually bound in cellular complexes with Hsp90 (Arlander et al 2006). The data in Figure 1 suggested that the PR and Chk1 are differentially dependent on the level of Hsp90 protein. To test this further, each client was reconstituted with its optimal chaperone mixture but with various amounts of Hsp90 (Fig 2). Using approximately the same amount of client protein (approximately 0.06 μM dimer), 1 μg of Hsp90 (0.06 μM dimer) was sufficient to reconstitute 70% of maximal Chk1 activity, and maximum activity was achieved with 5 μg (0.3 μM) of Hsp90 (top panel). In slight contrast, 1 μg of Hsp90 reconstituted only 40% of maximal PR activity, and 10 μg of Hsp90 (0.6 μM) were needed to achieve maximum hormone binding activity for PR. Since the data shown are from single-point experiments, we conclude that the Hsp90 requirements for the 2 clients are very similar. However, there are subtle differences that are reproducible among several experiments that suggest that Chk1 is more easily reconstituted than PR, particularly when the amount of Hsp90 is limiting. These observations may reflect differences in the stability of the 2 clients both in vitro and in the cell. For example, Hsp90 binding to the more labile steroid receptor requires additional stabilizations contributed by p23, which is also present in the cellular receptor complexes.
Fig 2.
Dose dependence of Chk1 and PR reconstitutions for Hsp90 are similar. Each client was reconstituted with its control amounts of chaperones as in Fig 1 but in the presence of increasing amounts of Hsp90
Geldanamycin sensitivity of client reconstitution
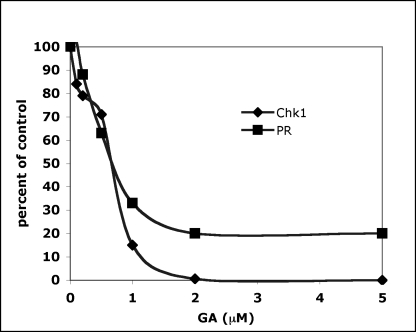
A standard test for determining whether a particular cellular protein is a client for Hsp90 is whether that protein is degraded in cells treated with the Hsp90 inhibitor geldanamycin (GA) (Neckers 2002; Zhang and Burrows 2004). In addition, some clients may be degraded more readily than others (Chen et al 1997; Citri et al 2006). One explanation for these differences might relate to the relative dependence of these clients on Hsp90 at 1 or more steps during their synthesis, folding, maintenance, and/ or activity. Alternatively, we wondered whether some inherent properties of various clients might dictate how readily they are degraded in GA-treated cells. The addition of GA to in vitro reconstitutions has been shown to mimic the effect on Hsp90 complexes in cells (Smith et al 1995). Since we found that the addition of p23, Cdc37, and CK2 had only positive effects on the reconstitution of their specific type of client and no detrimental effects on the other client, we used the ability to reconstitute very different clients under identical conditions to address whether the type of client affected the sensitivity of chaperoning to GA. The results (Fig 3) show that Chk1 and PR reconstitutions were inhibited by GA very similarly (IC50s ∼ 0.6 μM). GA (2 μM) abolished Chk1 reconstitution completely and also produced the maximum amount of PR inhibition. This amount of drug was fairly stoichiometric to the 1.2 μM amount of Hsp90 monomer used in the reactions. It is important to note that the signal-to-background ratios for the 2 assays differ markedly, There was also a level of PR hormone-binding activity that was resistant to inhibition by drug. Nonetheless, the data do not reveal any major differences in GA sensitivity of Hsp90-dependent folding that is attributable directly to the type of client. Thus, cellular differences in client sensitivity to GA likely reflect the direct dependence of that client on Hsp90.
Fig 3.
