Summary
We previously reported our experience in treating 56 patients with metastatic melanoma using a human anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody. Durable tumor regressions were seen that correlated with the induction of autoimmune toxicities. In this study, we treated 46 additional patients using an intrapatient dose escalation schema to test whether higher doses of anti–CTLA-4 antibody would induce increased autoimmunity and concomitant tumor regression. Twenty-three patients started anti–CTLA-4 antibody administration at 3 mg/kg and 23 patients started treatment at 5 mg/kg, receiving doses every 3 weeks. Patients were dose-escalated every other dose to a maximum of 9 mg/kg or until objective clinical responses or grade III/IV autoimmune toxicity were seen. Escalating doses of antibody resulted in proportionally higher plasma concentrations. Sixteen patients (35%) experienced a grade III/IV autoimmune toxicity. Five patients (11%) achieved an objective clinical response. Two of the responses are ongoing at 13 and 16 months, respectively. Flow cytometric analysis of peripheral blood revealed significant increases in both T-cell surface markers of activation and memory phenotype. Thus, higher serum levels and prolonged administration of anti–CTLA-4 antibody resulted in a trend toward a greater incidence of grade III/IV autoimmune toxicity than previously reported, but did not seem to increase objective response rates.
Keywords: autoimmunity, human, tumor immunity, cell activation, T-cells
Activation of a T cell requires binding of the T-cell receptor to an HLA-presented peptide on an antigen presenting cell, as well as costimulation of the T-cell CD28 receptor by its ligand B7 on the antigen presenting cell.1,2 The combination of these 2 interactions promotes T-cell activation, proliferation, and effector function.1,3–8 In the absence of a costimulatory CD28-B7 interaction, the T cell undergoes tolerance or cell death.9 Alternatively, if B7 molecules engage T-cell expressed cytotoxic T-lymphocyte antigen 4 (CTLA-4), instead of CD28, the lymphocyte undergoes cell cycle arrest.3,10–12 The overall purpose of these signals is to fine-tune the strength of the immune response and to maintain peripheral T-cell tolerance mechanisms.4,5,8,13
Whereas CD28 is constitutively expressed on the T-cell surface, CTLA-4 is only found constitutively on the surface of CD4+CD25+ cells, or must be induced by T-cell receptor binding and activation on other T cells.5,14–18 It has a higher avidity for B7 than CD28, and a shorter half life on the surface of the T cell.7,16,18 These findings led to 2 hypothetical models of CTLA-4 modulation of effector cell function.4,5 The threshold model proposes that CTLA-4 allows only strong signals capable of overcoming CTLA-4 inhibition to activate T cells, thereby narrowing the T-cell response. The attenuation model proposes that CTLA-4 differentially inhibits the most activated T cells, thereby broadening the immune response by preserving weakly avid T cells and inhibiting strongly avid T cells that may be responding to self-antigens. Both models predict that blockade of CTLA-4 would augment the effector functions of activated T cells.
The finding that CTLA-4 knockout mice developed a rapid and fatal lymphoproliferative disorder secondary to unopposed T-cell activation to self-antigens implied a role for CTLA-4 in attenuating the immune response.4,19–22 In preclinical murine models of CTLA-4 blockade using an anti–CTLA-4 antibody, autoimmune arthritis, colitis, depigmentation, diabetes, encephalomyelitis, pancreatitis, and thyroiditis were observed in strains of susceptible mice.23–29 CTLA-4 blockade in tumor-bearing mice also enhanced antitumor responses and resulted in tumor regressions in multiple models, implying that CTLA-4 blockade released the effector function of self-tumor antigen reactive cells.30–38 Preclinical39,40 and clinical models40–44 have established the ability of cancer-bearing hosts to recognize and mount an immune response against their own tumors. The ability to extract and expand tumor-reactive T cells from fresh human tumor digests and adoptively transfer them back into a host to affect an antitumor response confirmed the presence of tumor-reactive lymphocytes in cancer patients, existing in an “ignorant” or tolerant state.42 It is possible that CTLA-4-mediated inhibition may play a role in tolerizing such T cells.45 On the basis of this hypothesis, we investigated the role of CTLA-4 blockade in humans with metastatic melanoma to evaluate the immunologic impact on T-cell activation, tumor burden, and autoimmunity.
