Abstract
Heat shock proteins are induced under stress conditions and they act as molecular chaperones to refold denatured polypeptides. Stress resistances including thermotolerance generally are correlated with levels of the heat shock proteins. We investigated a fruit fly gene encoding a small heat shock protein, Hsp27, to determine if it functions in stress response of Drosophila melanogaster. A knockout Hsp27 allele was generated. Flies homozygous for this allele were viable, without obvious defects, and fertile, indicating Hsp27 is not essential for development. In stress-response tests, loss of the Hsp27 gene caused no defects in resistance to heat shock or oxidative treatments. However, a significant reduction in starvation resistance was associated with the genotype without a functional Hsp27 gene. The data suggest that the Drosophila HSP27 protein acts as a chaperone to provide cellular stress resistance, although its function may be limited to a subset of the stress response such as the starvation resistance.
INTRODUCTION
Heat shock response is a cellular mechanism that is induced upon exposure to increase in temperature and it provides thermotolerance to the cells (Lindquist 1986; Morimoto 1993). In response to heat shock, a large group of ubiquitous, phylogenetically conserved proteins, known as heat shock proteins (HSPs), are induced in both prokaryotes and eukaryotes. HSPs are molecular chaperones that confer proper protein folding and play important roles in stress response by preventing the accumulation of damaged or misfolded proteins in cells (Soti and Csermely 2000; Hartl and Mayer-Hartl 2002). Studies also have shown general correlations between overexpressing HSPs and elevated cellular resistance to stress conditions, such as heat shock and oxidative stress. (Parsell et al 1993; Feder et al 1996; Morrow et al 2004b; Wang et al 2004).
In normal nonstressed prokaryotic cells, several HSPs act as molecular chaperones in assisting the folding process of newly synthesized proteins (Hartl and Hayer-Hartl 2002). In multicellular organisms, the hsp gene expression is spatially and temporally regulated, suggesting that they play important roles under normal physiological conditions (Michaud et al 1997; Christians et al 2003; Krone et al 2003). Indeed, some of the HSPs are essential for development. For example, the Hsp83 gene of Drosophila is required for signal transduction, and loss of its function results in developmental failure (Van Der Straten et al 1997; Rutherford and Lindquist 1998). The mitochondrial Hsp60 gene of Drosophila is essential for fertility, and loss of its activity is detrimental to the fly development (Perezgasga et al 1999). Another gene closely related to the Hsp60 gene, Hsp60B, is required exclusively for Drosophila late spermatogenesis (Timakov and Zhang 2001). In mice, the Hsp70-2 gene also is required for spermatogenesis (Eddy 1999).
Small heat shock proteins ([sHSPs], ∼15–40 kDa) initially were found in Drosophila melanogaster (Tissieres et al 1974), but their physiological functions largely remain unknown. These ubiquitous proteins share the α-crystallin domain of approximately 90 amino acid residues near their C-termini (de Jong et al 1998). The N-termini and the tails at the C-termini of different sHSPs vary extensively. A number of sHSPs form large oligomers in cells and the oligomers bind to unfolded proteins (Van Montfort et al 2001; Giese and Vierling 2002). Investigations into the mechanisms of unfolding and refolding proteins suggest that the primary function of sHSPs is to protect denatured proteins from aggregation by forming complexes with malfolded polypeptides (Jakob and Buchner 1994; Ehrnsperger et al 2000; Morrow et al 2006). sHSPs are implicated in a variety of cellular activities, including thermotolerance, resistance to apoptosis, and eye lens transparency (Landry et al 1989; Arrigo 1998; Liang and MacRae 1999; Andley et al 2002). In this study we took a genetic knockout approach to investigate the sHSP functions in Drosophila melanogaster. We report the isolation and characterization of a null allele of the Hsp27 gene. The results show that the loss of the Hsp27 function caused no obvious developmental defects, because flies homozygous for the null allele were viable, normal-looking, and fertile. However, the homozygotes displayed a reduced stress response to starvation and a decrease in mean life span.
MATERIALS AND METHODS
Drosophila stocks
All flies were grown on standard cornmeal/agar media at 25°C. A stock containing a P-element insertion, EP(3)3583, was described previously (Timakov et al 2002). An isogenetic strain containing the w1118 allele initially was obtained from the Bloomington Drosophila Stock Center, and a pair of flies was taken from the stock to establish a fresh isogenic strain for the studies described here.
Mutagenesis to generate a null allele of the Hsp27 gene
To isolate a null allele of the Hsp27 gene, we activated the EP(3)3583 insertion in the promoter region of the Hsp27 gene by using a transposase source as described previously (Timakov et al 2002). We then monitored changes of the expression of a marker gene, the mini-white gene, that is carried within the EP(3)3583 element. The mini-white gene in P-element transformation vectors is derived from the X-linked white gene (Levis et al 1985; Pirrotta 1988). Regulatory elements necessary for high levels of white expression in the adult eyes are absent from the mini-white gene. Flies carrying a mini-white transgene displayed pale yellow to orange-red eyes, depending on the genomic sites where the transgene is inserted. We reasoned that the mini-white expression of the EP(3)3583 insertion could be modified as the flanking genomic sequence was changed through P-element–induced rearrangement (Zhang and Spradling 1993; Preston et al 1996). After activating the EP(3)3583 insertion by the transposase, flies displaying altered eye pigmentation were collected and individual stocks were established from these flies.