Inhibition of Chk1 and PR reconstitutions by geldanamycin (GA) are comparable. Clients were reconstituted in vitro using identical compositions of chaperones (Hsp70, Hsp40 [DJB1], Hop, Hsp90, Cdc37, CK2, p23) in the presence of various amounts of GA. Kinase (Chk1) or hormone-binding (PR) activities are expressed as percentages of that obtained for each client in the absence of drug
Hsp70/Hsp40 machinery—type II Hsp40 is preferred for Chk1 reconstitution
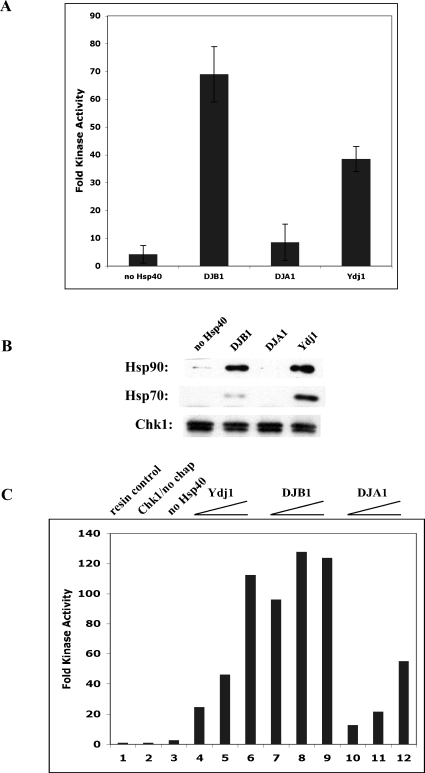
Hsp40 recognition of client has been shown to be the first step in the folding of PR (Hernandez et al 2002a). There are many types of Hsp40 cochaperones for Hsp70. Cintron and Toft (2006) recently showed that PR reconstitution is accomplished equally well in vitro using the yeast Ydj1 or either human Hsp40s DJB1 (previously referred to as HDJ1) or DJA1 (HDJ2), although the type I Hsp40 proteins Ydj1 and DJA1 bound into PR complexes more stably. We tested whether Chk1 reconstitution showed a preference for any of these Hsp40 proteins. The data in Figure 4 show that an Hsp40 protein was essential for kinase reconstitution (panel A, lane 1). The type II Hsp40 DJB1 promoted the reconstitution of the most kinase activity (lane 2), and this was correlated with better binding of Hsp90 (Fig 4B). Ydj1, the Hsp40 used in our previous studies, also functioned well to reconstitute Chk1 activity (lane 4). In contrast, DJA1 functioned poorly in Chk1 reconstitution (lane 2), and its presence failed to lead to stable Hsp90 binding (panel B). DJA1 was also less active in recruiting Hsp70 to the kinase client. The same pattern of Hsp40 preference was obtained when various amounts of protein were tested (panel C). These results reveal another difference in chaperoning requirements between PR and Chk1 and suggest that the client itself influences the type of Hsp40 used for folding and perhaps alters the way Hsp70 and its Hsp40 cochaperone interact.
Fig 4.
Chk1 reconstitution prefers the Hsp40 cochaperone DJB1. (A) Chk1 reconstitution using no Hsp40 or 2 μg of DJB1, DJA1, or Ydj1 as indicated. Error bars represent the deviation from the mean of 3 independent experiments. (B) Western blotting of Chk1 load and complexes from panel A to show binding of chaperones Hsp90 and Hsp70. (C) Chk1 reconstitution using control chaperone conditions lacking any Hsp40 (lane 3) or using 1, 2, or 5 μg of Ydj1 (lanes 4–6), DJB1 (lanes 7–9), or DJA1 (lanes 10–12)
Hop functions to transit the client from Hsp70 to Hsp90
Our in vitro data continue to support a model that Hsp70 and Hsp90 folding machines work together to fold both kinase and steroid receptor clients and that these machines are bridged by the cochaperone Hop. To test the requirement for Hop further, we examined Chk1 reconstitution with time in the presence or absence of Hop. The data in Figure 5 show not only that reconstitution of kinase activity was time dependent but also that the amount of functional kinase was greater and occurred much more quickly if Hop was present in the reaction (panel A, dark gray bars compared to light gray bars). The presence of Hop also resulted in an ability to detect Hsp90 at earlier times during reconstitution (see blot). When Hop was absent from the reaction, Cdc37 only partially compensated to fold the client (panel B, compare lanes 6 and 2). The addition of more Cdc37 protein did not support a further gain in kinase reconstitution (panel B, lanes 4, 5, 7, 8). Thus, the actions of these cochaperones do not overlap, and both seem to function to transition the kinase client from Hsp70 to Hsp90 during intermediate steps of folding.
Fig 5.
Hop enhances Hsp90 binding and Chk1 reconstitution. (A) GST-Chk11–265 kinase activity was reconstituted for various times, as indicated, in the absence (light gray bars) or presence (dark gray bars) of Hop. Chk1 kinase activity is expressed as in earlier figures. At the top, Western blotting of the final kinase complex for bound Hsp90. (B) GST-Chk11–265 kinase activity was reconstituted for 30 minutes in the presence or absence of Hop as indicated, with the addition of 2 (the control amount), 4, or 6 μg of Cdc37
Chk1 folding through a dynamic, stepwise series of chaperone complexes
The data from Figure 4 showed that Hsp70/Hsp40 interaction with client, in turn, affected client binding and chaperoning by Hsp90. Data from Arlander et al (2006) showed that, in addition to Hsp70, Cdc37 also bound to the kinase client at early times. Now that we established that p23 did not contribute to kinase folding and that DJB1 was a better Hsp40 to use, we set out to better understand how Chk1 progresses through early and late complexes. We first tried to preform Hsp70- and/or Cdc37-Chk1 complexes, remove any unbound protein, and complete the reconstitution by the addition of the other chaperones. The data in Figure 6 (bottom panel) show that the continued presence of all the chaperones was required to achieve the folding of a functional kinase (lanes 8, 9). In separate experiments we found that Chk1 does not lose its potential for chaperoning by the first incubation. That is, when Chk1 was incubated alone or with an incomplete chaperone mixture, it could be successfully reconstituted by a complete chaperone mixture in a second incubation (not shown). Figure 6 (top panel) also shows the pattern of protein binding. Prebound Cdc37 (lane 2) or prebound Hsp70 (lane 4 or 6) was stable to washing but was not sufficient to support kinase reconstitution when the remaining chaperones were added later (lanes 3, 5, 7). Prebound Cdc37 (lane 2) was displaced only by Hsp70 when Hsp70 and the other chaperones were added (lane 3). In contrast, prebound Hsp70 enabled the later recruitment of intermediate complexes containing Hop, Cdc37, and some Hsp90 (lanes 4, 5). Also, Hsp70 competed with Cdc37 for Chk1 binding, when added at the same time, to form an early complex (lane 6 vs lane 2) to which Hop and Hsp90 could later bind (lane 7). These data suggest that initial recognition of kinase client by Hsp70 is necessary to initiate folding steps that enable the client to bind and mature through additional chaperoning steps involving Hop, Cdc37, and Hsp90. And yet, without the continued presence of Hsp70, these client-chaperone complexes do not mature to the point of reconstituting an active client. Thus, each client-chaperone interaction is likely to be reiterative, and many intermediate steps may occur before the kinase folds appropriately to be functional.