We previously treated 56 HLA-A*0201 patients with anti–CTLA-4 antibody plus a gp100 peptide vaccine in 2 dose cohorts. Twenty-nine patients received antibody doses of 3 mg/kg, and 27 patients received a first dose at 3 mg/kg and subsequent doses at 1 mg/kg. An overall response rate of 12.5% (7/56 patients) and a 25% frequency of grade III/IV autoimmunity (14/56 patients) was seen, similar in both treatment groups.46 We then treated a further 36 patients with anti–CTLA-4 antibody combined with the administration of high-dose interleukin-2 (IL-2) to test the effect of combining the activating effects of IL-2 with the anti-inhibitory function of anti–CTLA-4 antibody. Three patients in each of 4 cohorts received increasing doses of antibody (0.1, 0.3, 1, and 2 mg/kg of antibody) with intravenous bolus IL-2 at 720,000 IU/k followed by 24 patients receiving 3 mg/kg of antibody with the same regimen of IL-2. There was an overall response rate of 22% (8/36 patients).47 In the previous study of 56 patients receiving anti–CTLA-4 antibody and peptide vaccine, there was a significant correlation between the induction of anti–CTLA-4 autoimmunity and tumor regression (P = 0.008). This association was not seen in patients who received antibody and IL-2 (P = 0.3), although it is possible that some of the objective responses in these patients were attributable to IL-2 administration independent of anti–CTLA-4 antibody.
Therefore, in the current study we explored the effect of intrapatient dose escalation of anti–CTLA-4 antibody on the incidence of major autoimmune toxicities and its impact on both T-cell activation and clinical response. A separate group of HLA-A*0201–positive patients are enrolled in a parallel ongoing study with randomization to receive or not to receive peptide vaccine. Therefore, we are currently reporting on all patients who were HLA-A*0201 negative and were treated with escalating doses of anti–CTLA-4 antibody.
METHODS
Patients and Treatment
Patients eligible for treatment with anti–CTLA-4 antibody (MDX-010, Medarex Inc, Bloomsbury, NJ) were HLA-A*0201 negative, had measurable stage IV melanoma, were ≥ 16 years of age, had a life expectancy of at least 6 months, absolute neurophil count ≥ 1500/mcl, creatinine <2.0 mg/dL, and bilirubin <1.4 mg/dL, an Eastern Cooperative Oncology Group performance status ≤2, and ≥ 3 weeks had elapsed since any previous systemic cancer therapy. Patients were excluded if they had autoimmune disease, active infection, were pregnant or nursing, had any concurrent medical condition requiring the use of systemic or topical steroids, or had received prior treatment with any anti–CTLA-4 antibody. All patients were treated on an Investigational Review Board-approved protocol in the Surgery Branch, National Cancer Institute in Bethesda, MD.
Antibody doses were escalated within each patient until the development of objective response, ≥ grade III autoimmunity, or another dose-limiting toxicity was reached. In the first 23 patients the starting dose was 3 mg/kg, similar to that in our previous studies,46,48 escalated every other dose to 5 mg/kg, and finally to 9 mg/kg. After treating 23 patients the starting dose was increased to 5 mg/kg for the next 23 patients. The 3-mg/kg dose was well tolerated and this change was made to enable more rapid escalation to higher doses of the antibody. A cycle was defined as 1-dose administration, and a course was defined as the administration of 2 doses at the same drug concentration.
After completion of a course at a given dose, patients who did not experience a decrease in tumor volume or dose-limiting toxicity were escalated to the next dose level. Patients who experienced a complete response were treated for 2 additional cycles at the same dose level. Patients who achieved a partial response, and continued to have tumor regression, were re-treated at the same dose level until they achieved a complete response or no longer had tumor shrinkage. Once a patient experienced a complete response or tumor size stabilized, they received 2 additional cycles at the same dose level. Patients who completed treatment with 2 cycles of antibody at each dose level, either 3, 5, and 9 mg/kg (patients 1 to 23) or 5 and 9 mg/kg (patients 24 to 46), and had disease progression were taken off study, whereas those that were stable received 1 additional course at 9 mg/kg.
Patients who experienced non–skin-related ≥ grade III adverse events, any autoimmune ocular toxicity, or required steroid therapy for toxicity attributable to anti–CTLA-4 antibody administration did not receive further therapy, regardless of their clinical response status. Patients who experienced skin-related toxicity ≤ grade III, or a non–skin-related toxicity <grade III were eligible to restart therapy after resolution of their toxicities.
