Abstract
Inhibition of protein folding in the endoplasmic reticulum (ER) causes ER stress, which triggers the unfolded protein response (UPR). To decrease the biosynthetic burden on the ER, the UPR inhibits in its initial stages protein synthesis. At later stages it upregulates components of ER-associated degradation (ERAD) and of the ubiquitin/proteasome system, which targets ER as well as cytosolic proteins for disposal. Here we report that, at later stages, the UPR also activates an alternative nonproteasomal pathway of degradation, which is resistant to proteasome inhibitors and is specific for ER substrates (assessed with uncleaved precursor of asialoglycoprotein receptor H2a and unassembled CD3δ) and not for cytosolic ones (p53). To mimic the initial inhibition of translation during UPR, we incubated cells with cycloheximide. After this treatment, degradation of ERAD substrates was no longer effected by proteasomal inhibition, similarly to the observed outcome of UPR. The degradation also became insensitive to abrogation of ubiquitination in a cell line carrying a thermosensitive E1 ubiquitin activating enzyme mutant. Of all protease inhibitors tested, only the metal chelator o-phenanthroline could block this nonproteasomal degradation. Preincubation of o-phenanthroline with Mn2+ or Co2+, but not with other cations, reversed the inhibition. Our results suggest that, upon inhibition of translation, an alternative nonproteasomal pathway is activated for degradation of proteins from the ER. This involves a Mn2+/Co2+-dependent metalloprotease or other metalloprotein. The alternative pathway selectively targets ERAD substrates to reduce the ER burden, but does not affect p53, the levels of which remain dependent on proteasomal control.
INTRODUCTION
Degradation of aberrant proteins from the endoplasmic reticulum (ER) involves an elaborate mechanism of recognition, reverse translocation to the cytosol, ubiquitination, and shuttling to cytosolic proteasomes, a series of processes termed ER-associated degradation ([ERAD], Lederkremer and Glickman 2005; Meusser et al 2005; Romisch 2005; Sayeed and Ng 2005). When the capacity of ERAD and the ER protein-folding machinery are exceeded, protein accumulation in the ER triggers the unfolded protein response (UPR) (Schroder and Kaufman 2005). This comes about when the cell encounters environmental insults that compromise protein folding or during the normal physiology of the cell, especially of secretory cells like plasma cells or pancreatic cells, when a large output of secretory proteins is required. The UPR initiates a series of steps to correct this situation of ER stress. As an immediate action, it causes arrest in protein synthesis in a mechanism involving the phosphorylation of translation initiation factor eIF2α, to stop the production of misfolded proteins (Harding et al 2000). Long-term the protein synthesis recovers and the UPR upregulates the expression of genes encoding for chaperones and other proteins participating in the ER folding machinery to attempt to repair the misfolded proteins. The UPR also upregulates proteasomal degradation components, to dispose of the aberrant proteins (Friedlander et al 2000; Travers et al 2000). This is a rather general strategy that targets to degradation ER proteins as well as cytosolic ones. We recently reported (Shenkman et al 2007) that, during the initial UPR stages, the arrest in protein synthesis blocks proteasomal degradation, which spares short-lived proteins from depletion. Here we describe a selective mechanism by which prolonged UPR induces additional nonproteasomal degradation pathways that target only proteins from the ER and not cytosolic ones. One of those pathways is induced as a long-term consequence of inhibition of protein synthesis.
MATERIALS AND METHODS
Materials
Rainbow [14C]-labeled methylated protein standards were obtained from Amersham Biosciences (Piscataway, NJ, USA). Pro-mix cell-labeling mixture ([35S]Met plus [35S]Cys, >1000 Ci/mmol) was from Perkin Elmer Life Sciences (Boston, MA, USA). Protein A-Sepharose was from Repligen (Needham, MA, USA) and Protein G from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). N-glycosidase F, N-acetyl-leucyl-leucyl-norleucinal (ALLN), N-acetyl-leucyl-leucyl-methional (ALLM), tosyl-L-lysine chloromethyl ketone (TLCK), and tosyl phenylalanyl chloromethyl ketone (TPCK) were obtained from Roche (Basel, Switzerland). N-carbobenzoxyl-leucinyl-leucinyl-leucinal (MG-132), cyclosporine-A, lactacystin, and cycloheximide (CHX) were from Calbiochem (La Jolla, CA, USA). Apstatin was a kind gift of W. Simmons (Loyola University, Maywood, IL, USA). Tunicamycin, thapsigargin, puromycin, aprotinin, leupeptin, phenylmethylsulfonyl fluoride (PMSF), E64D, iodoacetamide (IAA), N-ethyl-maleimide (NEM), pepstatin, bestatin, leucinethiol, 1,10-phenantroline, chloroquine, phosphoramidon, thiorphan, angiotensin II, Cpp-Ala-Ala-Phe-pAB-HCl, and other common reagents were from Sigma-Aldrich (St Louis, MO, USA).
Cells and culture
NIH 3T3 fibroblasts expressing asialoglycoprotein receptor (ASGPR) H2a (2–18 cells) (Shenkman et al 1997) were grown in Dulbecco's modified Eagle's medium plus 10% newborn calf serum under 5% CO2 at 37°C. A CHO cell line carrying a thermosensitive mutant E1 (ts20 cells; Kulka et al 1988) was grown similarly but with 10% fetal calf serum at 31°C. Plasmid pCDδM coding for CD3δ (see Frenkel et al 2003) was transfected into 3T3 cells in 60-mm dishes using FuGENE 6 transfection reagent (Roche) according to the provided protocol.
Antibodies
Polyclonal anti-H2a antibody against the region of the extra pentapeptide of ASGPR H2a as compared to H2b was the one used before (Tolchinsky et al 1996), as well as anti-C terminal CD3δ polyclonal (Frenkel et al 2003). Rabbit polyclonal anti-Sec61β was a kind gift of T. Rapoport and anti-p53 was from Santa Cruz.