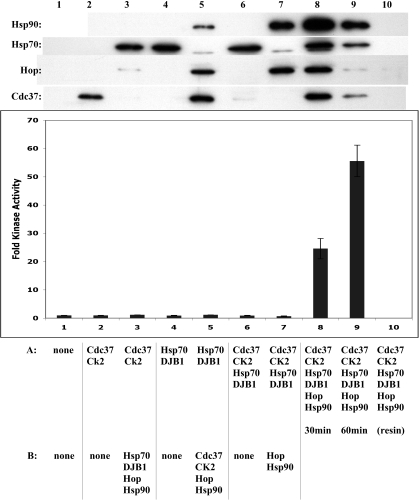
Fig 6.
Preformed Chk1-Cdc37 or Hsp70 complexes are not sufficient for reconstitution of an active kinase. Immobilized GST-Chk11–265 was incubated with chaperones listed as condition A for 30 minutes. After washing with GST buffer lacking NaCl, samples 2, 4, and 6 were kept on ice while additional chaperones in condition B were incubated with the client-chaperone complexes for another 30 minutes (samples 1, 3, 5, 7). Samples 8, 9, and 10 were incubated with all chaperones for 30 or 60 minutes. Kinase activity is shown as the average of 2 independent experiments. Western blots (top) show which chaperones were bound to the kinase under each condition
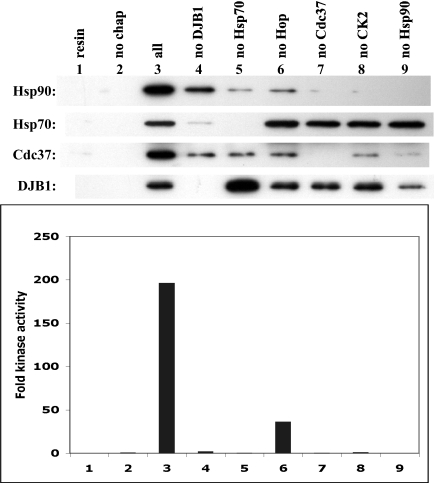
The data in Figure 6 supported the hypothesis that chaperones function in a dynamic manner to fold a kinase client. To determine the order of chaperone binding in a different way, we asked what chaperone complexes were formed when individual chaperones were left out of the reconstitution mixture. Assessment of Chk1 kinase activity confirmed that all 6 chaperones were required for reconstitution in vitro (Fig 7, bottom panel, lane 3). Western blotting of the Chk1-chaperone complexes (top panel) shows that Hsp90 binding was best when all chaperones were present in the reaction (lane 3). No Hsp90 was found bound to the kinase in the absence of Cdc37 (lane 7), and phosphorylation of Cdc37 was also required to promote Hsp90 binding (lane 8). Hsp90 binding was very much reduced in the absence of DJB1 (lane 4) or Hsp70 (lane 5), and what amount of Hsp90 that bound did not function to reconstitute kinase activity (bottom panel). These data are consistent with data in Figure 6 and reemphasize the idea that initial folding steps carried out by Hsp70 are necessary for later, Hsp90-mediated steps to occur. A small amount of Hsp90 binding and kinase activity was achieved in the absence of Hop, but these were significantly less than when Hop was present (as also shown in Fig 5). Similarly, Cdc37 binding was decreased by the absence of any 1 of the other chaperones. Thus, even though Cdc37 can bind Chk1 on its own (Fig 6), its interaction with client is more productive when Hop and Hsp70 are also present. In contrast, Hsp70 binding was detected in the absence of Hop, Cdc37, CK2, or Hsp90 (lanes 6–9) but not in the absence of DJB1 (lane 4). DJB1 binding was strongest in the absence of Hsp70 (lane 5). These data are consistent with Hsp40 being the first chaperone to recognize the client, followed by Hsp70 (whose binding is stabilized by Hsp40). Cdc37 and Hsp90 bind later and appear to be interdependent. Hop was difficult to detect in Chk1 complexes unless we used less stringent washing conditions (not shown) that tended to increase nonspecific binding. But since the absence of Hop decreased stable Cdc37 and Hsp90 binding, it is likely that Hop functions to organize Hsp70 and Hsp90 transiently into intermediate complexes that enable the formation of more stable mature kinase-Cdc37-Hsp90 complexes.
