Abstract
The Whitehall Study is a prospective epidemiological study of cardiovascular risk factors in healthy members of the British Civil Service, which has identified psychological distress as a major risk factor for coronary heart disease. The levels of circulating Hsp60 in 860 participants from the Whitehall cohort and 761 individuals diagnosed with diabetes have been measured and related to psychological, biological, and genetic factors. In the Whitehall participants, concentrations of Hsp60 ranged from undetectable to mg/mL levels. Circulating Hsp60 correlated with total and low-density lipoprotein (LDL) cholesterol and was positively associated with a flattened slope of cortisol decline over the day. Levels of this stress protein also correlated with measures of psychological stress including psychological distress, job demand, and low emotional support. Mass spectrometric analysis of circulating immunoreactive Hsp60 reveal that it is predominantly the intact protein with no mitochondrial import peptide, suggesting that this circulating protein emanates from mitochondria. The Hsp60 is stable when added to plasma and the levels in the circulation of individuals are remarkably constant over a 4-year period, suggesting plasma levels are partly genetically controlled. Sequence analysis of the HSP60-HSP10 intergenic promoter region identified a common variant 3175 C>G where the G allele had a frequency of 0.30 and was associated with higher Hsp60 levels in 761 type 2 diabetic patients. The extended range of plasma Hsp60 concentrations in the general population is genuine and is likely to be related to genetic, biological, and psychosocial risk factors for coronary artery disease.
INTRODUCTION
Accumulating evidence shows that psychosocial factors contribute to coronary heart disease (CHD) risk, with variables such as low socioeconomic status (SES), psychological distress, high work stress, and low levels of social support being associated with morbidity and mortality independently of conventional biological risk factors (Rosengren et al 2004; Everson-Rose and Lewis 2005; Kuper et al 2005). The mechanisms underlying these effects are thought to include changes in healthy habits or lifestyle, and direct biological influences on inflammatory factors, autonomic function, and the metabolic syndrome. For example, in the Whitehall II prospective epidemiological cohort, low SES has been associated with elevated plasma fibrinogen, interleukin 6 (IL-6) and C-reactive protein and reduced heart rate variability (Brunner et al 1996; Hemingway et al 2003; Hemingway et al 2005); chronic work stress predicts the development of the metabolic syndrome, and psychological distress is a risk factor for CHD in men (Stansfeld et al 2002; Chandola et al 2006).
One mechanistic hypothesis that has significant experimental support predicts that atherosclerosis is an autoimmune disease driven by crossreactive immunity to the mitochondrial molecular chaperone, heat shock protein 60 ([Hsp60], Wick et al 2004). It is therefore possible that psychosocial factors influence the presence in the plasma of Hsp60. In an analysis of a small sample of participants from the Whitehall II cohort, we identified Hsp60 in the plasma of a substantial proportion of these individuals (Lewthwaite et al 2002). Plasma Hsp60 levels were associated with psychological distress in women and with lower SES and social isolation in both men and women. This preliminary study left a number of key unanswered questions including (1) the reproducibility of the results; (2) the nature of the analyte being measured; (3) the reason for the enormous variation in levels in human circulation; and (4) whether the Hsp60 levels being measured are the result of short-term environmental factors or are genetically determined and stable. These questions have been addressed using a much larger cohort of British civil servants and also a cohort of patients with type 2 diabetes.
MATERIALS AND METHODS
Participants
Three groups were investigated. The main analyses were carried out on 860 members of the Whitehall II epidemiological cohort who were consecutive attendees at medical screening sessions that took place from 2003– 2004 (Marmot and Brunner 2005). We excluded those with a history of CHD, stroke, or transient ischaemic attack, and individuals who had been prescribed cardiovascular medication. The sample included 541 men and 319 women aged 50–72 years (mean 60.2 ± 5.7). Genetic analyses were carried out with a cohort of 761 Caucasian patients (457 men, 304 women) with type 2 diabetes recruited into the University College London (UCL) Diabetes and Cardiovascular Disease Study (UDACS) described elsewhere (Stephens et al 2004; Shamaei-Tousi et al 2006). The stability of plasma Hsp60 over 30 days was investigated in 14 patients suffering from severe chronic periodontitis being treated at the UCL Eastman Dental Hospital. Appropriate medical research ethics committees approved all studies, and participants gave written consent.
Biological measures
Height, weight, and waist and hip circumference were measured using standardized methods, and body mass index (BMI) and waist/hip ratio were calculated. A fasting blood sample was drawn for lipid analyses, and total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol were determined. Systolic and diastolic blood pressure were measured with the participant seated using an electronic sphygmomanometer. Salivary free cortisol was measured as a biological indicator of stress, with samples taken on 4 occasions over a typical day: 2.5, 8, and 12 hours after waking (means 9:18 hours, 14:52 hours, and 18:25 hours, respectively) and at bedtime (mean 23:08 hours). Cortisol was assayed using a time-resolved immunoassay with fluorescence detection. We analyzed the magnitude of cortisol decrease between the first and last sample, together with the coefficient of variation of all 4 samples. Smaller decreases and a smaller coefficient of variation over the day indicate a flatter cortisol profile, a pattern that is associated with chronic psychological stress (Abercrombie et al 2004; Barnett et al 2005). Complete cortisol data were available from 648 participants.