Primers and reverse transcriptase–polymerase chain reaction
Reverse transcription followed by polymerase chain reaction (RT-PCR) was used to analyze the level of BiP mRNA as an indicator of induction of the UPR. Total cell RNA was extracted with EZ-RNA kit (Biological Industries, Beit Haemek, Israel). ReddyMix (ABgene, Epsom, UK) was used for PCR. Reverse transcription was performed with a ProtoScript kit using random primers. An aliquot (5%) of the RT product was used for PCR with the following primers: TGCTTGTCGCTGGGCATCATTG and TTGGAATGACCCTTCGGTGCAG for mouse BiP; GTGGCCATCTCTTGCTCGAAGTC and GTTTGAGACCTCAACACCCC for mouse actin as a control for an mRNA that does not change its level upon UPR.
Prechelation of 1,10 phenanthroline
Phenanthroline was prechelated with different divalent cations as is done to determine which compensates its inhibitory activity by competing with the chelation of the same cation in the reaction being studied. This was done by mixing a 10 mM aqueous solution with an equal volume of 10 mM chlorides of divalent cations and incubating at room temperature for 1 h. The mixtures then were added at 1 mM final phenanthroline concentration to metabolically labeled cells during the chase period and assessing the effect on degradation of the protein being studied by immunoprecipitation.
[35S]Cys/Met metabolic labeling and immunoprecipitation
Metabolic labeling followed by immunoprecipitation was used to assess the changes in stability of ERAD substrates compared to p53, a cytosolic proteasome substrate. For stably expressed H2a, subconfluent (90%) cell monolayers in 60-mm dishes were labeled with [35S]Cys, lysed, and immunoprecipitated with anti-H2a antibody as described previously (Tolchinsky et al 1996; Shenkman et al 1997). Treatment with N-glycosidase F after immunoprecipitation to eliminate sugar chain heterogeneity and obtain more precise quantitation was performed as described previously (Shenkman et al 1997). Ts20 cells stably expressing H2a (Kamhi-Nesher et al 2001) were incubated at the permissive (31°C) or restrictive (40°C) temperatures before metabolic labeling and immunoprecipitation as above. The NIH 3T3 cells transiently expressing CD3δ were metabolically labeled 48 hours posttransfection with [35S]Cys plus [35S]Met mix and immunoprecipitated as before (Frenkel et al 2003). For p53, cells were labeled for 1 hour with [35S]Cys plus Met mix and lysed with lysis buffer containing 50 mM Tris pH 8.0, 5 mM ethylenediamine-tetraacetic acid (EDTA), 0.15 M NaCl, and 0.5% NP40. Immunoprecipitation was done using rabbit anti-p53 and protein A-sepharose. Washes were done with a buffer containing 5% sucrose, 50 mM Tris pH 7.4, 5 mM EDTA, 0.5 M NaCl, and 0.5% NP40.
For experiments involving UPR induction, labeling was done on 50–60% confluent cells to avoid eIF2α phosphorylation that takes place upon cell confluence.
Gel electrophoresis, fluorography, and quantitation
Reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed on 12% Laemmli gels except where indicated. The gels were analyzed by fluorography using 20% 2,5-diphenyloxazole and were exposed to Kodak BioMax MR film. Quantitation was performed in a Fuji BAS 2000 phosphorimager.
RESULTS
Alternative degradation pathway induced by the unfolded protein response
The UPR is known to activate components of proteasomal degradation and ERAD in its late stages (Schroder and Kaufman 2005). We analyzed the behavior of an established ERAD substrate, the uncleaved precursor of ASGPR H2a upon prolonged ER stress. We have seen recently that the UPR inhibits the degradation of ASGPR H2a at its early stages (Shenkman et al 2007). H2a initially is formed as a membrane-bound precursor that undergoes a nonproteasomal-dependent physiological cleavage creating a soluble ectodomain fragment (by signal peptidase; Yuk and Lodish 1993). This fragment matures through the Golgi complex to a soluble secreted form of the receptor in hepatocytes, where it is expressed endogenously. However, in other cell types, the exogenously expressed H2a precursor mostly remains uncleaved and thus is treated as an ERAD substrate and sent to degradation. The small number of ectodomain fragment molecules that are formed in these cells also are mostly degraded (Tolchinsky et al 1996).
To assess the effect of prolonged ER stress on ERAD of H2a, cells stably expressing the protein were first preincubated with the UPR inducers tunicamycin or thapsigargin for 16 h. This period of time is sufficient for total cell recovery from the transient translational block induced by these inhibitors (Shenkman et al 2007). They were then metabolically labeled and chased with or without MG-132. In control untreated cells, proteasomal inhibition prevented degradation of both H2a precursor and of the 35 kDa carboxyterminal ectodomain fragment (Fig 1, compare lane 2 with 3). Surprisingly, after prolonged ER stress, the proteasomal inhibitor had almost no inhibitory effect on the degradation (Fig 1, compare lane 5 with 6 and lane 8 with 9). It can be seen that, in untreated cells, H2a precursor and fragment appear with 3 or less N-linked sugar chains added (Tolchinsky et al 1996), whereas tunicamycin treatment prevents N-glycosylation resulting in a faster migration of unglycosylated precursor and fragment. Tunicamycin treatment also results in induction of UPR as indicated by significantly increased levels of BiP mRNA (Fig 1B).
Fig 1.