The human IgG1κ anti–CTLA-4 monoclonal antibody was administered as an intravenous bolus over 90 minutes for every 3 weeks. Before antibody administration and, when possible, 3 weeks after each course, peripheral blood mononuclear cells were obtained by apheresis, isolated by Ficoll-Hypaque separation, and cryopreserved at − 180°C in heat-inactivated human AB serum with 10% dimethy sulfoxide until further use.
Clinical Response Evaluation and On-study Evaluation
All patients underwent computed axial tomography of the chest, abdomen, and pelvis, and magnetic resonance image of the brain, within 28 days of starting treatment and after every 2 cycles of treatment. Response Evaluation Criteria in Solid Tumors (RECIST) criteria using the sum of the longest diameters of all measurable tumors were the criteria for radiographic response to treatment.49 A partial response was defined as a decrease of greater than or equal to 30% (but not 100%) of the sum of the longest diameters of index lesions, lasting at least 1 month with no growth of lesions or the appearance of new lesions. A complete response was defined as the disappearance of all lesions for greater than or equal to 1 month. Patients not achieving either a partial or a complete response were deemed nonresponders.
At the initial patient screening, patients underwent a physical examination, assessment of performance status, ophthalmologic examination, electrocardiography, pulmonary function tests, HLA typing, pheresis, rheumatoid factor, antinuclear antibody, human antihuman antibody, hematologic, biochemical, and thyroid function tests. After every dose cycle, a physical examination and thyroid, hematologic, and biochemical panels were performed. Diagnostic imaging, an autoimmune panel, urinalysis, pheresis, screen, and human antihuman antibody were obtained after every course, and a repeat ophthalmologic examination was performed after 4 cycles and as clinically indicated.
Pharmacokinetics and T-cell Phenotype Analysis
Blood samples were drawn immediately before each dose cycle and again 1 hour after antibody infusion. Quantitative enzyme-linked immunosorbent assay was used to determine plasma concentrations of anti–CTLA-4 antibody. Microtiter plates were coated with recombinant human CTLA-4-Ig, and bound anti–CTLA-4 antibody was detected with an alkaline phosphatase labeled goat antihuman IgG probe. Phenotypic analysis of T-cell activation markers was performed using antibodies from BD Biosciences (San Jose, CA) and analyzed using a FACS Calibur (Becton Dickinson) using standard flow analysis techniques by Esoterix Inc (East Windsor, NJ) on peripheral blood samples obtained immediately before each course.
RESULTS
Patient Characteristics
Forty-six HLA-A*0201–negative patients had stage IV melanoma with metastases to various sites (Table 1). Thirty-nine patients (85%) had visceral metastases. Thirty-two patients (70%) were male. Patients were heavily pretreated before enrollment in this study: 29 (63%) had received chemotherapy, 14 (30%) radiation therapy, 2 (4%) hormonal therapy, 38 (83%) immunotherapy, and 28 (61%) had received more than one therapy. Patients 1 to 23 began treatment with anti–CTLA-4 antibody at a concentration of 3 mg/kg and patients 24 to 46 started at a concentration of 5 mg/kg.
TABLE 1.