Analysis of circulating Hsp60
Concentrations of human Hsp60 in participants' plasma were assayed by the method described previously using highly purified recombinant Hsp60 (Lewthwaite et al 2002; Maguire et al 2003). To determine if human plasma contained substances that interfered with the assay of Hsp60 and produced artificially low or high results, plasma samples from individuals with low, medium, or high Hsp60 levels were spiked with known concentrations of recombinant Hsp60 and were assayed for Hsp60 levels.
Stability of Hsp60 in plasma
Plasma samples from individuals with undetectable, medium, or high Hsp60 levels were incubated with 11 μg recombinant Hsp60 in sealed tubes at 37°C for 48 hours. Samples were removed at 0.5, 1, 2, 4, 8, 24, and 48 hours and assayed for the presence of Hsp60 by Western blotting and 2-site enzyme-linked immunosorbent assay (ELISA).
Analysis of the presence of the Hsp60 mitochondrial import peptide
The peptide VFRQMRPVSRVLA, which represents amino acid 7 to 19 of the 30 residue N-terminal mitochondrial import peptide of Hsp60, was synthesized by Eurogentec (Seraing, Belgium) and used to raise a high titer rabbit polyclonal antiserum. This was used in Western blotting experiments and in conjunction with the LK-1 monoclonal capture antibody in an ELISA assay to determine if any of the circulating Hsp60 in human blood contains the mitochondrial import peptide.
Mass spectrometric analysis of circulating Hsp60
A number of plasma samples with high levels of Hsp60 were chosen for analysis of the nature of the circulating antigen. The Hsp60 in these samples was immunoprecipitated using Seize X protein G Immunoprecipitation Kit from Pierce (Rockford, IL, USA), according to the vendor. The protein(s) binding to columns was analyzed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) using the method of Laemmli (1970). Proteins were stained with colloidal Coomassie brilliant blue (Sigma, St Louis, MO, USA) and digested in-gel with trypsin prior to analysis of peptides by liquid chromatography/tandem mass spectrometry (LC-MS/MS) using a ProteomeX system (Thermo Electron, Hemel Hempstead, UK). Spectra were searched against the translated human database downloaded from the National Center for Biotechnology Information ([NCBI], http://www.ncbi.nlm.nih.gov) using Bioworks v3.1 with TurboSEQUEST software (Thermo Electron).
Determination of the variation in individual circulating Hsp60 levels
A group of 69 Whitehall II study participants whose blood had been taken in 1999/2000 and assayed for Hsp60 in our preliminary study (Lewthwaite et al 2002) were reassessed for this study in order to investigate stability over a 4-year period. Shorter-term stability was assessed in the dental patients. They provided blood on 6 occasions over a period of 30 days (baseline and 1, 3, 5, 7, and 30 days after periodontal therapy), and levels of plasma Hsp60 were assayed using ELISA.
Sequencing of the HSP60/HSP10 bidirectional promoter
Genomic DNA was prepared from the circulating mononuclear cells of 7 individuals with high circulating Hsp60 and 8 with low Hsp60 levels from the Whitehall II cohort. The promoter region and the first exons of both the genes were amplified (from position 2996 to 3957, accession number AJ250915) according to the method described by Hansen et al (2003). Sequencing of the polymerase chain reaction (PCR) products was performed with appropriate sequencing primers using protocols provided for the DYEnamic ET Dye Terminator Cycle Sequencing kit and the MegaBACE DNA analysis system (Amersham Biosciences, Little Chalfont, Bucks, UK).
Genotyping of the 3175 G>C and 3700 C>T variants
The sequence surrounding the 3175 G>C polymorphism contains a naturally occurring MspA1I restriction enzyme site. The 3175 G>C wild-type sequence (CAGCGG) contains this MspA1I site, which is not presented in the rare variant (CAGCCG). In the presence of the G allele, MspA1I digestion yields fragments of 82 base pairs (bp) and 46 bp, whereas in the presence of the C allele, digestion does not occur. Around the other polymorphism site, 3700 C>T, a naturally occurring TseI restriction enzyme site was found. In this case, the 3700 C>T rare variant (GCTGG) contains the TseI site, which is not present in the wild-type sequence (GCCGG). That would mean that, in the presence of the T allele, TseI digestion yields fragments of 96 and 56 bp, whereas in the presence of the C allele, digestion does not occur. Digested polymerase chain reaction fragments were separated as described elsewhere (Day and Humphries 1984).