Fig 7.
Chk1 reconstitution in the absence of individual chaperones suggests a stepwise assembly of client-chaperone complexes. GST-Chk11–265 was reconstituted with control conditions established above (lane 3) or in the absence of individual chaperones as indicated. Top panel: Western blotting for the binding of Hsp90, Hsp70, Cdc37, or DJB1 in the final kinase complex. Bottom panel: Chk1 kinase activity expressed as fold increase over the unchaperoned client (lane 2)
DISCUSSION
The molecular details for how chaperones and cochaperones function to fold client proteins are still emerging. Many of the existing models are based on structural studies of a subset of chaperones (usually protein fragments) or studies in cells and crude cellular extracts. In order to experimentally dissect the functions of molecular chaperones, we have focused on developing and refining our ability to reconstitute active clients in vitro with purified chaperones. In this study we have shown that the reconstitution of a model kinase client, Chk1, progresses in a stepwise manner from early chaperoning by Hsp70 and its cochaperone Hsp40 toward a more matured chaperone complex containing Hsp90 and Cdc37. This reconstitution can be accomplished using somewhat lower protein concentrations than our model system for steroid receptor reconstitution and does not utilize the cochaperone p23. The observation that PR is a less stable client explains why it requires constant chaperoning in the cell and in vitro, with the inclusion of more total chaperone proteins, more Hsp90, and the addition of p23.
With the exception of studies with the heme-regulated inhibitor (HRI) kinase in reticulocyte lysate (Uma et al 1999; Thulasiraman et al 2002), the importance of the Hsp70 chaperone machinery for the folding of kinase clients is generally underappreciated in the literature. Not only have our studies with Chk1 shown that Hsp70 is a necessary early step in kinase folding, but our testing of different Hsp40 cochaperones suggests that the initial recognition of client by Hsp40 governs how effectively the client is matured toward later steps in which Hsp90 binds. In contrast to our in vitro studies with a steroid receptor (PR), which can be chaperoned with a number of Hsp40 family members, in vitro chaperoning of Chk1 was better accomplished with the type II molecule DJB1. The reasons for this preference need to be explored in future studies. For example, we do not know whether the recombinant nature or method of purification of our Chk1 somehow influenced which type of Hsp40 recognized it best. Our source of steroid receptor is immunopurified PR expressed in Sf9 cells and stripped of endogenous chaperones with high salt and ATP. If kept cold, this PR is still active but quickly loses the ability to bind hormone when placed at 30°C. Thus, this PR is likely to be very near its native state when used for in vitro reconstitution. Our source of Chk1 is as a GST fusion protein expressed in E coli and purified under standard affinity conditions. This purified Chk1 is not associated with host chaperone proteins and has no activity. Thus, it could be that the physical natures of the 2 clients are different enough to be recognized preferentially by different Hsp40s. However, our results are similar to studies of the reverse transcriptase of hepatitis B virus, an Hsp90 client having some resemblance to protein kinases. That client was also shown to be chaperoned in vitro using DJB1 rather than Ydj1 or DJA1 (Hu et al 2004). As the many types of Hsp40 proteins become better characterized, it will be important to test whether these different types of clients are affected preferentially by different Hsp40s in the cell.
Reconstitution experiments in reticulocyte lysates have been an important complement to genetic studies in yeast showing the importance of Hsp40 (Ydj1) and Hop (Sti1) in client function (Hartson et al 2000; Lee et al 2004). However, recent investigations of kinase chaperoning and crystallographic-based modeling focus most of their attention on Hsp90 and Cdc37. Those studies were no doubt fueled by the novelty of Cdc37 as a kinase-specific cochaperone for Hsp90 (Stepanova et al 1996; Hunter and Poon 1997). The putative role of kinases in cancer etiology has greatly enhanced scientific interest in the once so-called housekeeping chaperones. Indeed, the discovery that the kinase inhibitor geldanamycin was actually an inhibitor of Hsp90 put Hsp90 in the center of a number of cell signaling pathways and thus a possible target for drug discovery for new cancer therapies (Neckers 2002; Pearl 2005; Chiosis et al 2006).