Patient Characteristics, Clinical Response, and Toxicity
| Age | Sex | Disease Sites | Previous Therapy | Doses (mg/kg) | Response (mo)* | Autoimmune Toxicity (Grade III/IV)† | |
|---|---|---|---|---|---|---|---|
| 1 | 47 | M | Mesenteric LN | S, I | 3, 3, 3, 3, 5, 5, 9, 9, 9, 9 | PR (16‡) | Hypophysitis |
| 2 | 50 | M | Cervical LN, lung, SQ | S, C, I | 3, 3 | NR | |
| 3 | 43 | M | Adrenal, ALN, lung | S, C, I | 3, 3, 5, 5, 9, 9, 9 | NR | Hypophysitis |
| 4 | 66 | M | Lung | S, I | 3, 3, 5, 5, 9, 9, 9, 9, 9, 9 | PR (4) | |
| 5 | 30 | M | Lung | S, I | 3, 5, 5, 9 | NR | Hypophysitis |
| 6 | 27 | F | ALN, brain, lung, RPLN, SQ, supraclavicular LN | R, S, I | 3, 3 | NR | |
| 7 | 48 | M | Aortic LN, peri-portal LN, RPLN | S, C, I | 3, 3, 5, 5, 9, 9, 9, 9, 9, 9 | PR (13‡) | |
| 8 | 33 | F | Clavicular LN, liver, lung, MLN, mandibular LN, muscle | S, I | 3, 5, 5 | NR | |
| 9 | 43 | M | ALN, MLN, liver, lung, peri-aortic LN, spleen, SQ | S, C, I | 3, 3, 5, 5, 9, 9 | NR | |
| 10 | 61 | M | Lung, muscle | S, C, H, I | 3, 3, 5, 5, 9, 9 | PR (9) | Hypophysitis |
| 11 | 34 | M | Lung | S, I | 3, 3, 5, 5 | NR | |
| 12 | 43 | M | Iliac LN, peri-aortic LN | S, I | 3, 3, 5, 5, 9, 9 | NR | Anterior Uveitis |
| 13 | 63 | M | Lung, MLN | S, C, I | 3, 3 | NR | |
| 14 | 68 | M | MLN, SQ | R, S, C, I | 3, 3, 5, 5, 9, 9 | NR | |
| 15 | 56 | F | Bone, gallbladder, lung, muscle, popliteal LN, SQ | R, S | 3 | NR | |
| 16 | 52 | F | Lung | S, C, I | 3, 3, 5, 5, 9, 9 | NR | |
| 17 | 35 | F | Adrenal, mesenteric LN, pelvis, RPLN | S, C, I | 3, 3, 5, 5, 9, 9, 9, 9 | NR | |
| 18 | 35 | M | Liver, pelvis, SQ, spleen | S, C, I | 3, 3, 5 | NR | Tubulo interstitial nephritis |
| 19 | 38 | M | Bone, MLN, parotid, SQ | R, S, I | 3 | NR | |
| 20 | 66 | M | Lung, MLN | R, S, I | 3, 3, 5, 5, 9, 9 | NR | |
| 21 | 44 | F | Iliac LN, liver, lung, MLN, peri-aortic LN | S, I | 3, 3, 5, 5 | NR | Arthritis |
| 22 | 64 | M | Bone, liver, lung, MLN, SQ | S, C, I | 3, 3, 5, 5, 9, 9 | NR | |
| 23 | 48 | M | Iliac LN, liver, MLN | S, C | 3, 3, 5, 5, 9, 9 | NR | Diarrhea, hypophysitis |
| 24 | 40 | M | Adrenal, ALN | S, C, I | 5 | NR | |
| 25 | 54 | M | Bladder, kidney, liver, MLN, pancreas, RPLN, SQ | R, S, C, I | 5, 5, 9 | NR | |
| 26 | 44 | F | ALN, SQ | S, C, I | 5, 5 | NR | Diarrhea |
| 27 | 46 | F | Liver, lung, caval LN | R, S, C, I | 5, 5, 9, 9, 9 | NR | |
| 28 | 57 | F | Liver, lung, MLN | S, C, I | 5, 5, 9, 9 | NR | |
| 29 | 52 | M | Lung, RPLN, SQ | S, C, I | 5, 5, 9, 9 | NR | |
| 30 | 62 | F | Iliac LN, lung | S, I | 5, 5, 9, 9, 9, 9, 9 | PR (7) | Diarrhea |
| 31 | 59 | M | Liver, lung, MLN | S | 5, 5, 9 | NR | |
| 32 | 49 | M | Lung, MLN | R, S, C, I | 5, 5, 9, 9 | NR | Hypophysitis |
| 33 | 55 | F | Bone, muscle, SQ | R, S, C | 5, 5, 9, 9 | NR | |
| 34 | 27 | M | Internal mammary LN | R, S, C | 5, 5, 9, 9 | NR | |
| 35 | 55 | M | Lung, SQ | S, C | 5, 5 | NR | Hypophysitis |
| 36 | 57 | M | Lung | S, C | 5, 5, 9, 9 | NR | |
| 37 | 59 | M | Lung, MLN | S, C | 5, 5, 9, 9, 9, 9 | NR | Hypophysitis |
| 38 | 35 | M | Kidney, MLN | R, S, C, I | 5, 5, 9, 9 | NR | |
| 39 | 50 | M | Liver, lung, pleura | S, I | 5, 5, 9, 9, 9 | NR | Colitis |
| 40 | 24 | F | Lung | R, S, C, I | 5, 9, 9 | NR | Colitis, transaminitis |
| 41 | 67 | M | Bone, breast, liver, lung, spleen, SQ | S, I | 5, 5, 9, 9, 9 | NR | |
| 42 | 49 | M | Adrenal, clavicular LN, lung, pleura | S, I | 5, 5 | NR | Dermatitis, Diarrhea |
| 43 | 59 | M | ALN, MLN | S, I | 5, 5, 9, 9 | NR | |
| 44 | 41 | M | Lung, RPLN, SQ | S, C, I | 5, 5, 9, 9 | NR | |
| 45 | 29 | F | ILN, lung, MLN | R, S, C, H, I | 5, 9, 9 | NR | |
| 46 | 46 | F | Adrenal, MLN, SQ | R, S, C, I | 5, 5, 9 | NR |
Responses as on January 1, 2005.