Psychosocial measures
Psychological distress was assessed using the General Health Questionnaire 30 (GHQ) (Goldberg 1972). In the full Whitehall II study, high scores on the GHQ have been found to predict future CHD (Stansfeld et al 2002). In the present analyses, the sample was divided into low-distress (0–4), moderate-distress (5–14), and high-distress (≥15) groups. Job demands were measured as a source of chronic stress in participants who were still in paid employment using a questionnaire developed in the Whitehall II study that also has been shown to predict future CHD in initially disease-free individuals (Kuper and Marmot 2003). Ratings could range from 0 to 100, with higher scores representing greater job demands. Emotional social support was assessed with the Close Person's Questionnaire (Stansfeld and Marmot 1992). A relationship between low emotional support and CHD has been observed in several longitudinal studies (Lett et al 2005). The job demands and emotional support scales were divided into tertiles for the purposes of analysis.
Analyses of socioeconomic status (SES) in the Whitehall II cohort primarily have been based on grade of employment within the civil service. However, only one-third of respondents still worked within the civil service at the time of testing, others having either retired or moved to another occupation. Household income therefore was used as the measure of SES, with the sample being divided into those with low (<£25,000), medium (£25,000–£70,000), and high (>£70,000) incomes.
Statistical analysis
The distribution of Hsp60 concentrations was markedly skewed (Fig 1), so associations with biological and psychosocial risk factors were analyzed in 2 ways. First, comparisons were made between participants with very high Hsp60 concentrations (≥1000 ng/mL) and the remainder. Second, we compared participants with and without any detectable plasma Hsp60. In each case, associations with biological and psychosocial factors were analyzed using χ2, followed by logistic regression, computing odds ratios with 95% confidence intervals (CIs) of high Hsp60 or detectable levels across the tertiles of risk factors, adjusted for potential confounders, including age, gender, BMI, and smoking status. Associations with Hsp60 genotypes were examined with a Mann-Whitney U test. Differences in prevalence of genotypes in subjects with Hsp60 below and above 1000 ng/mL were tested by χ2 test or Fisher's exact test. The linkage disequilibrium coefficient (D′) between FVII polymorphisms was calculated according to Chakravarti et al (1984).
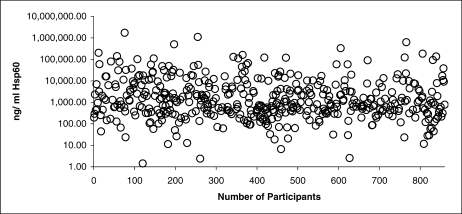
Fig 1.
Scatter plot showing the concentration of human Hsp60 in the plasma of each of the sequential 457 Whitehall II samples that gave a positive response in the enzyme-linked immunosorbent assay (ELISA). The concentration (ng/mL) of Hsp60 is on a logarithmic scale
RESULTS
Distribution of immunoreactive Hsp60 in Whitehall II cohort
Levels were below the limit of assay detection (approximately 1 ng/mL) in 403 individuals (46.9% of the sample; results not shown), whereas 229 (26.6%) had values ≥1000 ng/mL, and the remainder had intermediate levels. The distribution of plasma Hsp60 levels in the 467 consecutive individuals with measurable Hsp60 is shown in Figure 1.
Molecular nature of plasma Hsp60
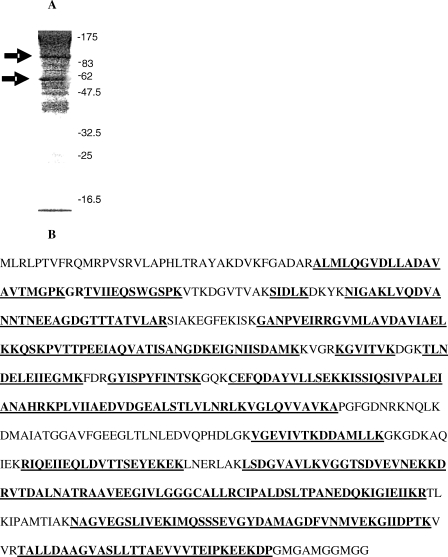
A polyclonal antisera against the Hsp60 mitochondrial import peptide, although able to identify the synthetic peptide, failed to bind to plasma samples containing high levels of Hsp60. Hsp60 immunoadsorbed by monoclonal antibody LK-1 was separated on SDS-PAGE, and 2 major bands were visualized in high titer samples, but not in samples in which Hsp60 was undetectable (Fig 2A). These bands were of 60 kDa and >60 kDa with a smaller band appearing in some samples. The 60 kDa and >60 kDa bands were subject to trypsin digestion and peptide mass fingerprinting. Both protein bands provided a large number of identifiable peptides (>70% coverage) covering the whole Hsp60 protein sequence from the N-terminus to the C-terminus but without identifying the mitochondrial import peptide. Thus both high molecular mass bands seen on SDS-PAGE are genuine and intact Hsp60, but lack the mitochondrial import peptide (Fig 2B).