The data in Figures 5 and 7 indicate that Hop, usually discussed in the context of steroid receptor chaperoning, is also very important for chaperoning kinases. Both Hop and Cdc37 inhibit Hsp90 adenosine triphosphatase activity (Siligardi et al 2002), and it was presumed from those data that Hop and Cdc37 would function in parallel ways to aid Hsp90 chaperoning for either a steroid receptor (in the case of Hop) or a kinase client (in the case of Cdc37). Genetic studies have shown that overexpression of Cdc37 compensates for the decrease in v-Src or Ste11 kinase activities in yeast lacking Hop (Δsti1; Lee et al 2004). We still lack some technical tools to further dissect the details of how Hop and Cdc37 function during kinase folding. However, our data suggest a cooperative model for these cochaperones, consistent with other biochemical evidence of Sti1-Cdc37 complexes in yeast or reticulocyte lysates (Hartson et al 2000; Abbas-Terki et al 2002).
A crystallography- and microscopy-based model for Hsp90 in complex with Cdc37 and the kinase Cdk4 has recently been described (Vaughan et al. 2006). It is tempting to speculate how this model might accommodate both Hop and Cdc37. Hop binds to the carboxy terminal MEEVD of Hsp90, which is either in its “apo” or its adenosine 5′-diphosphate (ADP) conformation (Johnson et al 1998; Brinker et al 2002). Cdc37 binds to exposed surfaces in the amino terminal (N) and middle (M) domains of the opened Hsp90 “clamp” (Roe et al 2004). The structure described by Vaughan and colleagues contained a dimer of Hsp90 in which 1 Hsp90 molecule was held in the “open” conformation and bound to 1 molecule of Cdc37. The other molecule of Hsp90 was in the “closed,” ATP-bound conformation and associated with the client Cdk4. The complexes were isolated by a multistep fractionation of cellular lysates. It was not determined whether the Cdk4 kinase in the published structure was active. Thus, it is possible that the published structure represents an inactive client-chaperone complex or a more stable complex derived from a previous Cdk4-Cdc37-Hop-Hsp90 complex that was lost during cell fractionation. If the Cdk4 had been active, our data would suggest that cellular Hsp70 played a critical role in folding that client as well. Clearly, we do not yet understand the dynamic interactions that occur during kinase folding. Nonetheless, the mature kinase complex requires a Cdc37-dependent binding of Hsp90, and full kinase activity is not acquired until Hsp70 and Hop are less abundant in the complex, as is also the case with steroid receptors (Pratt and Toft 2003).
Our data raise additional questions regarding the timing of Cdc37 phosphorylation during kinase chaperoning. For instance, some binding of Cdc37 to Chk1 was detected when CK2 was left out of the reconstitution mixture (Fig 7), and a low amount of Cdc37 binding to Chk1 was also observed in the absence of Hsp40 or Hsp70 or Hop. The absence of Hsp90 from the reconstitution eliminated that level of Cdc37 binding. These results might indicate that Cdc37 binds Chk1 after Hsp90 binds to the client. But such an explanation is inconsistent with time course experiments published previously (Arlander et al 2006). An alternative explanation may be that initial binding of Cdc37 is relatively weak. Hsp90, along with Hsp70 and Hop, might serve to mature the client-Cdc37 complex to one that is more stable, perhaps by fostering the phosphorylation of Cdc37. In our hands, Cdc37 will bind GST-Chk1 in the absence of other proteins and CK2 (not shown). It may be that phosphorylated Cdc37 is most critical when it is functioning in the presence of the other chaperones and thus under circumstances in which several reiterative binding events are required before stable association is achieved. Only then can the client-chaperone complex and the active kinase itself withstand the experimental washing conditions we employ or withstand the conditions used by others to isolate and determine the physical structure of Cdk4-Cdc37-Hsp90 complex (Vaughan et al 2006). This rationale is in line with data of the folding of Lck in cellular lysates during which the client passes through a salt-stable intermediary complex with Hsp90 and Cdc37 (Hartson et al 2000).
Figure 8 incorporates the data from the experiments above and attempts to integrate them into models for the folding of both steroid receptor and kinase clients. Both Hsp90-dependent pathways for client folding are initiated by events chaperoned by Hsp70 and Hsp40. In the case of steroid receptors, the Hsp40 of choice appears to be a type I Hsp40 (DJA1 or Ydj1). The type II Hsp40 (ie, DJB1) is clearly preferred for chaperoning the model kinase Chk1. The early chaperoning of clients by Hsp70 then progresses through 1 or more intermediate complexes that contain Hop. Cdc37 is also a critical component of these intermediate complexes in the case of kinase clients. Cdc37 needs to be phosphorylated by CK2 in order to chaperone the kinase client, and we show this to be a relatively early step for kinase folding. As the receptor matures through its interactions with Hsp70/Hsp40/ Hop and as the kinase matures through its interactions with Hsp70/Hsp40/Hop/Cdc37, Hsp90 is recruited to these clients. The less stable PR client requires additional chaperoning by p23, which further stabilizes Hsp90 binding. This mature complex keeps the receptor in a conformation that is readily able to bind hormone. The role of p23 in kinase chaperoning is less clear. p23 has little influence on the in vitro chaperoning of Chk1; however, this may or may not be the case for all kinases or in cells. Functional or physical interactions of p23 have been shown with several protein kinases, including v-Src (Fang et al 1998), HRI (Xu et al 1997), Fes (Nair et al 1996), and Wee1/Cdc2 (Munoz et al 1999).