After prolonged ER stress, degradation of ASGPR H2a cannot be blocked by a proteasomal inhibitor. (A) NIH 3T3 cells stably expressing H2a were preincubated for 16 hours with complete medium in the absence (lanes 1–3) or presence of 10 μg/mL tunicamycin ([tun], lanes 4–6) or 2 μg/mL thapsigargin ([thap], lanes 7–9). They were then metabolically labeled with [35S] Cys and chased for 3 hours with complete medium with or without 20 μM MG-132 as indicated. Tunicamycin or thapsigargin were added also during starvation, pulse, and chase periods. Cell lysates were immunoprecipitated with anti-H2a antibodies and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by fluorography. On the right are indicated molecular masses in kilodaltons. On the left are the migrations of H2a precursor and cleaved ectodomain fragment. Bands below the upper H2a precursor and fragment species in this and other figures are underglycosylated molecules (with less than the total of 3 sugar chains). This heterogeneity is eliminated in samples preincubated with tunicamycin that blocks N-glycosylation (migration of unglycosylated species is indicated on the left). Cell viability was not very affected by the long preincubation with the unfolded protein response (UPR) inducers, because it did not have a significant effect on the metabolic labeling. (B) RNA was extracted from H2a-expressing cells pretreated with 10 μg/mL tunicamycin for the indicated times and used for reverse transcriptase polymerase chain reaction (RT-PCR) analysis (20 cycles) with primers for BiP mRNA (upper panel) compared to actin (lower panel).These experiments, as well as those in other figures, are representative of at least 3 similar experiments
Transient inhibition of the degradation of ERAD substrates but extended block in the degradation of p53 by inhibition of protein synthesis
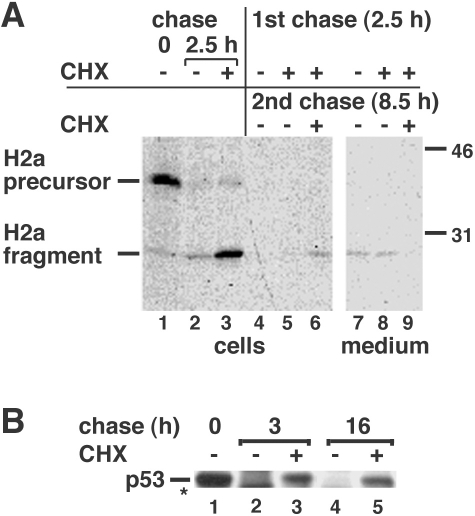
To mimic the transient block in protein synthesis that occurs in early UPR (Schroder and Kaufman 2005), we pulse-labeled cells, then incubated them for a short chase period (2.5 h) with CHX. We recently reported that short-term inhibition of protein synthesis (during early UPR or by cell treatment with CHX) causes arrest of protein ubiquitination and proteasomal degradation (Shenkman et al 2007). In the case of our model ERAD substrate H2a, this prevents degradation of the membrane-bound precursor and leads to its cleavage and accumulation of the ectodomain fragment mentioned above (Fig 2A, lanes 1–3). Similarly we had seen accumulation of the precursor and fragment upon proteasomal inhibition (Kamhi-Nesher et al 2001).
Fig 2.
Inhibition of protein synthesis blocks only transiently the degradation of H2a but extendedly that of p53. (A) NIH 3T3 cells stably expressing H2a (2–18 cell line) were metabolically labeled for 20 min with [35S] Cys and chased for 0 or 2.5 hours with complete medium in the absence or presence of 300 μM cycloheximide (CHX) as indicated. Some samples (lanes 4–9) then were rinsed and incubated for an additional 8.5 hours in the absence or presence of 300 μM CHX. At the end of the pulse or chase periods, the cells were lysed; the lysates (cells) or cell supernatants (medium) were immunoprecipitated with anti-H2a antibody, treated with N-glycosidase F, and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by fluorography. On the right are indicated molecular masses in kilodaltons. On the left are the migrations of H2a precursor and cleaved ectodomain fragment. (B) NIH 3T3 cells were metabolically labeled for 1 hour with [35S] Cys+Met mix and chased for the indicated times in the presence or absence of CHX. Cell lysates were immunoprecipitated with anti-p53 antibodies and analyzed by SDS-PAGE and fluorography. The asterisk indicates a cleavage product
We then removed CHX, following the 2.5-h incubation, which, as expected, allowed resumption of degradation (Fig 2A, lane 5). In the control experiment (continued incubation of cells in the presence of CHX), we expected that inhibition of degradation would be maintained. Surprisingly, after longer incubations with CHX (an 11-h chase), degradation resumed and reached almost the levels seen in the absence of the inhibitor (Fig 2A, lanes 4–6). Only a small amount of secretion of the ectodomain fragment was observed in the absence of CHX and inhibited in its presence (Fig 1A, lanes 7–9). Therefore, the large decrease in levels of the intracellular protein in the prolonged presence of CHX must be attributed to resumption of protein degradation. In this experiment and in most of the following ones, immunoprecipitated H2a was treated with N-glycosidase F to eliminate heterogeneity in the run due to the variable degree of glycosylation.
As we had seen before (Shenkman et al 2007), degradation of the cytosolic proteasomal substrate p53 was inhibited following incubation of cells with CHX for short periods. However, in contrast to H2a, the degradation of p53 still was inhibited even after very long chase periods (16 h) in the presence of CHX (Fig 2B, lanes 3 and 5).
Taken together our results suggest that, upon prolonged inhibition of protein synthesis, an alternative degradation pathway not dependent on protein synthesis is induced for ERAD substrates but not for cytosolic ones.
The degradation pathway induced after inhibition of protein synthesis is nonproteasomal
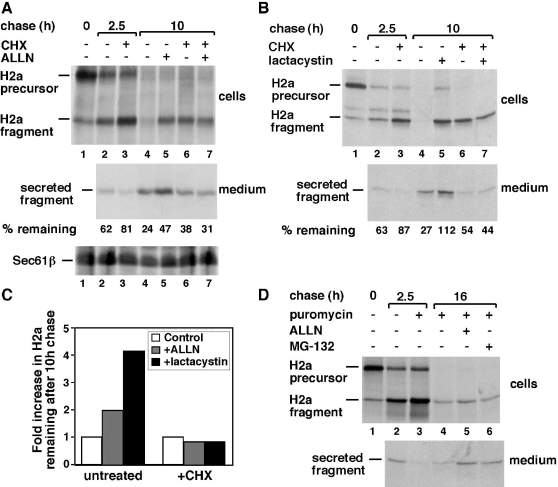
Strikingly, the proteasomal inhibitor ALLN could not inhibit the degradation that occurred following prolonged incubation with CHX (Fig 3A top panel, compare lane 6 with 7 and Fig 3C). This suggests activation of an alternative proteasome-independent pathway, similar to what we observed upon prolonged ER stress. In the absence of CHX, incubation with ALLN resulted in protection from degradation and subsequent increased secretion of the H2a ectodomain fragment (Fig 3A, top and middle panels, lanes 4 and 5). As we had seen before, the cleavage forming the ectodomain fragment is insensitive to proteasomal inhibitors such as ALLN (Kamhi-Nesher et al 2001).