Polymerase chain reactions primers
| The polymerase chain reaction (PCR) primers were | |
|---|---|
| Phsp27-a | 5′-ACATTGGGTGTGTTGTGG-3′ |
| Phsp27-b | 5′-GGATCATGACTACCGCAC-3′ |
| Pg-R | 5′-GAGCCAGAAGATGCGAGA-3′ |
| Pp31 | 5′-CGACGGGACCACCTTATGTTATTTCATCATG-3′ (the 31-bp inverted terminal repeat of the P element and with an outward orientation) |
| Pactin-a | 5′-CGCTCATTTCCGATGGTG-3′ |
| Pactin-b | 5′-GGCTCCTTTGAACCCCAAG-3′ |
| Hsp27RT-upl | 5′-GGTCGTCGTCGTTATTCG-3′ |
| Hsp27RT-lowl | 5′-TTGAACTGCGACACATCC-3′ |
| Rp49-fwd | 5′-TGTCCTTCCAGCTTCAAGATGACCATC-3′ |
| Rp49-ref | 5′-CTTGGGCTTGCGCCATTTGTG-3′ |
Reverse transcriptase–polymerase chain reaction and real-time PCR
Total RNA was isolated from thirty 2-d-old files. For paraquat treatment, flies were fed with 5% sucrose containing 20 mM paraquat for 18 h. For starvation treatment, flies were kept in a plastic vial containing a wet filter paper for 24 h.
Total RNA was isolated by using an Absolutely RNA™ reverse transcriptase–polymerase chain reaction (RT-PCR) Miniprep Kit (Stratagene Inc., La Jolla, CA, USA) following the manufacture's instructions. cDNA libraries for different stress treatments were made by using a GeneAmp® RNA PCR kit (Roche Inc., Indianapolis, IN, USA). For regular RT-PCR, polymerase chain reactions were carried out using GeneAmp system (PE Applied Biosystem Inc., Foster City, CA, USA) under standard conditions on a Robocycler (Stratagene Inc.). For real-time PCR, quantification of mRNA expression was performed using a MyiQ real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA). SYBR Green (Bio-Rad Laboratories) was used as a double-stranded DNA-specific fluorescent dye. Each of the quantitative reactions was carried out in triplicates. To generate relative values of the Hsp27 abundance in the cDNA libraries, the comparative cycle threshold method was use (Pfaffl 2001). Value of the Hsp27 expression was determined by calculating the difference of comparative cycle thresholds between the Hsp27 expression under each of the three conditions (nonstress treatment, oxidative treatment, or starvation treatment) and a calibrator gene, Rp49.
Sequencing
PCR product amplified from the genomic DNA was loaded on a gel of 0.7% low melting temperature agarose (FMC Bioproducts Inc., Philadelphia, PA, USA) in the TAE buffer, and the band containing the amplified DNA was cut out. The gel-purified DNA was used as a template for a second round of PCR with the same primers used in the first PCR reaction. The PCR product was purified using a QIAquick PCR Purification Kit (Qiagene Inc., Valencia, CA, USA) and was used in a sequencing reaction (PE Applied Biosystem Inc.) with the same pair of primers.
Heat shock treatment
Two separate tests were used to examine the effect of heat shock treatments. In the first test, 3- to 4-d-old males were transferred into empty plastic vials containing filter papers saturated with 5% sucrose. Flies in the vials (20 per vial) were pretreated at 35°C by immersing the vials in a circulated water bath for 30 min and were allowed to recover for 1 h at 25°C. The flies then were transferred into a water bath at 37°C for various lengths and thermotolerance was measured as the percentage of flies displaying body movement 12 h after the heat shock treatment.
In the second test, vials with a thin layer of fresh medium were warmed to the experimental temperature at 35°C. Three- to four-day-old males were introduced into the vials and exposed to heat stress in a circulated hot-air chamber set at 35°C and 50–60% relative humidity. Mortality was examined every 4 h. A total of 100 flies in 5 groups were tested for each genotype. Mean survival time was calculated and subjected to nonparametric Wilcoxon signed-rank statistical test. Similarly, mean survivorship data from paraquat and starvation treatment were subjected to the same statistical test by using the SAS programs.
Paraquat treatment
Three-day-old males were starved in empty vials for 6 h before the oxidative treatment with paraquat. The flies then were transferred into vials containing filter papers saturated with 200 μl of 5% sucrose, 20 mM paraquat solution (Sigma Chemical Co., St Louis, MO, USA). Mortality was recorded every 4 h. A total of 100 flies in 5 groups for each genotype were tested.
Starvation treatment
Three-day-old males were starved in empty vials with cotton plugs saturated with water. A total of 100 flies in 5 groups were tested for each genotype. Mortality was recorded every 4 h.
Life span assay
Newly hatched males and females were collected and transferred to fresh bottles. They were kept in the bottles for 3 d to allow mating. Male and female flies then were separated. The males were maintained at 25°C, transferred to vials with fresh food every 2–3 d, and dead flies were counted upon each transfer. A total of ∼270 flies were examined for each genotype. The assay was repeated once and similar data were obtained.
RESULT
Isolation of a null Hsp27 allele
The Hsp27 gene is present in a single copy in Drosophila melanogaster, and it is located within the 67B region of Chromosome 3. A P-element insertion, EP(3)3583, was mapped to the promoter region of the Hsp27 gene and its insertion site is 89 bp 5′ to the Hsp27 transcription initiation site (Timakov et al 2002). Thus, the ORF of the hsp27 allele associated with the P-element insertion remains intact. Flies homozygous for the chromosome with the insertion were viable and fertile. Because P-elements insert preferentially into 5′ ends of genes and generate mutant alleles that partially disrupt transcriptional activities of the affected genes (Spradling et al 1995), we asked if the allele associated with EP(3)3583 is transcriptionally active. By using RT-PCR with a pair of primers derived from the Hsp27 transcript, we have determined that the Hsp27 allele associated with EP(3)3583 was transcribed, though its mRNA level was lower than that of a wild type allele, indicating that the Hsp27 allele is leaky (Fig 1).