Fig 2.
Figure 2A shows the profile of the proteins on polyacrylamide gel electrophoresis (PAGE), which have been isolated from one of the human plasma samples with a high Hsp60 concentration by immunoadsorption with monoclonal antibody LK-1. The protein bands (58 kdDa and >82 kDa) were dissected from the gel and digested with trypsin and subject to matrix-assisted laser desorption ionisation-time of flight (MALDI-TOF) mass spectrometry to identify the peptide fingerprint. Figure 2B shows the peptides (underlined) that were identified as belonging to the human Hsp60 protein sequence. Over 70% of the Hsp60 sequence was identified in these analyses. However, in none of the mass spectrometric analyses was the N-terminal mitochondrial import peptide identified
Stability of Hsp60 in blood and presence of interfering substances
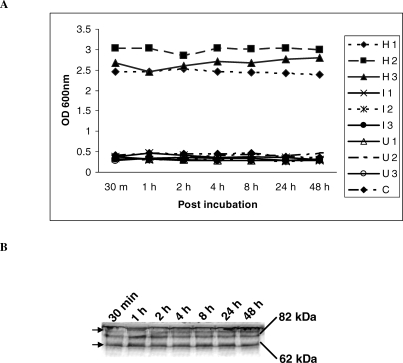
Incubation of known concentrations of Hsp60 in human plasma at 37°C for up to 48 hours revealed no loss of immunoreactive material (Fig 3A) and no significant protein breakdown as assessed by Western immunoblotting (Fig 3B). Recovery was complete of the spiked Hsp60 from plasma samples containing low, intermediate, or high levels of Hsp60.
Fig 3.
Stability of Hsp60 in plasma. Eleven micrograms of recombinant Hsp60 protein was added to each of 9 samples (H1–H3: >1000 ng/mL Hsp60; I1–I3: 1–1000 ng/mL Hsp60; and U1–U3 with undetectable levels of plasma Hsp60). Samples were removed at various time points up 48 hours after incubation. The samples were analyzed by two-site enzyme-linked immunosorbent assay (ELISA; Fig 2A) and Western blotting. Figure 3B shows a representative Western blotting assay of 1 of 3 samples with high Hsp60 concentration. For clarity, the Hsp60 monomer (58 kDa) and oligomer (82 kDa) are depicted
Temporal variation in plasma Hsp60 concentrations
The correlation between plasma Hsp60 in 69 individuals retested after 4 years was significant (Spearman r = 0.52, P < 0.001), and 68% of those with Hsp60 ≥1000 ng/mL when originally tested showed similar values in the second sample taken 4 years later. Acute variations in plasma Hsp60 levels also were assessed in 14 dental patients from whom blood was taken on 6 occasions over 30 days. Six patients had measurable levels of Hsp60 that varied from 100 ng/mL to >100 μg/mL. For each individual, the plasma levels of Hsp60 were stable over the 30-day period of analysis (results not shown).
Analysis of the Hsp60/Hsp10 promoter
The entire Hsp60/Hsp10 promoter region (from position 3054 to 3712) was sequenced in 8 individuals with undetectable Hsp60 blood levels and 7 with Hsp60 blood levels >1000 ng/mL. As shown in Figure 4A, 2 previously reported (Hansen et al 2003) common variants were detected in these samples, a 3700 C>T change and a 3175 G>C change. These changes were found in subjects with both high and low levels of Hsp60; however, although the prevalence of the 3700T allele was similar in those with low and high levels, there was a suggestion that the 3175G allele was of low prevalence in those with undetectable Hsp60 levels, and particularly the GG genotype was absent from this group. To examine this further, we genotyped a cohort of individuals with type 2 diabetes. The distribution of Hsp60 levels in the type 2 diabetes patients was similar to that of the Whitehall II sample, as previously described (Shamaei-Tousi et al 2006). For both genotypes the distributions observed were as expected from Hardy Weinberg proportions. The frequency of the 3700T allele was 0.19 and, for the 3175G allele, was 0.30. For the 3700C>T genotype, there was no statistically significant difference in median Hsp60 levels (CC n = 328, 37.9 [0–1227]; CT n = 157, 41.9 [0–1391]; TT n = 13, 0 [0– 2186], P = 0.36). However, for the 3175 C>G genotype, there was significant association between the G allele and higher levels of Hsp60 (CC n = 246, 28.9 [0–1211]; CG n = 210, 31.6 [0–820]; GG n = 50, 76.9 [0–5546]; CC+CG vs GG P = 0.05). As shown in Figure 4B, this was reflected in a significantly higher proportion of the GG subjects having Hsp60 levels >1000 ng/mL (P = 0.02) compared to those with other genotypes combined, whereas there was no such effect associated with 3700 C>T genotype. These associations were not affected by adjustment for potential confounders such as age, gender, or duration of diabetes (data not shown).