Including severe ocular toxicity.
Indicates ongoing response.
ALN indicates axillary lymph node; C, chemotherapy; CR, complete response; F, female; H, hormonal therapy; I, immunotherapy; LN, lymph node; M, male; MLN, mediastinal lymph node; NR, no response; PR, partial response; R, radiation therapy; RP, retroperitoneal; S, surgery; SQ, subcutaneous.
Clinical Responses
Five of 46 patients (11%) experienced objective clinical responses (Table 1), and all of these patients reached criteria for objective clinical responses after treatment at 9 mg/kg (Table 2). Two of the 5 responses are ongoing at 13 and 16 months, respectively.
TABLE 2.
Incidence of Antitumor Responses and Autoimmunity in Patients by Dose Level
| Dose | 3 mg/kg | 5 mg/kg | 9 mg/kg | All Doses |
|---|---|---|---|---|
| Responders | 0 | 0 | 5 | 5/46 (11%) |
| Grade III/IV or severe ocular autoimmunity | 0 | 5 | 11 | 16/46 (35%) |
| Responders with autoimmunity | 0 | 0 | 3 | 3* |
P = 0.32, Fisher exact test comparing responders with autoimmunity to nonresponders with autoimmunity.
Patient 1 achieved an objective partial response in lymph nodes of the retroperitoneum, preaortic, and peri-pancreatic areas after receiving 4 doses at 3 mg/kg, 2 doses at 5 mg/kg, and 2 doses at 9 mg/kg. He received a final course at 9 mg/kg before experiencing hypophysitis, and remains a responder at over 16 months. Patient 4 experienced regression of multiple lung metastases after 2 doses each at 3, 5, and 9 mg/kg. He became a partial responder but recurred at 4 months. Patient 7 achieved complete regression of peri-portal (Fig. 1) and peri-pancreatic lymph nodes, and partial regression of a peri-aortic lymph node after 2 doses each at 3, 5, and 9 mg/kg. He received 2 additional courses at 9 mg/kg and remains a responder at over 13 months. Patient 10 experienced complete regression of an intramuscular lesion (Fig. 2) and partial responses in multiple lung lesions after receiving 2 doses each at 3, 5, and 9 mg/kg, at which time he also experienced hypophysitis. He remained a partial responder for 9 months before growth of new lung lesions. Patient 30 achieved a partial response in an iliac lymph node and a lung lesion after receiving 2 doses at 5 mg/kg, and 4 doses at 9 mg/kg. She received an additional dose at 9 mg/kg before experiencing grade III autoimmune diarrhea, but recurred at 7 months.
FIGURE 1.