Fig 1.
Hsp27 transcription from the allele associated with the EP(3)3583 element. Reverse transcriptase–polymerase chain reaction (RT-PCR) amplified from a pair of primers derived from the hsp27 transcript, Phsp27-a and Phsp27-b, are shown. Three cDNA dilutions were 1:6, 1:3, and 1:1. Optimized PCR conditions were allowed for 28 cycles to ensure DNA amplification within the exponential range. RNA was extracted from young adult flies without or with heat shock pretreatment for 1 h at 37°C and recovery for 30 min at 25°C. As positive control for cDNA synthesis, amplification of a Drosophila actin cDNA with a pair of primers, Pactin-a and Pactin-b, also was shown. Wild type control was no heat shock (Lane 1– 3) and heat shock (Lanes 7–9). The allele associated with the EP(3)3583 element was no heat shock (Lanes 4–6) and heat shock (Lanes 10–12)
To address the question of whether the Hsp27 gene is essential for Drosophila development, we took a further step to generate a null allele for the Hsp27 gene. As described in the Materials and Methods, we activated the EP(3)3583 element and established a total of 145 lines that displayed altered eye pigmentation and therefore were candidates for harboring a null Hsp27 allele. We employed standard PCR to isolate a null allele among these lines. Genomic DNA was isolated from each of the candidate strains and used as a template for PCR with a pair of primers, Pp31 and Phsp27-a (Fig 2). The Pp31 primer is derived from the 31-bp terminal inverted repeats of the P-element, and the Phsp27-a primer is derived from the Hsp27 gene. All but one of the strains produced a 1,078 bp PCR product that was generated between Phsp27-a and the starting EP(3)3583 element. The exceptional strain produced a ∼0.4 kb PCR product, which was much smaller than that of the normal product of 1,078 bp with the Pp31 and Phsp27-a primer pair (Fig 2, lanes 1 and 2). The result from PCR experiments with another pair of primers, Pp31 and Pg-R, indicated that the 5′ end of the starting EP(3)3583 element was retained in this line, because the size of the PCR product from this line was the same as that of the starting line (241 bp, Fig 2, lanes 3 and 4). Further characterization by Southern blot analysis suggested that the starting element was retained, but a deletion was present between the Pp31 and Phsp27-a primers (data not shown). We determined the molecular structure of the deletion by sequencing the ∼0.4 kb PCR product with the Pp31 and Phsp27-a primers. The data showed a 707 bp deletion within the Hsp27 gene. The deletion removed the entire coding region of the Hsp27 gene (213 aa). Sequencing data also revealed three additional nucleotides (GAA) at the junction of the deletion breakpoints (Fig 2). This Hsp27 null allele was designated as hsp27−0.7.
Fig 2.
The hsp27−0.7 null allele. (A) A schematic drawing of the genomic DNA flanking the EP(3)3583 element. The relative positions of the Hsp27 transcript and the deletion in the hsp27−0.7 allele are shown. The breakpoints of the deletion (707 basepair, from +179 to +885) are separated by an insertion of 3 nucleotides (GAA, underlined). (B) Amplification of genomic DNA by polymerase chain reaction (PCR). With the Pp31 and Phsp27-a primer pair a fragment of ∼1.1 bp was produced from the starting chromosome with EP(3)3583 (control, Lane 1), whereas a fragment of ∼0.4 kb was produced from the hsp27−0.7 allele (Lane 2). With the Pp31 and Pg-R primer pair a fragment of ∼250 bp was produced from the starting chromosome with EP(3)3583 (control, Lane 3) and from the hsp27−0.7 allele (Lane 4)
The Hsp27 null allele caused no obvious developmental defects
Two steps were taken to introduce the hsp27−0.7 null allele into a near isogenic background. First, genetic crosses with balancers were used to introduce an isogenic Chromosome X and an isogenic Chromosome 2 from an isogenic w1118 strain (see Materials and Methods section) into the strain carrying the hsp27−0.7 allele. Second, females carrying the hsp27−07 allele in the isogenic background for Chromosome X and Chromosome 2 were allowed to backcross to males of the isogenic w1118 strain for 10 generations. During this introgression process, the hsp27−0.7 allele was recovered by recognizing its mini-white marker gene expression from the retained EP element in hsp27−0.7. After 10 generations with meiotic recombination, a single fly carrying the hsp27−0.7 allele, which was confirmed by PCR (Fig 2), was used to establish a fresh stock in a near isogenic background.
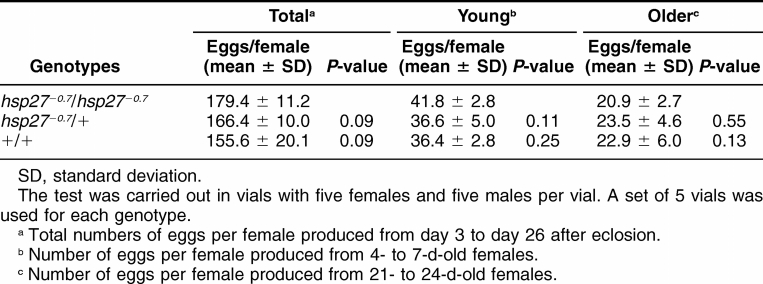
Homozygotes for the hsp27−0.7 allele in the near isogenic background were healthy with no obvious visible defects. Fertility tests also showed that both of homozygous males and females were fertile, at levels comparable to wild type controls. The numbers of eggs produced from the homozygotes, heterozygots, and w1118 wild type controls were monitored and the rates of the egg productions were determined (Table 1). The homozygotes produced 179.4 ± 11.2 (Mean ± standard deviation [SD]) eggs per female from day 3 to day 26 after eclosion. Though it was slightly more than that of the heterozygotes (166.4 ± 10.0), or the wild type control (155.6 ± 20.1), the difference is statistically insignificant (P = 0.09 in both cases, Table 1). The rates of egg production were similar among the homozygotes, heterozygotes, and wild type controls for the young females (4- to-7 d-old) or the older females (21- to-24 d-old; Table 1). These data indicate that the null allele did not affect the egg production. Furthermore, from a cross between +/hsp27−0.7 siblings, the progeny showed a ratio of 1:2:1 among the +/+, +/hsp27−0.7, and hsp27−0.7/hsp27−0.7 genotypes (952, 2065, 945, or very close to the 1:2:1 ratio). Taken together, these results show that the Hsp27 gene is not required for fly development under the laboratory conditions.