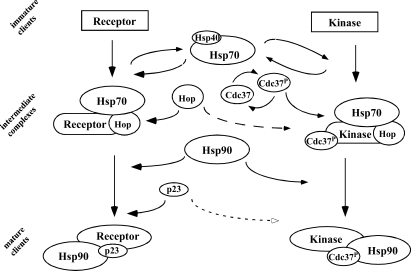
Fig 8.
A model to illustrate similarities and differences for in vitro reconstitutions of kinase and steroid receptor clients. Immature clients are initially recognized by Hsp40, and this interaction recruits and is stabilized by Hsp70. The type of Hsp40 may vary between types of clients. Interactions with Hop (in the case of steroid receptors) or Hop and Cdc37 (in the case of protein kinases) establish intermediate complexes to which Hsp90 binds. The cochaperone p23 is required to establish and maintain a mature steroid receptor complex capable of binding hormone. On the other hand, kinases mature through complexes that contain Cdc37 and Hsp90, and the requirement for p23 is less clear. Some matured kinases continue to be bound in Hsp90/Cdc37 complexes, whereas others, such as the full-length Chk1, are released from the chaperones
Based on their different needs for chaperoning in the cell, kinase clients seem to fall into at least 2 categories. Kinases such as ERB2, Akt, IKK, and Raf can be isolated from cells still bound—and supposedly maintained—in a complex with Hsp90 and Cdc37 (Schulte et al 1995; Chen et al 2002; Solit et al 2003; Xu et al 2007). The kinases Lck and Chk1 are more difficult to detect in cellular Hsp90 complexes. Reconstitution experiments using rabbit reticulocyte lysate showed that immature complexes of HRI or Lck contain Hsp90 and Cdc37 but that the kinase then matures to a form that no longer requires Hsp90 (Hartson et al 2000; Shao et al 2001). Chk1 (1–265 as well as full length) also requires Hsp90 and Cdc37 for folding in vitro. The full-length kinase is released in a conformation that no longer requires constant chaperoning (Arlander et al 2006). These findings explain why Chk1 and some other Hsp90 clients that are degraded in cells treated with the Hsp90 inhibitor 17-AAG are difficult to isolate bound to Hsp90 in lysates from untreated cells.
In conclusion, we now have 2 very powerful in vitro systems with which to study molecular chaperone function. Future refinements in techniques will, it is hoped, allow us to uncover more details of chaperone-chaperone and client-chaperone interactions and to analyze the in vitro activities of many additional cochaperones that are known to participate in these processes in the cell.
Acknowledgments
We acknowledge Sonnet Arlander for her participation and helpful insights during the early parts of this work. We also are grateful to Ahmed Chadli, Nela Cintron, and Bill Sullivan for helpful discussions and to Bridget Stensgard and Becky Bruisma for protein purification. Sf9 cell maintenance and protein expression were performed with the assistance of Kurt Christensen in the Recombinant Protein Expression/Proteomics Core, Baylor College of Medicine. This research was supported by National Institutes of Health grants CA104378 (L.M.K.) and DK46249 (D.O.T).