Fig 3.
Proteasome inhibitors do not block the degradation of H2a after prolonged inhibition of protein synthesis. (A) Upper and middle panels are similar to Figure 2A, but in this case with chases in the absence or presence of 300 μM cycloheximide (CHX), 100 μM N-acetyl-leucyl-leucyl-norleucinal (ALLN) or with both drugs simultaneously (lane 7). Supernatants of the immunoprecipitation of H2a from cell lysates were immunoprecipitated with anti-Sec61β and run on 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by fluorography (lower panel). The values at the bottom of the middle panel represent total percent of pulse-labeled H2a remaining, including intracellular precursor and fragment and secreted fragment, calculated from a phosphorimager quantitation of the gel. (B) Similar to (A) but using 10 μM lactacystin instead of ALLN. (C) Graph comparing fold increase in percent of pulse-label remaining after 10 hours of chase by incubation with the proteasomal inhibitors, relative to the control untreated samples in the presence or absence of CHX. Values were calculated from the phosphorimager quantitations of the gels in (A) and (B). (D) Similar to (A) but using 50 μg/mL puromycin instead of CHX, alone or together with 100 μM ALLN or 20 μM MG-132 as indicated
To assess whether the novel nonproteasomal pathway induced by long-term incubation with CHX could degrade a stable ER-resident protein in addition to ERAD substrates, we analyzed the fate of the endogenous translocation channel subunit Sec61β. The levels of labeled Sec61β after the chase period were similar, irrespective of either the presence or absence of cell treatment with CHX (Fig 3A, bottom panel).
A more specific proteasome inhibitor, lactacystin, showed similar results to ALLN and demonstrated a strong inhibitory effect on the degradation of H2a (Fig 3B, compare lane 4 with 5). In line with our proposed novel nonproteasomal pathway, lactacystin did not inhibit the degradation that occurred after a long chase period in the presence of CHX (Fig 3B, compare lane 6 with 7 and Fig 3C).
Treatment with fivefold higher concentrations of the proteasomal inhibitors (compared with those used in Fig 3A,B, which are the saturating concentrations usually utilized to inhibit the proteasome) also failed to inhibit the long-term CHX-induced protein degradation (data not shown).
A different protein synthesis inhibitor, puromycin, showed an effect similar to CHX. After 2.5-h chase, puromycin inhibited degradation and secretion of H2a (Fig 3D, compare lane 2 with 3), but after a long chase period degradation resumed (Fig 3D, lane 4). The proteasomal inhibitors ALLN and MG-132 had very little effect in inhibiting the degradation that occurred after long chases in the presence of puromycin (Fig 3D, lanes 4–6).
Taken together, our results show that the novel nonproteasomal pathway cannot be inhibited by three different proteasomal inhibitors (ALLN, lactacystin, and MG132) and is induced by two diverse protein synthesis inhibitors, CHX and puromycin.
The nonproteasomal degradation pathway requires a Mn2+/Co2+ metalloprotein
To try to determine the type of protease involved in the novel degradation pathway, cells incubated with CHX were treated with saturating concentrations of many cell-permeable protease inhibitors. They included inhibitors specific for lysosomal, cysteine-, aspartyl-, metallo-, serine-proteases, caspases, and others. Of all compounds tested at saturating concentrations (E64D, leupeptin, NH4Cl, chloroquine, DTT, pepstatin, ZVAD-FMK, IAA, NEM, PMSF, TLCK, TPCK, lactacystin, MG-132, ALLN, ALLM, bestatin, leucinethiol) only the metalloprotease inhibitor (metal chelator) 1,10-phenanthroline (o-phananthroline) protected H2a from degradation in the presence of CHX. Figure 4A shows a representative experiment where short-term treatment with CHX (2.5 h) totally blocked the degradation of H2a precursor and its intracellular fragment (Fig 4A, lane 8). After a long chase (10 h) in the presence of CHX, degradation resumed and only 33% of the pulse label remained. Dramatically, phenanthroline completely inhibited this degradation (Fig 4A, compare lane 9 with 13), whereas other inhibitors showed no effect. We tried to block the degradation with peptide inhibitors specific for several intracellular metalloproteases (phosphoramidon for endothelin converting enzyme, thiorphan for neutral endopeptidase, Cpp-Ala-Ala-phe-pAB-HCl for thimet oligopeptidase, angiotensin II for microsomal endopeptidase, apstatin for aminopeptidase P) but they all failed to inhibit the novel degradation pathway. The degradation of H2a also was inhibited by phenanthroline in the absence of CHX (Fig 4A, lane 7), suggesting that it also can block the proteasomal pathway or that the nonproteasomal pathway acts to a certain degree in normal conditions in parallel with proteasomal degradation.
Fig 4.