Table 1.
Total number of eggs and the egg production rate
Heat and oxidative stress resistance
To study the roles of the Hsp27 gene in stress response, 3 series of experiments were carried out to examine the effect of the hsp27−0.7 null allele on stress resistance under heat shock stress, oxidative stress, or starvation conditions. Young flies of the same age (3- to 4-d-old adults) were used in all experiments. In addition, each test on the effect of the stress resistance was repeated at least twice.
Thermotolerance was shown to increase in severe heat shock treatment, if a mild heat shock, known as pretreatment, was administrated (Mitchell et al 1979). To examine the effect of the loss of the Hsp27 gene activity on thermotolerance, we constructed 3 genotypes, the hsp27−0.7/ hsp27−0.7 homozgyote, its heterozygous sibling, and a wild type control. After a pretreatment, a recovery period, and severe heat shock treatment (see Materials and Method section), the differences of the survivorships among the genotypes were small and statistically insignificant (Fig 3A). The thermotolerance tests with pretreatment indicate that loss of the Hsp27 activity does not cause a significant reduction in heat shock resistance.
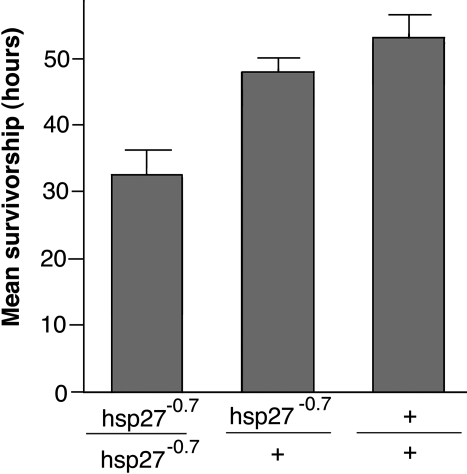
Fig 3.
Effect of the hsp27−0.7 allele on thermotolerance. (A) Thermotolerance at 37°C with a mild pretreatment. Survivals (%) of 3 genotypes are shown. (B) Thermotolerance under conditions of optimal sHSP induction (35°C). Mean survivals (hours) of 3 genotypes are shown. Genotypes: hsp27−0.7/hsp27−0.7, hsp27−0.7/+, and +/+. The w1118 strain was used as a wild type control. Values are means with 95% confidence intervals
High levels of the HSP70 protein generally are correlated with increased thermotolerance in Drosophila (Parsell et al 1993; Feder et al 1996). In addition, elevated thermotolerance also was seen when sHSPs were overexpressed (Morrow et al 2004b; Wang et al 2004). We asked if stress-induced high levels of Hsp27 also could increase thermotolerance. In Drosophila cell culture, heat shock induces the expression of sHSP genes, including Hsp27 with an optimal temperature of approximately 35°C (Yost and Lindquist 1986). To examine the effect of the Hsp27 gene on thermotolerance under conditions where its induction is optimal, genotypes with or without the Hsp27 gene were treated continuously at 35°C. The rate of adult death under the heat shock condition was determined for each genotype. When exposed to 35°C, the hsp27−0.7/hsp27−0.7 homozgyotes displayed a mean survivorship of 31.9 ±1.0 h, although its heterozygous siblings and wild type controls showed 30.4 ± 1.5 h and 31.3 ± 1.5 h of mean survivorship, respectively (Fig 3B). Thus, loss of the Hsp27 function appeared to have no adverse effect on the heat-induced death rate at 35°C. In conjunction with the thermotolerance data at 37°C obtained from the tests with pretreatment, these results suggest that Hsp27 is not required in heat shock resistance.
We also examined the effect of the hsp27−0.7 null allele on oxidative stress resistance by exposing flies to paraquat, an oxidative reagent. The hsp27−0.7/hsp27−0.7 homozygotes showed oxidative resistance at levels comparable to that of wild type controls (a mean survivorship of 63.5 ± 13.0 h for Hsp27 null homozygotes vs. 65.2 ± 8.6 h for a wild type control, Fig 4). The oxidative resistance levels of the hsp27−0.7/hsp27−0.7 homozygotes were also similar to that of the hsp27−0.7 heterozygotes (mean survivorship, 72.1 ± 8.3 h, Fig 4). The data indicate that loss of the Hsp27 function had no significant effect on the oxidative resistance.
Fig 4.