Fig 4.
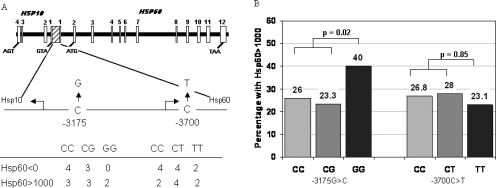
(A) schematic presentation of the common promoter region of the Hsp10 and Hsp60 genes and the associated introns and exons of Hsp60, showing the region sequenced and the genotype distribution of the two detected single nucleotide polymorphisms (SNPs) in subjects with 0 and >1000 ng/mL Hsp60. For −3175 C>G, the P-value for the comparison of genotype frequencies = 0.35, and the frequencies of the G allele are 0.21 and 0.44, respectively (P = 0.07). For −3700 C>T, the genotype frequency comparison between groups is 0.4 and the frequencies of the T allele are 0.36 and 0.50, respectively (P = 0.43). (B) Histogram showing the percentage of Type 2 Caucasian diabetic subjects with Hsp60 >1000 ng/mL with each different SNP genotype. Numbers shown at the base of each bar is the total number with each genotype
Association of plasma Hsp60 with biological risk factors
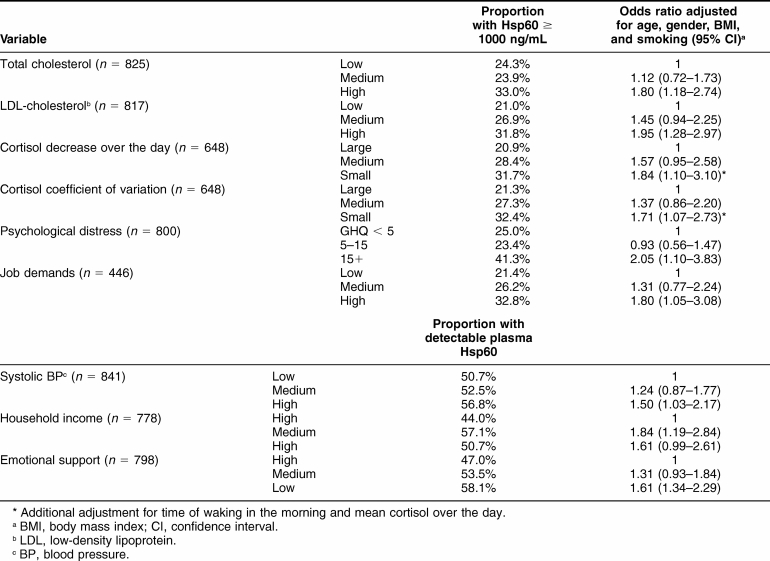
The presence of high plasma Hsp60 (≥1000 ng/mL) in the Whitehall II cohort was associated with total and LDL cholesterol. After adjustment for age, gender, BMI, and smoking status, the odds of a high Hsp60 concentration were 1.80 (CI 1.18 to 2.74 P < 0.001) for participants in the high compared with low total cholesterol tertile (Table 1). The corresponding odds for participants with high LDL cholesterol were 1.95 (1.28 to 2.97 P < 0.001). Hsp60 was not related to HDL cholesterol. Salivary cortisol averaged 10.6 ± 6.4, 7.07 ± 4.8, 3.51 ± 2.8, and 3.24 ± 4.2 nmol/L in the 4 samples collected over the day. The slope of cortisol decrease over the day was inversely associated with Hsp60 ≥1000 ng/mL; 31.7% of participants in the lowest tertile of cortisol decreases had elevated Hsp60, compared with 20.9% of those with the largest decrease. The odds of Hsp60 ≥1000 ng/mL in the lowest decrease tertile were 1.84 (1.10 to 3.10, P = 0.021) after adjusting not only for age, gender, BMI, and smoking status, but also for time of waking and average cortisol over the day. Similarly, participants with a low coefficient of variation of cortisol were more likely to have Hsp60 ≥1000 ng/mL (odds ratio 1.71, 1.07 to 2.73, P = 0.023). Systolic blood pressure was not associated with high plasma Hsp60. However, the presence of any Hsp60 in plasma was more likely in participants with higher systolic blood pressure, with odds for the top versus bottom blood pressure tertile of 1.50 (1.03 to 2.17, P = 0.04) after adjustment for age, gender, BMI, and smoking status.
Table 1.
Factors associated with plasma Hsp60 in Whitehall Cohort
Plasma Hsp60 and psychosocial risk factors
Two psychosocial risk factors were associated with the presence of Hsp60 ≥1000 ng/mL (Table 1). First, 41.3% of participants with GHQ psychological distress scores ≥15 had elevated Hsp60, compared with 25% of those with low scores. The odds of a high Hsp60 were 2.05 (1.10 to 3.83, P = 0.024) in the elevated GHQ group after adjustment for age, gender, BMI, and smoking status. Second, an association was observed with job demands in the 440 men and women who were still working. The adjusted odds of an Hsp60 concentration ≥1000 ng/mL were 1.80 (1.05 to 3.08) for participants in the high compared with low job demand tertile (P = 0.032).