Mn2+/Co2+ dependence of the cycloheximide (CHX)-induced nonproteasomal degradation pathway. (A) NIH 3T3 stably expressing H2a (2–18 cells) were metabolically labeled with [35S] Cys and chased for the indicated times with the cysteine protease inhibitors N-ethyl-maleimide ([NEM], 10 μM) and iodoacetamide ([IAA], 10 μM), the serine protease inhibitor phenylmethylsulfonyl flouride ([PMSF], 2 mM), or the metalloprotease inhibitor (metal chelator) o-phenanthroline (1 mM; all inhibitors at concentrations usually used for maximal effect), in the absence or presence of 300 μM CHX. Cell lysates (upper panel) and cell supernatants (lower panel) were immunoprecipitated with anti-H2a antibodies, treated with N-glycosidase F, and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and fluorography. (B) O-phenanthroline was prechelated with the indicated divalent cations as described in the Materials and Methods section before addition at 1 mM final concentration in a 3-h chase period to cells expressing H2a that had been labeled metabolically with [35S] Cys. Cell lysates (upper panel) and cell supernatants (lower panel) were processed as in (A). (C) An experiment similar to that in (B) was performed, where 10 mM o-phenanthroline was prechelated with the indicated increasing concentrations of MgCl2, MnCl2, or CoCl2 and then incubated at a final concentration of 1 mM during the chase period. Values from phosphorimager quantitation of the gel were used to calculate the percent of inhibition of H2a degradation with prechelated phenanthroline as compared to inhibition by phenanthroline that had been preincubated without any added cations (=100)
To determine which metal ion is necessary for the nonproteasomal degradation pathway, phenanthroline was prechelated with CaCl2, MgCl2, MnCl2, or ZnCl2. Phenanthroline binds weakly to Ca2+ and with higher affinity to other divalent cations. After prechelation it was added at a final concentration of 1 mM phenanthroline to NIH 3T3 cells expressing H2a. Prechelation with the same metal cation that is a cofactor in the cells would lead to competition and abrogation of the effect of phenanthroline. Preincubation of the drug with Ca2+, Zn2+, and Mg2+ failed to inhibit its activity (Fig 4B, lanes 4, 5, and 7), but preincubation with Mn2+ reversed its blockage of degradation (Fig 4B, lane 6). Preincubation of phenanthroline with CoCl2 had an effect similar to MnCl2 (Fig 4C). The results suggest that a Mn2+/Co2+ metalloprotease or accessory metalloprotein that is activated by inhibition of protein synthesis is involved in the degradation of H2a.
The alternative nonproteasomal degradation pathway is independent of ubiquitination
To see whether the ubiquitination machinery plays a role in the nonproteasomal degradation pathway, we used ts20 cells, which carry a temperature-sensitive mutation in the ubiquitin-activating enzyme, E1 (Kulka et al 1988). Inhibition of ubiquitination at the restrictive temperature caused a marked protection from degradation and accumulation of H2a, especially of the ectodomain fragment (Fig 5A, compare lane 2 with 5, Fig 5B).
Fig 5.

The nonproteasomal degradation pathway is functional in ts20 cells harboring a temperature-sensitive mutant E1 ubiquitin activating enzyme. (A) A CHO cell line carrying a thermosensitive mutant E1 (ts20 cells) stably expressing H2a (Kamhi-Nesher et al 2001) was grown at 31°C and then incubated for 4 hours at 31°C (permissive temperature) or 40°C (restrictive), followed by metabolic labeling with [35S] Cys and chase for the indicated times in the absence or presence of cycloheximide (CHX) and processing as in Figure 2A. Shifts to faster migrating bands after chase are due to mannose trimming of the sugar chains (Frenkel et al 2003). Secretion of H2a fragment from these cells was extremely low and is not shown. (B) Values from a phosphorimager quantitation of the gel in (A) representing percent of pulse-label remaining after the chase period
Treatment of cells at the permissive temperature with CHX increased degradation (Fig 5A, lane 3). Likewise, there was increased degradation in the presence of CHX at the restrictive temperature (Fig 5A, lane 6, Fig 5B), suggesting that ubiquitination is not needed for the alternative degradation pathway. The alternative nonproteasomal pathway can overcome the inhibition of degradation that results from the dysfunctional E1 at the restrictive temperature.
A nonproteasomal phenanthroline-resistant degradation pathway induced by the UPR
We next analyzed whether the nonproteasomal degradation induced by the UPR showed characteristics similar to those of the CHX-induced pathway. We performed an experiment similar to that in Figure 1 and, after preincubation with the UPR inducer tunicamycin, we pulse-labeled cells and treated them with phenanthroline during the chase period. Surprisingly, after preincubation with tunicamycin, phenanthroline did not inhibit the degradation (Fig 6A, compare lane 9 with 13 and Fig 6B). Because we know the proteasomal pathway is enhanced long-term by the UPR (Travers et al 2000), it is possible that both pathways (proteasomal and nonproteasomal) are activated and operate simultaneously. Alternatively, an additional nonproteasomal degradation pathway might be activated, perhaps lysosomal or autophagic (which relies on degradation of substrates by lysosomal enzymes after fusion of autophagosomes with lysosomes). To test if lysosomal degradation is induced, we incubated cells with the lysosomal enzyme inhibitors ammonium chloride or leupeptin. They had no effect before or after preincubation with tunicamycin (Fig 6A, lanes 3, 4, 10, and 11). Likewise, combined simultaneous treatment of cells with MG-132, phenanthroline, and leupeptin did not inhibit the degradation after UPR induction (Fig 6A, lane 14). This suggests that, instead of or in addition to the phenanthroline-sensitive nonproteasomal pathway induced by CHX, a different nonproteasomal pathway is turned on by the UPR that cannot be inhibited either by MG-132, phenanthroline, or lysosomal inhibitors.
Fig 6.