Effect of the hsp27−0.7 allele on paraquat treatment. Mean survivals (hours) of 3 genotypes are shown: hsp27−0.7/hsp27−0.7, hsp27−0.7/+, and +/+. The w1118 strain was used as a wild type control. The 20 mM paraquat was in 5% sucrose solution. Values are means with 95% confidence intervals
Effect of the Hsp27 null allele on starvation
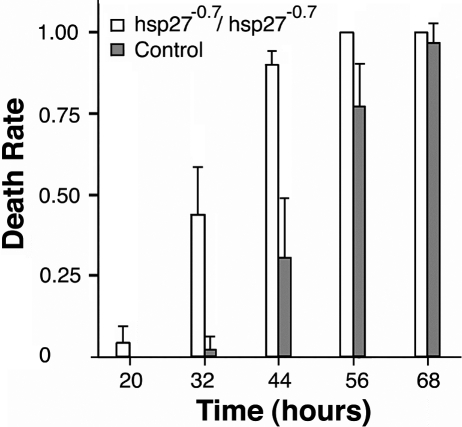
The effect of the hsp27−0.7 null allele on starvation resistance also was measured in the rate of survivorship. The mean survivorship of a wild type control under starvation was 53.1 ± 2.7 h (Fig 5). In contrast, the mean survivorship of the hsp27−0.7/hsp27−0.7 homozygotes under the starvation test was only 32.6 ± 3.0 h, which was a decrease of 39% (P < 0.01). Similarly, the starvation-resistance levels of the hsp27−0.7/hsp27−0.7 homozygotes were significantly lower than those of the heterozygotes for the null allele (a mean survivorship of 47.9 ± 1.6 h, P < 0.01, Fig 5). The reduced resistance was seen for the null homozygotes shortly after administrating the starvation treatment. Approximately 20 h into the starvation, the homozygotes displayed weakness in resisting the stress and began to die (Fig 6). At 32 h into the treatment, >40% of the homozygotes were dead, and its death rate was ∼11 times higher than that of the control. These data suggest that the Hsp27 gene plays a significant role in starvation resistance.
Fig 5.
Effect of the hsp27−0.7 allele on starvation treatment. Mean survivals (hours) of 3 genotypes are shown: hsp27−0.7/hsp27−0.7, hsp27−0.7/+, and +/+. The w1118 strain was used as a wild type control. Values are means with 95% confidence intervals
Fig 6.
The differential death rates on starvation. The rate of death was determined for two genotypes, hsp27−0.7/hsp27−0.7 and a wild type control (w1118), at various time periods (hours after the beginning of starvation). Values are means with 95% confidence intervals
Loss of the Hsp27 gene activity and life span
In addition to increase stress resistance, overexpressing several sHSPs in Drosophila extended the life span (Walker and Lithgow 2003; Morrow et al 2004b; Wang et al 2004). To address a question of whether loss of the Hsp27 gene influences the aging process, we examined the male life spans of three genotypes, hsp27−0.7/hsp27−0.7 homozygote, hsp27−0.7/+ heterozygote, and a wild type control at 25°C. Compared with the wild type or the heterozygous controls, there was an early acceleration of death for the hsp27−0.7/hsp27−0.7 homozygotes at the age of approximately 35 d old (Fig 7). Although the wild type controls and the hsp27−0.7/+ heterozygotes had mean life spans of 56.4 d and 61.3 d, respectively, the hsp27−0.7/hsp27−0.7 homozygotes only had a mean life span of 45.2 d. Thus, male hsp27−0.7/hsp27−0.7 homozygotes showed a 19.8% (P < 0.001) decrease in the mean life span when compared to that of the wild type control, or a 26.2% (P < 0.001) decrease of the mean life span when compared to the hsp27−0.7/+ heterozygote. In addition, the reduction in the hsp27−0.7/hsp27−0.7 life span was correlated with an increase of deaths that began at ∼30 d of age, although the maximum life span appeared to be unaffected.
Fig 7.
Effect of the hsp27−0.7 allele on life span. Flies were maintained in a humidified and temperature-controlled environmental chamber (25°C, 50–60% relative humidity). A total of 279 hsp27−0.7/ hsp27−0.7 homozygous males and 268 w1118 control males were tested. Two-sample T-test showed a significant difference of lifespan between the homozygotes and the control (P < 0.001)
Inductions of the Hsp27 transcription under oxidative or starvation treatment
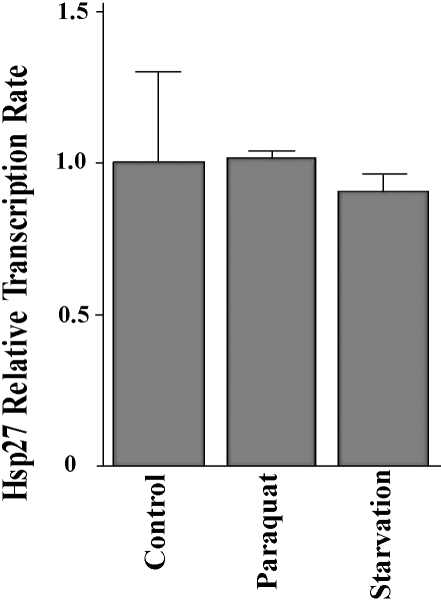
The influence of either an oxidative treatment with paraquat, or starvation treatment, on the transcription of the Hsp27 gene was examined by using real-time PCR. The quantitative analysis shows that the abundance of the Hsp27 transcript is not affected significantly by either of the stress treatments, when total mRNA isolated from the adults is examined (Fig 8). When compared to a nonstressed control, the related abundance of the Hsp27 transcript for the paraquat-treated sample is 1.02 ± 0.01, indicating no change of the Hsp27 transcript level between the control and the oxidative treated sample. For the starvation-treated sample, the related abundance of the Hsp27 transcript is 0.91 ± 0.02. Though it appears to be slightly lower than the control (1.00 ± 0.12), the difference is not significant (P = 0.24).
Fig 8.