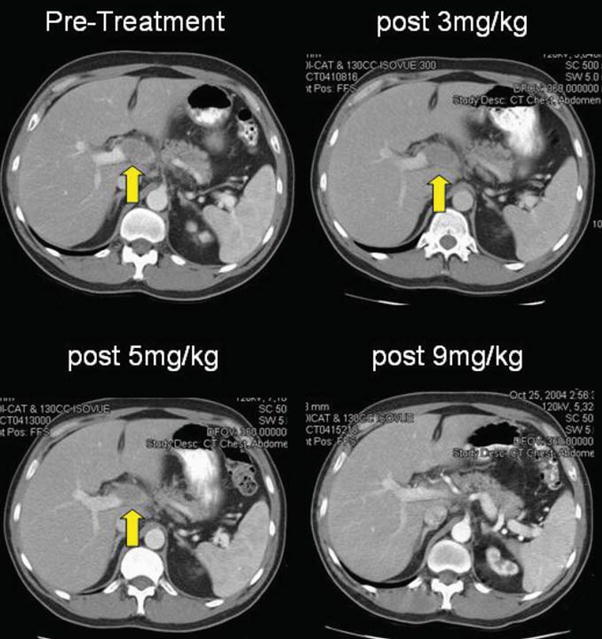
Patient 7 experienced complete regression of a large peri-portal lesion after receiving 9 mg/kg of anti–CTLA-4 antibody. He remains a responder at over 11 months.
FIGURE 2.
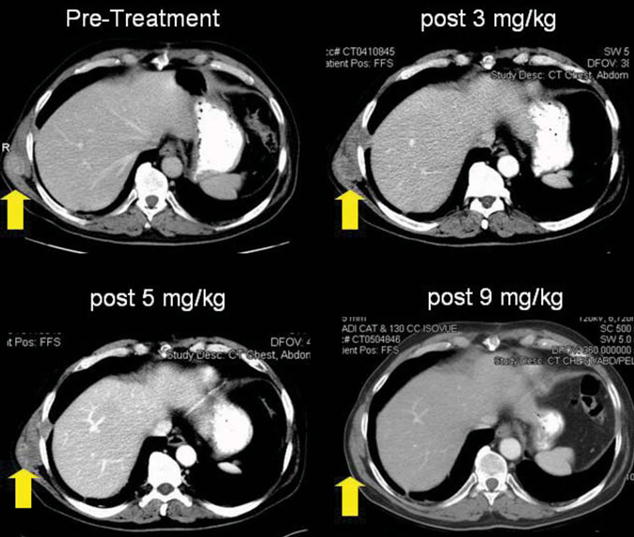
Patient 10 experienced complete regression of an intramuscular lesion after receiving 9 mg/kg of anti–CTLA-4 antibody. He was a responder for 9 months until the growth of new lung lesions.
Autoimmune Effects
Sixteen patients (35%) experienced 19 grade III/IV autoimmune events or severe ocular toxicity requiring steroid treatment (Tables 1 and 2). These events consisted of: anterior uveitis (1), arthritis (1), colitis/diarrhea (6), dermatitis (1), hypophysitis (8), hepatitis (1), and tubulointerstitial nephritis (1) (Tables 1 and 3). Of these 16 patients who experienced major autoimmune toxicities, 3 (17%) were objective clinical responders, whereas of the 30 patients who did not experience severe autoimmunity, 2 (17%) were responders (P = 0.32, Fisher exact test). In addition, 16 patients experienced 22 grade I/II autoimmune events including alopecia areata (1), anterior uveitis/episcleritis or conjunctivitis (2), arthritis (2), diarrhea (2), dermatitis (13), hypothyroidism (1), and vitiligo (1) (Table 3). Five of these patients had concurrent grade III/IV autoimmune toxicities. Thus, 27 patients experienced autoimmunity at any grade. Five of 27 (19%) patients with any autoimmunity experienced tumor regression, and 0/19 patients without any autoimmunity experienced tumor regression (P = 0.07, Fisher exact test).
TABLE 3.