No relationship between Hsp60 ≥1000 ng/mL and income or social support was observed, but these factors were associated with the presence of detectable Hsp60 in the plasma. As shown in Table 1, people with low emotional support were more likely to have detectable Hsp60, with adjusted odds of 1.61 (1.34 to 2.29, P = 0.007). Additionally, household income was inversely related to the presence of Hsp60 in plasma; 50.7% of participants with low incomes and 57.1% of those with medium incomes had detectable Hsp60, compared with only 44% of high-income respondents (P = 0.006). Thus, compared to the initial study of 291 Whitehall II individuals (Lewthwaite et al 2002) in which Hsp60 levels only correlated with psychological stress in females, this larger study shows that this relationship between blood levels of Hsp60 and stress holds both for men and women.
DISCUSSION
Immunity to Hsp60 has been proposed to be involved in the pathogenesis of atherosclerosis (Xu et al 1992; Wick et al 2004). The discovery that Hsp60 is a potent cell-cell signaling protein, able to activate myeloid and vascular endothelial cells (Maguire et al 2002; Verdegaal et al 1996) and modulate T and B cell function (Cohen-Sfady et al 2005) forms part of a new paradigm that proposes that molecular chaperones act as homeostatic signaling proteins involved in controlling key systems such as immunity (Henderson and Pockley 2005). This raised the possibility that human Hsp60 could directly participate in the pathogenesis of atherosclerosis.
This hypothesis has received some support from the finding that Hsp60 is elevated in individuals with evidence of atherosclerosis (Xu et al 2000; Pockley et al 2000). The authors also have found a correlation between high Hsp60 levels and endothelial dysfunction, as assessed by flow-mediated vasodilatation, in a cohort of 13–16 year olds (Halcox et al 2005) and associations with carotid atherosclerosis in young adults have been reported as well (Knoflach et al 2003).
The present study confirms and extends the associations between levels of plasma Hsp60 and psychosocial factors we observed in our preliminary study (Lewthwaite et al 2002). We previously found that Hsp60 levels were elevated among women reporting high levels of psychological distress. This association was confirmed in this larger sample using the same measure of distress. However, in addition, we have found that there was also an association between circulating Hsp60 and psychological stress in men. Our earlier observation of a positive association between Hsp60 and social isolation was corroborated with measures of emotional social support. The relationship between Hsp60 and lower socioeconomic status also was confirmed with measures of household income. In addition, we found high levels of Hsp60 in individuals experiencing intense job demands. Job demands have been related to future CHD in the Whitehall II cohort and other studies (Stansfeld et al 2002; Kuper et al 2005).
The association between Hsp60 and cortisol secretion over the day argues for a relationship with neuroendocrine as well as psychological markers of stress. A flatter profile of cortisol over the day previously has been observed in people reporting marital stress (Barnett et al 2005) and low social support (Abercrombie et al 2004). The association was independent of the average level of cortisol, suggesting dysregulation of the circadian rhythm of neuroendocrine function, rather than differences in absolute levels of secretion or clearance. Collectively, these findings suggest that one mechanism through which psychosocial factors may increase risk of coronary artery disease is by promoting inflammatory processes involving Hsp60. The relationship between plasma Hsp60 and elevated total and LDL cholesterol indicates that these inflammatory processes may act synergistically with lipids in accelerating atherogenesis. However, it should be emphasized that these results are cross-sectional, and longitudinal analyses are required to confirm whether plasma Hsp60 is a risk factor for CHD.
Hsp60 was below the limit of assay detection (approximately 1 ng/mL) in 403 individuals (46.9% of the sample). In contrast, 229 people (26.6%) had values ≥1000 ng/mL, with the remainder (26.5%) having intermediate levels. The concentrations of circulating Hsp60 show an extraordinary million-fold range, from 1 ng/mL to >1 mg/mL. This raises the obvious question of whether the results are an artifact of the assay. Immunoprecipitation of high titer plasma with the monoclonal antibody used in the Hsp60 ELISA-isolated proteins of between 60 and 120 kDa. Bands of 60 and 120 kDa were subject to mass spectrometric peptide fingerprinting and both were identified as Hsp60. The peptide mass fingerprints contained 70% sequence coverage but did not identify the N-terminal mitochondrial import peptide. A specific antiserum to this mitochondrial import peptide failed to react with any of the plasma samples tested. The conclusion is that the circulating material identified by ELISA is mainly the full-length Hsp60 protein minus the mitochondrial import peptide. This supports the hypothesis that circulating Hsp60 emanates from the mitochondria of some unidentified cell population.