Hsp27 transcription under starvation and oxidative conditions. cDNA libraries were constructed from wild type adult flies. Levels of transcription for the Hsp27 gene were determined using real-time polymerase chain reaction (PCR), under stress conditions of oxidative treatment with paraquat, and starvation treatment. The Hsp27 transcription level of a nonstress control is set at 1.0 (100%). Values are means with 95% confidence intervals. Pairs of primers for the amplification of the Hsp27 transcript were Hsp27RT-upI and Hsp27RT-lowI. The expression level of the calibrator gene, Rp49, in each of the cDNA libraries was determined with a pair of primers Rp49-fwd and Rp49-rev
DISCUSSION
The spatially and temporally regulated expression patterns of the sHSPs and the proposed sHSP chaperone activities suggest that sHSPs carry out important physiological functions as chaperones under normal and nonstressed conditions. During the Drosophila larval development, Hsp27 is expressed primarily in the brain and gonads (Pauli et al 1990; Michaud et al 1997). In the adult tissues it is localized in the Drosophila central nervous system and the germ line cells of both males and females. The present studies showed that loss of the Hsp27 function caused no obvious developmental defects in Drosophila. Flies homozygous for the loss-of-function allele were viable and fertile. Thus, our results demonstrated that the Drosophila Hsp27 gene is required for neither viability nor fertility. Moreover, because the null allele causes no obvious phenotypes, we conclude that the Hsp27 gene does not play significant roles in Drosophila development under the laboratory conditions. Similar results were obtained for a sHSP gene in S. cerevisiae, Hsp26, showing that it is not required for physiological functions (Susek and Lindquist 1989). In Drosophila melanogaster, there are four well-characterized sHSP genes, including Hsp22, Hsp23, Hsp26, and Hsp27. It is possible that they overlap functionally during development. Thus removing Hsp27 from the gene family may cause no detectable developmental defects. In addition to the sHsp genes in Drosophila, a database search revealed that there are at least 6 additional genes related to sHSP genes because each carries a recognizable α-crystallin domain. They are distributed on various chromosome locations, with one on the X, another on Chromosome 2, and 3 more on Chromosome 3.
The involvement of sHSPs in the cellular stress-response mechanisms has been implicated in many studies. Mutation analyses of the bacterial sHSP genes revealed essential functions of several sHSP chaperones in stress resistance (Servant and Mazordier 1995; Kitagawa et al 2000; Lee et al 2000). A growing body of evidence also indicates that sHSP are involved in a variety of cellular stress-response activities in multicellular organisms. Studies in Drosophila showed that upregulating the sHSP expression was associated with elevated resistance to a number of stress treatments. Flies that carried constructs of overexpressing Hsp26, or Hsp27, displayed increased stress resistance against oxidative, heat, or starvation treatments (Wang et al 2004). Although high levels of Hsp22 expression in adults might be toxic (Bhole et al 2004), there was also a correlation between levels of the Hsp22 expression and stress resistance (Morrow et al 2004a, 2004b).
Our results showed that loss of the Hsp27 function was associated with a significant decrease of stress response to starvation. However, loss of the Hsp27 function did not show a significant effect on the stress resistance to heat shock, or to paraquat oxidative treatment, under the test conditions. Thus, overexpressing Hsp27 influenced Drosophila resistance to heat shock and oxidative stress conditions, but loss of the Hsp27, function had no significant effect on these stress conditions. These different stress responses between the Hsp27 overexpression and the loss of the Hsp27 function could be due to an ectopic nature of gene induction associated with the overexpression system, which activates the Hsp27 transcription ubiquitously in the adult cells (Wang et al 2004). The ectopic Hsp27 expression also might induce a gain-of-function mechanism of stress response in some tissues that normally do not express Hsp27. Because loss of the Hsp27 function was associated with a defect in starvation resistance, but not in heat or oxidative resistance, our data suggest that individual sHSPs perform distinct cellular functions in response to various stress conditions.
Although the stress tests reveal a significant influence of the Hsp27 gene activity on starvation, our quantitative RT-PCR analyses show no significant change of the Hsp27 transcript level under conditions where the adult flies were treated with starvation. It is possible that the effect of the Hsp27 gene on starvation resistance does not require a transcriptional response on the Hsp27 gene. Several phosphorylated isoforms of the HSP27 protein were reported previously (Marin et al 1996). Posttranscriptional modifications such as phosphorylation to the HSP27 protein, which could be downstream of a primary stress-response mechanism, might modulate the HSP27 protein, giving rise to the effect of the Hsp27 gene on starvation. To reconcile the difference between the results from the stress tests and quantitative RT-PCR, it is also conceivable that, in response to a starvation treatment, an induction of the Hsp27 transcription is restricted in a particular tissue, but the tissue-specific induction accounts for only a small fraction of the Hsp27 transcript in the whole adult body. Thus, the real-time RT-PCR analyses, which were carried out using total adult cDNA libraries, might be unable to detect the small tissue specific induction.
Overexpressing a number of the sHSPs, including Hsp16 in Caenorhabditis elegans and Hsp22, Hsp26, and Hsp27 in Drosophila, extended the life span, in addition to the increase in stress resistance (Walker and Lithgow 2003; Morrow et al 2004b; Wang et al 2004). Knock-down mutations of the sHSP genes also were shown to be associated with shortened life span in C. elegans (Lin et al 2001; Hsu et al 2003). Thus, genetic manipulations have shown a direct correlation between level of the sHSP expression and the life span. Consistent with this correlation, our data showed that loss of the Hsp27 function was associated with a decrease in stress resistance and life span. The sHSP genes are targets of a transcription factor, FOXO, a key member of the insulinlike signaling pathway that influences aging (Hsu et al 2003; Hwangbo et al 2004). Studies have further indicated that activation of the pathway in the brain is sufficient to control the life span in Drosophila and C. elegans (Wolkow et al 2000; Hwangbo et al 2004). Because it is localized in the nervous system of both larvae and adults, the Drosophila Hsp27 may act as a downstream factor of the pathway in the brain to influence aging.