The unfolded protein response (UPR) –induced nonproteasomal degradation of endoplasmic reticulum–associated degradation (ERAD) substrates is insensitive to o-phenanthroline and to lysosomal inhibitors. (A) NIH 3T3 cells stably expressing H2a were preincubated for 16 hours with complete medium in the absence (lanes 1–7) or presence of 10 μg/mL tunicamycin (lanes 8–14) and then metabolically labeled with [35S] Cys and chased for 3 hours without or with 0.4 mg/mL leupeptin, 20 mM NH4Cl, 20 μM MG-132, or 1 mM o-phenanthroline as indicated. Samples in lanes 7 and 14 were chased in the presence of a combination of leupeptin, MG-132, and phenanthroline. Secretion of H2a fragment is extremely low after 3 hours chase (see Fig 4) and is not shown. (B) Quantitative analysis comparing values from a phosphorimager quantitation of the gel in (A), representing percent of pulse-label remaining. (C) NIH 3T3 cells were transiently transfected with an expression vector encoding CD3δ. After 48 hours they were preincubated with tunicamycin or thapsigargin as in (A) and then metabolically labeled for 20 min with [35S] Cys+Met mix and chased for the indicated times. Cell lysates were immunoprecipitated with anti-CD3δ antibodies and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and fluorography. (D) Graph comparing values from a phosphorimager quantitation of the gel in (C), representing percent of pulse-label remaining
We tested whether a similar pathway exists for another well-established ERAD substrate, unassembled CD3δ subunit of the T-cell antigen receptor. In this case the ER quality control machinery senses the lack of assembly of the subunit of the oligomeric receptor targeting it to ERAD (Yang et al 1998; Frenkel et al 2003). After UPR induction by preincubation of cells expressing CD3δ with tunicamycin or thapsigargin, its degradation was much accelerated (Fig 6C,D). After UPR induction there was little or no effect of MG-132 (Fig 6C, lanes 7 and 11). Same as for H2a, phenanthroline following UPR induction also had little or no effect on the degradation of CD3δ (Fig 6C, lanes 8 and 12). Therefore, a similar alternative nonproteasomal degradation pathway is induced by the UPR for unassembled CD3δ.
To determine whether the degradation pathways activated upon UPR include the pathway induced by CHX, we preincubated cells with tunicamycin and thapsigargin for 16 hours and added CHX after pulse labeling. As can be seen in Figure 7, after UPR induction there was no effect of CHX on the degradation of H2a after a short chase period (compare lanes 3 and 4 with 6 and 7) or after a long chase (compare lanes 9 and 10 with 12 and 13). The fact that CHX after UPR induction cannot inhibit degradation after a short chase can be explained by a preactivation of the nonproteasomal pathways. As a result of this preactivation, blockage of the proteasomal pathway after a short chase (2.5 h) with CHX shows no inhibitory effect on the degradation. The fact that degradation after UPR induction is not increased after a long chase (8 h) in the presence of CHX suggests that the phenanthroline-sensitive nonproteasomal pathway (induced by inhibition of protein synthesis) already was activated in addition to a phenanthroline-resistant pathway.
Fig 7.
Cycloheximide (CHX) shows no effect on degradation of H2a after induction of the unfolded protein response (UPR). Experiment similar to that in Figure 1A, but with samples chased in the absence or in the presence of 300 μM CHX as indicated. Immunoprecipitates from cell supernatants (medium) were treated with N-glycosidase F, whereas those from cell lysates (cells) were not
The UPR-induced nonproteasomal degradation does not exist for p53
When a pulse-chase analysis of p53 was performed after prolonged ER stress, in an experiment similar to those in Figure 6, MG-132 had a strong inhibitory effect also after a long (16 h) preincubation with tunicamycin (Fig 8A,B), suggesting that the nonproteasomal pathways do not function for p53. Furthermore, this result strengthens the conclusion that the alternative UPR-induced degradation pathways for ERAD substrates are indeed nonproteasomal. It is known that the UPR also induces proteasomal degradation, but this pathway still can be inhibited by MG-132 as shown by the fate of p53.
Fig 8.
Upon unfolded protein response (UPR), degradation of p53 is still only proteasomal. (A) Experiment similar to that in Fig. 6A except that, after preincubation with tunicamycin, the cells were metabolically labeled for 1 hour with [35S] Cys-Met mix and chased for the indicated times; lysates were immunoprecipitated with anti-p53 antibody and analyzed by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). (B) Quantitative analysis of the effect of the protease inhibitors on p53 degradation by phosphorimager quantitation of the gel in (A). Bars represent p53 label remaining as a percentage of the initial pulse-label. (C) Experiment similar to that in (A) but with samples preincubated in the absence or presence of tunicamycin or thapsigargin, then labeled and chased for the indicated times in the absence or in the presence of 300 μM cycloheximide (CHX) alone or together with 20 μM MG-132 as indicated
Phenanthroline, which strongly inhibited degradation of H2a and CD3δ, did not have any effect on the degradation of p53 in the absence or presence of UPR induction (Fig 8A, lanes 4 and 8, Fig 8B) leading to the conclusion that the Mn2+/Co2+-dependent pathway, sensitive to the action of phenanthroline, is not involved in the degradation of p53. The result also suggests that phenanthroline does not affect in general the proteasomal pathway and that it must affect some step specific to the ERAD pathways. A similar result was obtained in the presence of thapsigargin (data not shown).
In contrast to the result obtained with the ERAD substrate (Fig 7), CHX still blocked degradation of p53 after long preincubation with tunicamycin or thapsigargin (Fig 8C, lanes 8 and 13). Proteasomal degradation, which is inhibited during the transient arrest in protein synthesis at the initial stages of the UPR (Shenkman et al 2007), is restored and activated after long-term incubation with UPR inducers (Travers et al 2000). The proteasomal degradation of p53 thus can be blocked by inhibition of protein synthesis also after prolonged UPR and, as the results indicate, the UPR-induced nonproteasomal pathways that serve the ERAD substrates (Figs 1–7) do not act on p53 (Fig 8C).