The stability of Hsp60 levels in blood also was measured. Incubating recombinant Hsp60 in plasma at 37°C for up to 48 hours revealed no significant degree of breakdown. The reassessment of plasma Hsp60 levels over 4 years indicated moderate stability over this time period, although repeated assessments over 30 days showed no significant changes in any participants.
These findings indicate that, despite the probable influence of psychosocial factors, plasma Hsp60 is rather stable. One simple explanation is that circulating levels are partly under genetic control. We examined the intergenic region between the Hsp60 and Hsp10 genes, which contains the promoter for both genes, in a small number of Whitehall II participants and in the 760 diabetic patients. Hansen et al (2003) identified 4 sequence variants in this region, of which we found only the 2 that they report as the most common. The 3700C>T occurs downstream of the start of transcription and is not within any recognized sequence element for a nuclear transcription factor. By contrast, 3175C>G, which in this study was associated with significant effects on circulating Hsp60 levels, occurs within a unique 25 base pair insertion of DNA that is not found in either the mouse or rat gene. Hansen et al (2003) reported no sequence changes altering amino acids in 20 alleles sequenced, although they did report that examination of expressed sequence tag libraries suggest 3 different exon-1 sequences are spliced to exon-2. They suggested that possible roles of the alternatively spliced 5′ untranslated regions (UTRs) could be the modification of the translation or initiation site, the different stability of the transcripts, or the possibility of using diverse mechanisms for transcription initiation. All of these possibilities warrant further examination, as well as more detailed examination of intron/exon junctions and the 3′ UTR of the gene, because the 2 SNPs examined here clearly are not explaining the large population variation in Hsp60 levels, which probably are controlled by multiple factors.
Acknowledgments
The financial support of the British Heart Foundation (grant PG 03/029) to B.H., A.S., M.M., and A.R.M.C. is gratefully acknowledged.
Footnotes
Current address for Jeffrey W. Stephens: The Medical School, University of Wales Swansea, Swansea, SA2 8PP, UK.
REFERENCES
- Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003.0306-4530(2004)029[1082:FCRIMB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Barnett RC, Steptoe A, Gareis KC. Marital-role quality and stress-related psychobiological indicators. Ann Behav Med. 2005;30:36–43. doi: 10.1207/s15324796abm3001_5.0883-6612(2005)030[0036:MQASPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brunner E, Davey Smith G, Marmot M, Canner R, Beksinska M, O'Brien J. Childhood social circumstances and psychosocial and behavioral factors as determinants of plasma fibrinogen. Lancet. 1996;347:1008–1013. doi: 10.1016/s0140-6736(96)90147-6.0140-6736(1996)347[1008:CSCAPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Buetow KH, Antonarakis SE, Waber PG, Boehm CD, Kazazian HH. Nonuniform recombination within the human beta-globin gene cluster. Am J Hum Genet. 1984;36:1239–1258.0002-9297(1984)036[1239:NRWTHB]2.0.CO;2 [PMC free article] [PubMed] [Google Scholar]
- Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332:521–525. doi: 10.1136/bmj.38693.435301.80.1468-5833(2006)332[0521:CSAWAT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Sfady M, Nussbaum G, Pevsner-Fischer M, Mor F, Carmi P, Zanin-Zhorov A, Lider O, Cohen IR. Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. J Immunol. 2005;175:3594–3602. doi: 10.4049/jimmunol.175.6.3594.0022-1767(2005)175[3594:HSPABC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Day IN, Humphries SE. Electrophoresis for genotyping: microtiter array diagonal gel electrophoresis on horizontal polyacrylamide gels, hydrolink, or agarose. Anal Biochem. 1994;222:389–395. doi: 10.1006/abio.1994.1507.0003-2697(1994)222[0389:EFGMAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542.0163-7525(2005)026[0469:PFACD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Goldberg D Manual of the General Health Questionnaire. Windsor: NFER-Nelson. 1972. [Google Scholar]
- Halcox JP, Deanfield J, Shamaei-Tousi A, Henderson B, Steptoe A, Coates AR, Singhal A, Lucas A. Circulating human heat shock protein 60 in the blood of healthy teenagers: a novel determinant of endothelial dysfunction and early vascular injury? Arterioscler Thromb Vasc Biol. 2005;25:e141–142. doi: 10.1161/01.ATV.0000185832.34992.ff.1079-5642(2005)025[e141:CHHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hansen JJ, Bross P, and Westergaard M. et al. 2003 Genomic structure of the human mitochondrial chaperonin genes: HSP60 and HSP10 are localized head to head on chromosome 2 separated by a bidirectional promoter. Hum Genet. 112:71–77. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Shipley M, Brunner E, Britton A, Malik M, Marmot M. Does autonomic function link social position to coronary risk? The Whitehall II study. Circulation. 2005;111:3071–3077. doi: 10.1161/CIRCULATIONAHA.104.497347.0009-7322(2005)111[3071:DAFLSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hemingway H, Shipley M, and Mullen MJ. et al. 2003 Social and psychosocial influences on inflammatory markers and vascular function in civil servants (the Whitehall II study). Am J Cardiol. 92:984–987. [DOI] [PubMed] [Google Scholar]