Acknowledgments
We thank the Center for Applied Genetics and Technology at University of Connecticut for technological support and Xiaoru Liu for invaluable technical assistance. This work was partially supported by a grant from the National Science Foundation and a grant from the University of Connecticut Research Advisory Council.
REFERENCES
- Andley U, Patel H, Xi J. The R116C mutation in αA-crystallin diminishes its protective activity against stress-induced lens epithelial cell apoptosis. J Biol Chem. 2002;277:10178–10186. doi: 10.1074/jbc.M109211200.0021-9258(2002)277[10178:TRMIAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Arrigo A. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. J Biol Chem. 1998;379:19–26.0021-9258(1998)379[0019:SSPCTA]2.0.CO;2 [PubMed] [Google Scholar]
- Bhole D, Allikian M, Tower J. Doxycycline-regulated overexpression of hsp22 has negative effects on stress resistance and life span in adult Drosophila melanogaster. Mech Ageing Dev. 2004;125:651–663. doi: 10.1016/j.mad.2004.08.010.0047-6374(2004)125[0651:DOOHHN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Christians E, Zhou Q, Renard J, Benjamin I. Heat shock proteins in mammalian development. Semin Cell Dev Biol. 2003;14:283–290. doi: 10.1016/j.semcdb.2003.09.021.1084-9521(2003)014[0283:HSPIMD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- de Jong W, Caspers G, Leunissen J. Genealogy of the α-crystallin small heat shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0.0141-8130(1998)022[0151:GOTCSH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Eddy E. Role of heat shock protein HSP70-2 in spermatogenesis. Rev Reprod. 1999;4:23–30. doi: 10.1530/ror.0.0040023.1359-6004(1999)004[0023:ROHSPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Gaestel M, Buchner J. Analysis of chaperone properties of small Hsps. Methods Mol Biol. 2000;99:421–429. doi: 10.1385/1-59259-054-3:421.1064-3745(2000)099[0421:AOCPOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Feder M, Cartano N, Milos L, Krebs R, Lindquist S. Effect of engineering Hsp70 copy number on Hsp70 expression and tolerance of ecologically relevant heat shock in larvae and pupae of Drosophila melanogaster. J Exp Biol. 1996;199:1837–1844. doi: 10.1242/jeb.199.8.1837.0022-0949(1996)199[1837:EOEHCN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Giese K, Vierling E. Changes in oligomerization are essential for the chaperone activity of a small heat shock protein in vivo and in vitro. J Biol Chem. 2002;277:46310–46318. doi: 10.1074/jbc.M208926200.0021-9258(2002)277[46310:CIOAEF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl F, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408.0193-4511(2002)295[1852:MCITCF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hsu A, Murphy C, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701.0193-4511(2003)300[1142:ROAAAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hwangbo D, Gersham B, Tu M, Palmer M, Tatar M. Drosophila dFOXO controls life span and regulates insulin signaling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549.1476-4687(2004)429[0562:DDCLSA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jakob U, Buchner J. Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem Sci. 1994;19:205–211. doi: 10.1016/0968-0004(94)90023-x.0376-5067(1994)019[0205:ASTROH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Matsumura Y, Tsuchido T. Small heat shock proteins, IbpA and IbpB, are involved in resistances to heat and superoxide stresses in Escherichia coli. FEMS Microbiol Lett. 2000;184:165–171. doi: 10.1111/j.1574-6968.2000.tb09009.x.0378-1097(2000)184[0165:SHSPIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Krone P, Evans T, Blechinger S. Heat shock gene expression and function during zebrafish embryogenesis. Semin Cell Dev Biol. 2003;14:267–274. doi: 10.1016/j.semcdb.2003.09.018.1084-9521(2003)014[0267:HSGEAF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Landry J, Chretien P, Lambert H, Hickey E, Weber L. Heat shock resistance conferred by expression of the human Hsp27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7.0021-9525(1989)109[0007:HSRCBE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Owen H, Prochaska D, Barnum S. HSP16.6 is involved in the development of thermotolerance and thylakoid stability in the unicellular cyanobacterium, Synechocystis sp. PCC 6803. Curr Microbiol. 2000;40:283–287. doi: 10.1007/s002849910056.0343-8651(2000)040[0283:HIIITD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Levis R, Hazelrigg T, Rubin G. Separable cis-acting control elements for expression of the white gene of Drosophila. EMBO J. 1985;4:3489–3499. doi: 10.1002/j.1460-2075.1985.tb04108.x.1460-2075(1985)004[3489:SCCEFE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, MacRae T. The synthesis of a small heat shock/α-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol. 1999;207:445–456. doi: 10.1006/dbio.1998.9138.1095-564X(1999)207[0445:TSOASH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850.1061-4036(2001)028[0139:ROTCEL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443.0066-4154(1986)055[1151:THSR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Marin R, Landry J, Tanguay RM. Tissue-specific posttranslational modification of the small heat shock protein HSP27 in Drosophila. Exp Cell Res. 1996;223:1–8. doi: 10.1006/excr.1996.0052.0014-4827(1996)223[0001:TPMOTS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Michaud S, Marin R, Tanguay R. Regulation of heat shock gene induction and expression during Drosophila development. Cell Mol Life Sci. 1997;53:104–113. doi: 10.1007/PL00000572.1420-682X(1997)053[0104:ROHSGI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell H, Moller G, Petersen N, Lipps-Sarmiento L. Specific protection from phenocopy induction by heat shock. Dev Genet. 1979;1:181–192.1520-6408(1979)001[0181:SPFPIB]2.0.CO;2 [Google Scholar]