DISCUSSION
Our results show that an initial block in proteasomal degradation by inhibition of protein synthesis (Shenkman et al 2007) is compensated long term by activation of an alternative nonproteasomal degradation pathway for ERAD substrates. This pathway involves a metalloprotease or other metalloprotein that uses Mn2+/Co2+, as these were the only cations that, after presaturation, abrogated the activity of phenanthroline as an inhibitor of the degradation (Fig 4). We do not know at present if this degradative pathway is luminal or cytosolic, a subject that should be addressed in future studies. In yeast it had been shown that the Mn2+/Ca2+ Pmr1 pump is required for efficient degradation of the ERAD substrate mutant cpy* (Durr et al 1998; Vashist et al 2002). This could be a result of the need for Mn2+ for the degradation and, therefore, we could speculate that the nonproteasomal pathway may exist in yeast, in which case the requirement of Mn2+ would be luminal. A metalloprotease that has been implicated in trimming of peptides produced by the proteasome is thimet oligopeptidase (Saric et al 2004) and it could have been a candidate for the nonproteasomal degradation that we identified. However, a specific peptide inhibitor of this enzyme had no effect on the degradation. Leucine aminopeptidases, inhibited by bestatin or leucinethiol, also have been implicated in peptide trimming for antigen presentation or to amino acids (Serwold et al 2002; Kloetzel and Ossendorp 2004; Saric et al 2004) but these inhibitors showed no effect on H2a degradation. The lack of effect of dithiothreitol and of apstatin rule out the involvement of the Mn2+-dependent cytosolic aminopeptidase P (Cottrell et al 2000). Whatever the enzymes involved, the nonproteasomal degradation pathway induced by inhibition of protein synthesis obviously cannot require novel protein expression. It probably is activated by degradation of an inhibitor of the participating proteases or accessory proteins or of a modifying enzyme like a phosphatase or kinase that normally keeps the nonproteasomal pathway partially inactive. Activation of this pathway can have important consequences and should be taken into account in the interpretation of experiments where long incubations with protein synthesis inhibitors are used, for example as in so-called CHX chases.
Nonproteasomal degradation from the ER has been described for other proteins (Adeli et al 1997; Moriyama et al 1998; Cabral et al 2000; Myers et al 2004) but further research is needed to determine if these pathways are common or substrate-specific.
The inhibitory effect of phenanthroline, in the absence of CHX or UPR induction, on degradation of ERAD substrates (Figs 4 and 6) and see (Wileman et al 1991) but not on degradation of the cytosolic substrate (Fig 8) suggests that it inhibits a step in the ERAD pathways but not the final proteasomal degradation. This step might be shared by proteasomal ERAD and the alternative nonproteasomal pathway.
As prolonged UPR leads to apoptosis, an alternative degradation pathway could become activated during programmed cell death. However, the general caspase inhibitor, ZVAD-FMK, had no inhibitory effect, making a link to apoptosis unlikely. In fact, it was reported that, during apoptosis, proteasomal degradation is inhibited through inactivation by caspases (Sun et al 2004); even in these conditions the nonproteasomal degradation of ER substrates could persist. Autophagy also is induced upon stress. However, autophagosomes ultimately fuse with lysosomes, which degrade the engulfed proteins. Lysosomal inhibitors showed no effect in inhibiting the alternative degradation pathway (Fig 6), which would exclude the involvement of autophagy.
Figure 9 shows a model summarizing the participation of the proteasomal and nonproteasomal pathways of degradation. Before cell treatment degradation of ER proteins is mostly proteasomal, although some level of constitutive nonproteasomal degradation would exist, which might explain why degradation of ERAD substrates usually cannot be completely blocked by proteasome inhibitors. After short-term (1–3 h) UPR induction or inhibition of protein synthesis, proteasomal degradation is blocked. Prolonged UPR (>6 h) activates proteasomal and nonproteasomal degradation pathways for ER proteins. Long-term inhibition of protein synthesis activates a nonproteasomal pathway for ER proteins but proteasomal degradation remains blocked.
Fig 9.
Model for proteasomal and nonproteasomal degradation of endoplasmic reticulum (ER) and cytosolic substrates. Changes in relative degradation rates are suggested, compared to proteasomal degradation in untreated cells. (A) Proteasomal degradation of ER and cytosolic proteins is blocked upon short-term (1–3 h) unfolded protein response (UPR) induction. Prolonged ER stress (>6 h) activates both proteasomal and nonproteasomal degradation pathways, the latter only for ER proteins. (B) A short-term block in translation inhibits proteasomal degradation of ER and cytosolic proteins whereas long-term inhibition of protein synthesis induces a nonproteasomal Mn2+/Co2+-dependent pathway for ER proteins but not cytosolic ones and does not activate proteasomal degradation
The nonproteasomal pathways induced by the UPR or by protein synthesis inhibition are specific for ER substrates and not for cytosolic ones (at least not for p53). They might be luminal or possess a mechanism for tagging of ER substrates, different than ubiquitination that is not required (Fig 5). This substrate selectivity provides a mechanism for continued proteasomal control of the levels of p53 and probably of other cytosolic proteins after UPR induction, although accumulated misfolded ER proteins are disposed of by separate nonproteasomal pathways.
Acknowledgments
We are grateful to W. Simmons and T. Rapoport for reagents, to M. Kondratyev for technical assistance, and to E. Bacharach and D. Wreschner for helpful advice in writing the manuscript. This work was supported by a grant from the U.S.–Israel Binational Science Foundation.