- Henderson B, Pockley AG 2005 Molecular Chaperones and Cell Signaling. Cambridge: Cambridge University Press. [Google Scholar]
- Knoflach M, Kiechl S, and Kind M. et al. 2003 Cardiovascular risk factors and atherosclerosis in young males: ARMY study (atherosclerosis risk factors in male youngsters). Circulation. 108:1064–1069. [DOI] [PubMed] [Google Scholar]
- Kuper H, Marmot M. Job strain, job demands, decision latitude, and risk of coronary heart disease within the Whitehall II study. J Epidemiol Community Health. 2003;57:147–153. doi: 10.1136/jech.57.2.147.0143-005X(2003)057[0147:JSJDDL]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper H, Marmot M, and Hemingway H 2005 Systematic review of prospective cohort studies of psychosocial factors in the aetiology and prognosis of coronary heart disease. In: Coronary Heart Disease Epidemiology, 2nd ed, ed Elliott P, Marmot M. Oxford: Oxford University Press, 363–413. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0.1476-4687(1970)227[0680:COSPDT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lett HS, Blumenthal JA, Babyak MA, Strauman TJ, Robins C, Sherwood A. Social support and coronary heart disease: epidemiologic evidence and implications for treatment. Psychosom Med. 2005;67:869–878. doi: 10.1097/01.psy.0000188393.73571.0a.0033-3174(2005)067[0869:SSACHD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lewthwaite J, Owen N, Coates A, Henderson B, Steptoe A. Circulating human heat shock protein 60 in the plasma of British civil servants: relationship to physiological and psychosocial stress. Circulation. 2002;106:196–201. doi: 10.1161/01.cir.0000021121.26290.2c.0009-7322(2002)106[0196:CHHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Maguire M, Coates AR, Henderson B. Chaperonin 60 unfolds its secrets of cellular communication. Cell Stress Chaperones. 2002;7:317–329. doi: 10.1379/1466-1268(2002)007<0317:cuisoc>2.0.co;2.1466-1268(2002)007[0317:CUISOC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M, Coates AR, Henderson B. Cloning, expression, and purification of three Chaperonin 60 homologues. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;786:117–125. doi: 10.1016/s1570-0232(02)00732-8.0378-4347(2003)786[0117:CEAPOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol. 2005;34:251–256. doi: 10.1093/ije/dyh372.0300-5771(2005)034[0251:CPTWIS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frostegard J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–307. doi: 10.1161/01.hyp.36.2.303.0194-911X(2000)036[0303:CHSPIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rosengren A, Hawken S, and Ounpuu S. et al. 2004 Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 364:953–962. [DOI] [PubMed] [Google Scholar]
- Shamaei-Tousi A, Stephens JW, Bin R, Cooper JA, Steptoe A, Coates AR, Henderson B, Humphries SE. Association between plasma levels of heat shock protein 60 and cardiovascular disease in patients with diabetes mellitus. Eur Heart J. 2006;27:1565–1570. doi: 10.1093/eurheartj/ehl081.0195-668X(2006)027[1565:ABPLOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stansfeld SA, Fuhrer R, Shipley MJ, Marmot MG. Psychological distress as a risk factor for coronary heart disease in the Whitehall II Study. Int J Epidemiol. 2002;31:248–255. doi: 10.1093/ije/31.1.248.0300-5771(2002)031[0248:PDAARF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stansfeld S, Marmot M. Deriving a survey measure of social support: the reliability and validity of the Close Persons Questionnaire. Soc Sci Med. 1992;35:1027–1035. doi: 10.1016/0277-9536(92)90242-i.0277-9536(1992)035[1027:DASMOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stephens JW, Hurel SJ, Cooper JA, Acharya J, Miller GJ, Humphries SE. A common functional variant in the interleukin-6 gene is associated with increased body mass index in subjects with type 2 diabetes mellitus. Mol Genet Metab. 2004;82:180–186. doi: 10.1016/j.ymgme.2004.04.001.1096-7192(2004)082[0180:ACFVIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Verdegaal ME, Zegveld ST, van Furth R. Heat shock protein 65 induces CD62e, CD106, and CD54 on cultured human endothelial cells and increases their adhesiveness for monocytes and granulocytes. J Immunol. 1996;157:369–376.0022-1767(1996)157[0369:HSPICC]2.0.CO;2 [PubMed] [Google Scholar]
- Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644.0732-0582(2004)022[0361:AAIMIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992;12:789–799. doi: 10.1161/01.atv.12.7.789.1049-8834(1992)012[0789:IOAINR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xu Q, Schett G, and Perschinka H. et al. 2000 Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 102:14–20. [DOI] [PubMed] [Google Scholar]