- Morimoto R. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637.0193-4511(1993)259[1409:CISTAO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morrow G, Battistini G, Zhang P, Tanguay R. Decreased lifespan in the absence of expression of the mitochondrial small heat shock protein Hsp22 in Drosophila. J Biol Chem. 2004a;279:43382–43385. doi: 10.1074/jbc.C400357200.0021-9258(2004)279[43382:DLITAO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morrow G, Heikkila J, Tanguay R. Differences in the chaperone-like activities of the four main small heat shock proteins of Drosophila melanogaster. Cell Stress & Chaperones. 2006;11:51–60. doi: 10.1379/CSC-166.1.1466-1268(2006)011[0051:DITCAO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, Samson M, Michaud S, Tanguay R. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J. 2004b;18:598–599. doi: 10.1096/fj.03-0860fje.0892-6638(2004)018[0598:OOTSMH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Parsell D, Taulien J, Lindquist S. The role of heat shock proteins in thermotolerance. Philos. Trans R Soc Lond. 1993;339:279–286. doi: 10.1098/rstb.1993.0026. [DOI] [PubMed] [Google Scholar]
- Pauli D, Tonka C, Tissieres A, Arrigo A. Tissue-specific expression of the heat shock protein Hsp27 during Drosophila melanogaster development. J Cell Biol. 1990;111:817–828. doi: 10.1083/jcb.111.3.817.0021-9525(1990)111[0817:TEOTHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perezgasga L, Segovia L, Zurita M. Molecular characterization of the 5′ control region and of two lethal alleles affecting the hsp60 gene in Drosophila melanogaster. FEBS Lett. 1999;456:269–273. doi: 10.1016/s0014-5793(99)00963-1.0014-5793(1999)456[0269:MCOTCR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45.0305-1048(2001)029[e45:ANMMFR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V 1988 Vectors for P-mediated transformation in Drosophila. In: Vectors: A Survey of Molecular Cloning Vectors and Their Uses, ed Rodriguez R, Denhardt, D Butterworths. Boston, 437–445. [DOI] [PubMed] [Google Scholar]
- Preston C, Sved J, Engels W. Flanking duplications and deletions associated with P-induced male recombination in Drosophila. Genetics. 1996;144:1623–1638. doi: 10.1093/genetics/144.4.1623.0016-6731(1996)144[1623:FDADAW]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550.1476-4687(1998)396[0336:HAACFM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Servant P, Mazodier P. Characterization of Streptomyces albus 18-kilodalton heat shock–responsive protein. J Bacteriol. 1995;177:2998–3003. doi: 10.1128/jb.177.11.2998-3003.1995.0021-9193(1995)177[2998:COSAKH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soti C, Csermely P. Molecular chaperones and the aging process. Biogerontology. 2000;1:225–233. doi: 10.1023/a:1010082129022.1389-5729(2000)001[0225:MCATAP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Spradling A, Stern D, and Kiss L. et al. 1995 Gene disruptions using P transposable elements: an integral component of the Drosophila genome project. Proc Natl Acad Sci U S A. 92:10824–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek R, Lindquist S. hsp26 of Saccharomyces cerevisiae is related to the superfamily of small heat shock proteins but is without a demonstrable function. Mol Cell Biol. 1989;9:5265–5271. doi: 10.1128/mcb.9.11.5265.1098-5549(1989)009[5265:HOSCIR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timakov B, Liu X, Turgut I, Zhang P. Timing and targeting of P-element local transposition in the male germ line cells of Drosophila melanogaster. Genetics. 2002;160:1011–1022. doi: 10.1093/genetics/160.3.1011.0016-6731(2002)160[1011:TATOPL]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timakov B, Zhang P. The hsp60B gene of Drosophila melanogaster is essential for the spermatid individualization process. Cell Stress & Chaperones. 2001;6:71–77. doi: 10.1379/1466-1268(2001)006<0071:thgodm>2.0.co;2.1466-1268(2001)006[0071:THGODM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissieres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1.0022-2836(1974)084[0389:PSISGO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Van Der Straten A, Rommel C, Dickson B, Hafen E. The heat shock protein 83 (Hsp83) is required for Raf-mediated signaling in Drosophila. EMBO J. 1997;16:1961–1969. doi: 10.1093/emboj/16.8.1961.1460-2075(1997)016[1961:THSPHI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Montfort R, Slingsby C, Vierling E. Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem. 2001;59:105–156. doi: 10.1016/s0065-3233(01)59004-x.0065-3233(2001)059[0105:SAFOTS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Walker G, Lithgow G. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x.1474-9718(2003)002[0131:LEICEB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wang H, Kazemi-Esfarjani P, Benzer S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proc Natl Acad Sci U S A. 2004;101:12610–12615. doi: 10.1073/pnas.0404648101.1091-6490(2004)101[12610:MAFIOD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow C, Kimura K, Lee M, Ruvkun G. Regulation of C. elegans life span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147.0193-4511(2000)290[0147:ROCELS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yost HJ, Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986;45:185–193. doi: 10.1016/0092-8674(86)90382-x.0092-8674(1986)045[0185:RSIIBH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhang P, Spradling A. Efficient and dispersed local P-element transposition from Drosophila females. Genetics. 1993;133:361–373. doi: 10.1093/genetics/133.2.361.0016-6731(1993)133[0361:EADLPT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]