REFERENCES
- Abbas-Terki T, Briand P-A, Donze O, Picard D. The Hsp90 co-chaperones Cdc37 and Sti1 interact physically and genetically. Biol Chem. 2002;383:1335–1342. doi: 10.1515/BC.2002.152.1431-6730(2002)383[1335:THCCAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Arlander SJ, Eapen AK, Vromen BT, McDonald RJ, Toft DO, Karnitz LM. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J Biol Chem. 2003;278:52572–52577. doi: 10.1074/jbc.M309054200.0021-9258(2003)278[52572:HIDCAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Arlander SJ, Felts SJ, Wagner JM, Stensgard B, Toft DO, Karnitz LM. Chaperoning checkpoint kinase 1 (Chk1), an Hsp90 client, with purified chaperones. J Biol Chem. 2006;281:2989–2998. doi: 10.1074/jbc.M508687200.0021-9258(2006)281[2989:CCKCAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brinker A, Scheufler C, Von Der Mulbe F, Fleckenstein B, Herrmann C, Jung G, Moarefi I, Hartl FU. Ligand discrimination by TPR domains: relevance and selectivity of EEVD-recognition in Hsp70-Hop-Hsp90 complexes. J Biol Chem. 2002;277:19265–19275. doi: 10.1074/jbc.M109002200.0021-9258(2002)277[19265:LDBTDR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;9:401–410. doi: 10.1016/s1097-2765(02)00450-1.1097-2765(2002)009[0401:TRAAOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen H-S, Singh SS, Perdew GH. The Ah receptor is a sensitive target of geldanamycin-induced protein turnover. Arch Biochem Biophys. 1997;348:190–198. doi: 10.1006/abbi.1997.0398.0003-9861(1997)348[0190:TARIAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chiosis G, Rodina A, Moulick K. Emerging Hsp90 inhibitors: from discovery to clinic. Anticancer Agents Med Chem. 2006;6:1–8. doi: 10.2174/187152006774755483.1871-5206(2006)006[0001:EHIFDT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cintron NS, Toft D. Defining the requirements for Hsp40 and Hsp70 in the Hsp90 chaperone pathway. J Biol Chem. 2006;281:26235–26244. doi: 10.1074/jbc.M605417200.0021-9258(2006)281[26235:DTRFHA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Citri A, Harari D, and Shohat G. et al. 2006 Hsp90 recognizes a common surface on client kinases. J Biol Chem. 281:14361–14369. [DOI] [PubMed] [Google Scholar]
- Fang Y, Fliss AE, Rao J, Caplan AJ. SBA1 encodes a yeast Hsp90 cochaperone that is homologous to vertebrate p23 protein. Mol Cell Biol. 1998;18:3727–3734. doi: 10.1128/mcb.18.7.3727.1098-5549(1998)018[3727:SEAYHC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad I, McKee TA, and Ludwig SM. et al. 2006 The Hsp90 cochaperone p23 is essential for perinatal survival. Mol Cell Biol. 26:8976–8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408.0193-4511(2002)295[1852:MCITCF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartson SD, Irwin AD, Shao J, Scroggins BT, Volk L, Huang W, Matts RL. p50(Cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules. Biochemistry. 2000;39:7631–7644. doi: 10.1021/bi000315r.0006-2960(2000)039[7631:PIANHC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hernandez MP, Chadli A, Toft DO. HSP40 binding is the first step in the HSP90 chaperoning pathway for the progesterone receptor. J Biol Chem. 2002a;277:11873–11881. doi: 10.1074/jbc.M111445200.0021-9258(2002)277[11873:HBITFS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hernandez MP, Sullivan WP, Toft DO. The assembly and intermolecular properties of the hsp70-Hop-hsp90 molecular chaperone complex. J Biol Chem. 2002b;277:38294–8304. doi: 10.1074/jbc.M206566200.0021-9258(2002)277[38294:TAAIPO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hu J, Flores D, Toft DO, Wang X, Nguyen D. Requirement of heat shock protein 90 for human hepatitis B virus reverse transcriptase function. J Virol. 2004;78:13122–13131. doi: 10.1128/JVI.78.23.13122-13131.2004.1098-5514(2004)078[13122:ROHSPF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Poon RYC. Cdc37: a protein kinase chaperone? Trends Cell Biol. 1997;7:157–161. doi: 10.1016/S0962-8924(97)01027-1.0962-8924(1997)007[0157:CAPKC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Johnson BD, Schumacher RJ, Ross ED, Toft DO. Hop modulates Hsp70/Hsp90 interactions in protein folding. J Biol Chem. 1998;273:3679–3686. doi: 10.1074/jbc.273.6.3679.0021-9258(1998)273[3679:HMHIIP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kosano H, Stensgard B, Charlesworth MC, McMahon N, Toft DO. The assembly of progesterone receptor-hsp90 complexes using purified proteins. J Biol Chem. 1998;273:32973–32979. doi: 10.1074/jbc.273.49.32973.0021-9258(1998)273[32973:TAOPRC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lee P, Shabbir A, Cardozo C, Caplan AJ. Sti1 and Cdc37 can stabilize Hsp90 in chaperone complexes with a protein kinase. Mol Biol Cell. 2004;15:1785–1792. doi: 10.1091/mbc.E03-07-0480.1059-1524(2004)015[1785:SACCSH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning GD, Whyte B, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762.0193-4511(2002)298[1912:TPKCOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Munoz MJ, Bejarano ER, Daga RR, Jimenez J. The Identification of Wos2, a p23 homologue that interacts with Wee1 and Cdc2 in the mitotic control of fission yeasts. Genetics. 1999;153:1561–1572. doi: 10.1093/genetics/153.4.1561.0016-6731(1999)153[1561:TIOWAP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SC, Toran EJ, Rimerman RA, Hjermstad S, Smithgall TE, Smith D. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor Hsf1, and the aryl hydrocarbon receptor. Cell Stress Chaperones. 1996;1:237–250. doi: 10.1379/1466-1268(1996)001<0237:apomci>2.3.co;2.1466-1268(1996)001[0237:APOMIC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends in Molecular Medicine. 2002;8:S55–S61. doi: 10.1016/s1471-4914(02)02316-x.1471-4914(2002)008[S55:HIANCC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pearl LH. Hsp90 and Cdc37—a chaperone cancer conspiracy. Curr Opin Genet Dev. 2005;15:55–61. doi: 10.1016/j.gde.2004.12.011.0959-437X(2005)015[0055:HACCCC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Picard D. Chaperoning steroid hormone action. Trends Endocrinol Metab. 2006;17:229–235. doi: 10.1016/j.tem.2006.06.003.1043-2760(2006)017[0229:CSHA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303.0163-769X(1997)018[0306:SRIWHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201.0071-3384(2003)228[0111:ROSPFA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roe SM, Ali MM, Meyer P, Vaughan CK, Panaretou B, Piper PW, Prodromou C, Pearl LH. The mechanism of Hsp90 regulation by the protein kinase-specific cochaperone p50(cdc37) Cell. 2004;116:87–98. doi: 10.1016/s0092-8674(03)01027-4.0092-8674(2004)116[0087:TMOHRB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585.0021-9258(1995)270[24585:DOTRMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shao J, Grammatikakis N, Scroggins BT, Uma S, Huang W, Chen J-J, Hartson SD, Matts RL. Hsp90 regulates p50/Cdc37 function during the biogenesis of the active conformation of the heme-regulated eIF1a kinase. J Biol Chem. 2001;276:206–214. doi: 10.1074/jbc.M007583200.0021-9258(2001)276[0206:HRCFDT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Siligardi G, Panaretou B, Meyer P, Singh S, Woolfson DN, Piper PW, Pearl LH, Prodromou C. Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J Biol Chem. 2002;277:20151–20159. doi: 10.1074/jbc.M201287200.0021-9258(2002)277[20151:ROHAAB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith DF, Sullivan WP, Marion TN, Zaitsu K, Madden B, McCormick DJ, Toft DO. Identification of a 60-kilodalton stress-related protein, p60, which interacts with Hsp90 and Hsp70. Mol Cell Biol. 1993;13:869–876. doi: 10.1128/mcb.13.2.869.1098-5549(1993)013[0869:IOAKSP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Whitesell L, Nair SC, Chen S, Prapapanich V, Rimmerman RA. Progesterone receptor structure and function altered by geldanamycin, an Hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804.1098-5549(1995)015[6804:PRSAFA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solit DB, Basso AD, Olshen AB, Sher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003;63:2139–2144.0008-5472(2003)063[2139:IOHSPF]2.0.CO;2 [PubMed] [Google Scholar]
- Stepanova L, Leng X, Parker SB, Harper JW. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491.0890-9369(1996)010[1491:MPIAPK]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sullivan WP, Beito TG, Proper J, Krco CJ, Toft DO. Preparation of monoclonal antibodies to the avian progesterone receptor. Endocrinology. 1986;119:1549–1557. doi: 10.1210/endo-119-4-1549.0013-7227(1986)119[1549:POMATT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thulasiraman V, Yun B-G, Uma S, Gu Y, Scroggins BT, Matts RL. Differential inhibition of Hsc70 activities by two Hsc70-binding peptides. Biochemistry. 2002;41:3742–3753. doi: 10.1021/bi012137n.0006-2960(2002)041[3742:DIOHAB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Uma S, Thulasiraman V, Matts RL. Dual role for Hsc70 in the biogenesis and regulation of the heme-regulated kinase of the α subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 1999;19:5861–5871. doi: 10.1128/mcb.19.9.5861.1098-5549(1999)019[5861:DRFHIT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CK, Gohlke U, and Sobott F. et al. 2006 Structure of an Hsp90-Cdc37-Cdk4 complex. Mol Cell. 23:697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegele H, Muller L, Buchner J. Hsp70 and Hsp90—a relay team for protein folding. Rev Physiol Biochem Pharmacol. 2004;151:1–44. doi: 10.1007/s10254-003-0021-1.0303-4240(2004)151[0001:HAHRTF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xu W, Yuan X, Beebe K, Xiang Z, Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol Cell Biol. 2007;27:220–228. doi: 10.1128/MCB.00899-06.1098-5549(2007)027[0220:LOHAUS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Pal JK, Thulasiraman V, Hahn HP, Chen J-J, Matts RL. The role of the 90-kDa heat-shock protein and its associated cohorts in stabilizing the heme-regulated eIF-1a kinase in reticulocyte lysates during stress. Eur J Biochem. 1997;246:461–470. doi: 10.1111/j.1432-1033.1997.t01-1-00461.x.0014-2956(1997)246[0461:TROTKH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Young JC, Moarefi I, Hartl FU. Hsp90: a specialized but essential protein-folding tool. J Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079.0021-9525(2001)154[0267:HASBEP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med. 2004;82:488–499. doi: 10.1007/s00109-004-0549-9.0946-2716(2004)082[0488:TMSTPT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhao R, Houry WA. Hsp90: a chaperone for protein folding and gene regulation. Biochem Cell Biol. 2005;83:703–710. doi: 10.1139/o05-158.1208-6002(2005)083[0703:HACFPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]