REFERENCES
- Adeli K, Macri J, Mohammadi A, Kito M, Urade R, Cavallo D. Apolipoprotein B is intracellularly associated with an ER-60 protease homologue in HepG2 cells. J Biol Chem. 1997;272:22489–22494. doi: 10.1074/jbc.272.36.22489.0021-9258(1997)272[22489:ABIIAW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cabral CM, Choudhury P, Liu Y, Sifers RN. Processing by endoplasmic reticulum mannosidases partitions a secretion-impaired glycoprotein into distinct disposal pathways. J Biol Chem. 2000;275:25015–25022. doi: 10.1074/jbc.M910172199.0021-9258(2000)275[25015:PBERMP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Hooper NM, Turner AJ. Cloning, expression, and characterization of human cytosolic aminopeptidase P: a single manganese(II)-dependent enzyme. Biochemistry. 2000;39:15121–15128. doi: 10.1021/bi001585c.0006-2960(2000)039[15121:CEACOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Durr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Cell Biol. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149.1098-5549(1998)009[1149:TMIPPS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel Z, Gregory W, Kornfeld S, Lederkremer GZ. Endoplasmic reticulum-associated degradation of mammalian glycoproteins involves sugar chain trimming to Man6-5GlcNAc2. J Biol Chem. 2003;278:34119–34124. doi: 10.1074/jbc.M305929200.0021-9258(2003)278[34119:ERDOMG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Friedlander R, Jarosch E, Urban J, Volkwein C, Sommer T. A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat Cell Biol. 2000;2:379–384. doi: 10.1038/35017001.1465-7392(2000)002[0379:ARLBEP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8.1097-2765(2000)006[1099:RTICSG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kamhi-Nesher S, Shenkman M, Tolchinsky S, Fromm SV, Ehrlich R, Lederkremer GZ. A novel quality control compartment derived from the endoplasmic reticulum. Mol Cell Biol. 2001;12:1711–1723. doi: 10.1091/mbc.12.6.1711.1098-5549(2001)012[1711:ANQCCD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel PM, Ossendorp F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr Opin Immunol. 2004;16:76–81. doi: 10.1016/j.coi.2003.11.004.0952-7915(2004)016[0076:PAPFIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kulka RG, Raboy B, Schuster R, Parag HA, Diamond G, Ciechanover A, Marcus M. A Chinese hamster cell cycle mutant arrested at G2 phase has a temperature-sensitive ubiquitin-activating enzyme, E1. J Biol Chem. 1988;263:15726–15731.0021-9258(1988)263[15726:ACHCCM]2.0.CO;2 [PubMed] [Google Scholar]
- Lederkremer GZ, Glickman MH. A window of opportunity: timing protein degradation by trimming of sugars and ubiquitins. Trends Biochem Sci. 2005;30:297–303. doi: 10.1016/j.tibs.2005.04.010.0376-5067(2005)030[0297:AWOOTP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766.1465-7392(2005)007[0766:ETLRTD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Moriyama T, Sather SK, McGee TP, Simoni RD. Degradation of HMG-CoA reductase in vitro. Cleavage in the membrane domain by a membrane-bound cysteine protease. J Biol Chem. 1998;273:22037–22043. doi: 10.1074/jbc.273.34.22037.0021-9258(1998)273[22037:DOHRIV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Myers MP, Khanna R, Lee EJ, Papazian DM. Voltage sensor mutations differentially target misfolded K+ channel subunits to proteasomal and nonproteasomal disposal pathways. FEBS Lett. 2004;568:110–116. doi: 10.1016/j.febslet.2004.05.023.0014-5793(2004)568[0110:VSMDTM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romisch K. Endoplasmic reticulum–associated degradation. Annu Rev Cell Dev Biol. 2005;21:435–456. doi: 10.1146/annurev.cellbio.21.012704.133250.1081-0706(2005)021[0435:ERD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Saric T, Graef CI, Goldberg AL. Pathway for degradation of peptides generated by proteasomes: a key role for thimet oligopeptidase and other metallopeptidases. J Biol Chem. 2004;279:46723–46732. doi: 10.1074/jbc.M406537200.0021-9258(2004)279[46723:PFDOPG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sayeed A, Ng DT. Search and destroy: ER quality control and ER-associated protein degradation. Crit Rev Biochem Mol Biol. 2005;40:75–91. doi: 10.1080/10409230590918685.1040-9238(2005)040[0075:SADEQC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134.0066-4154(2005)074[0739:TMUPR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Serwold T, Gonzalez F, Kim J, Jacob R, Shastri N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419:480–483. doi: 10.1038/nature01074.1476-4687(2002)419[0480:ECPFMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shenkman M, Ayalon M, Lederkremer GZ. Endoplasmic reticulum quality control of asialoglycoprotein receptor H2a involves a determinant for retention and not retrieval. Proc Natl Acad Sci U S A. 1997;94:11363–11368. doi: 10.1073/pnas.94.21.11363.1091-6490(1997)094[11363:ERQCOA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkman M, Tolchinsky S, Kondratyev M, Lederkremer GZ. Transient arrest in proteasomal degradation during inhibition of translation in the unfolded protein response. Biochem J. 2007;404:509–516. doi: 10.1042/BJ20061854.0264-6021(2007)404[0509:TAIPDD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XM, Butterworth M, MacFarlane M, Dubiel W, Ciechanover A, Cohen GM. Caspase activation inhibits proteasome function during apoptosis. Mol Cell. 2004;14:81–93. doi: 10.1016/s1097-2765(04)00156-x.1097-2765(2004)014[0081:CAIPFD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tolchinsky S, Yuk MH, Ayalon M, Lodish HF, Lederkremer GZ. Membrane-bound versus secreted forms of human asialoglycoprotein receptor subunits—role of a juxtamembrane pentapeptide. J Biol Chem. 1996;271:14496–14503. doi: 10.1074/jbc.271.24.14496.0021-9258(1996)271[14496:MVSFOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1.0092-8674(2000)101[0249:FAGARA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vashist S, Frank CG, Jakob CA, Ng DT. Two distinctly localized p-type ATPases collaborate to maintain organelle homeostasis required for glycoprotein processing and quality control. Mol Cell Biol. 2002;13:3955–3966. doi: 10.1091/mbc.02-06-0090.1098-5549(2002)013[3955:TDLPAC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T, Kane LP, Terhorst C. Degradation of T-cell receptor chains in the endoplasmic reticulum is inhibited by inhibitors of cysteine proteases. Cell Regul. 1991;2:753–765. doi: 10.1091/mbc.2.9.753.1044-2030(1991)002[0753:DOTRCI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Omura S, Bonifacino JS, Weissman AM. Novel aspects of degradation of T-cell receptor subunits from the endoplasmic reticulum (ER) in T-cells: importance of oligosaccharide processing, ubiquitination, and proteasome-dependent removal from ER membranes. J Exp Med. 1998;187:835–846. doi: 10.1084/jem.187.6.835.0022-1007(1998)187[0835:NAODOT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk MH, Lodish HF. Two pathways for the degradation of the H2 subunit of the asialoglycoprotein receptor in the endoplasmic reticulum. J Cell Biol. 1993;123:1735–1749. doi: 10.1083/jcb.123.6.1735.0021-9525(1993)123[1735:TPFTDO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]