Number of Autoimmune Toxicities by Severity
| Toxicity | Grade I/II | Grade III/IV |
|---|---|---|
| Alopecia areata | 1 | 0 |
| Anterior uveitis or conjunctivitis | 2 | 1 |
| Arthritis | 2 | 1 |
| Colitis/diarrhea | 2 | 6 |
| Dermatitis | 13 | 1 |
| Hypophysitis | 0 | 8 |
| Hypopigmentation | 1 | 0 |
| Transaminitis | 0 | 1 |
| Hypothyroidism | 1 | 0 |
| Tubulointerstitial nephritis | 0 | 1 |
| 22 | 19 |
The specific findings and treatment of anti–CTLA-4 antibody induced autoimmune events seen in this trial were similar to those in patients in previous reported trials46,48 with the exception of tubulointerstitial nephritis (Fig. 3) and alopecia areata seen in one patient each. Eight patients experienced hypophysitis on this study as evidenced by decreased levels of pituitary and secondary hormone levels (Fig. 4); 7 occurring at a dose level of 9 mg/kg and one at 5 mg/kg. This is in contrast to 1 of 56 patients developing this complication in the previous study where only 3 or 1 mg/kg was administered (P = 0.01, Fisher exact test).46 All patients with grade III/IV non–skin-related or joint-related autoimmunity were treated with systemic high-dose steroids with resolution of their symptoms. Severe anterior uveitis in patient 12 was successfully treated with ophthalmologic steroid drops. It is not yet clear whether the administration of steroids can correct the biochemical abnormalities associated with hypophysitis. As in previous studies, the use of high-dose steroids to treat anti–CTLA-4 antibody induced major autoimmune events did not seem to adversely impact the durability of objective clinical responses (Table 4).
FIGURE 3.
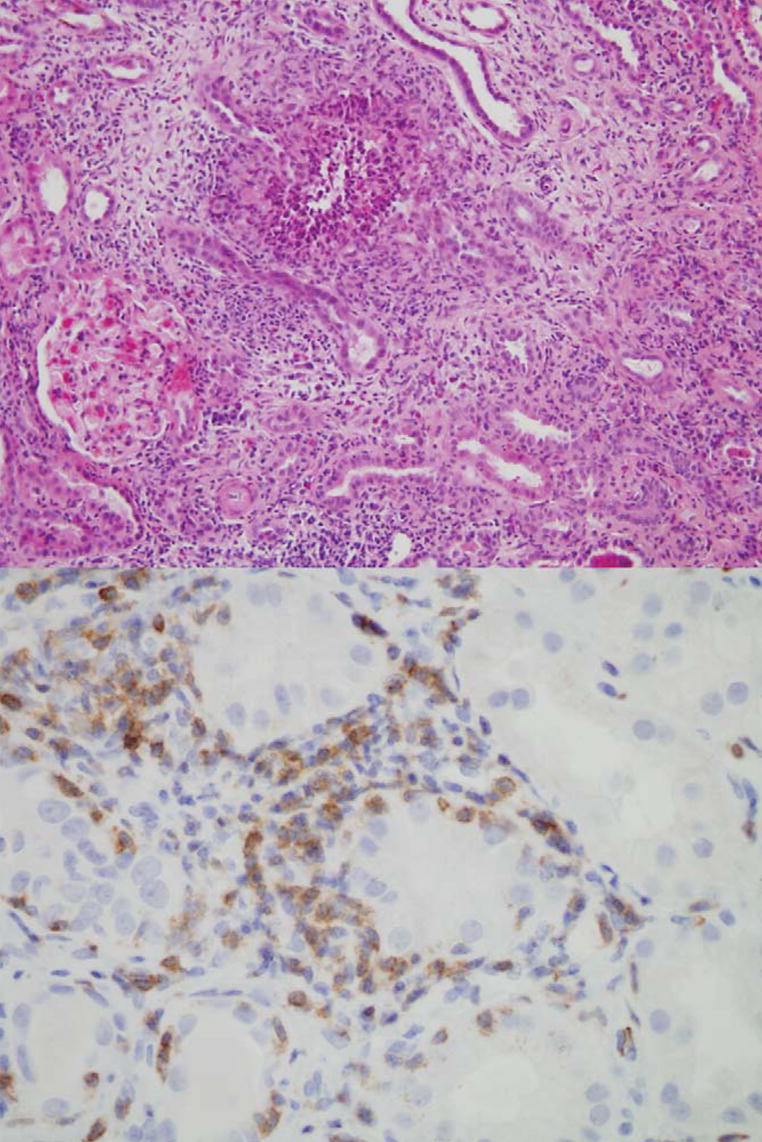
Patient 18 experienced autoimmune nephritis after receiving 3 doses (3, 3, and 5 mg/kg) of anti–CTLA-4 antibody. The renal stroma was edematous and infiltrated with plasma cells, eosinophils, and T lymphocytes. The tubule epithelium was abnormal with scant cytoplasm (upper panel, magnification 200 ×). A CD3 stain revealed lymphocytic invasion into the tubules, similar to acute transplant rejection (lower panel, magnification 600 ×).
FIGURE 4.
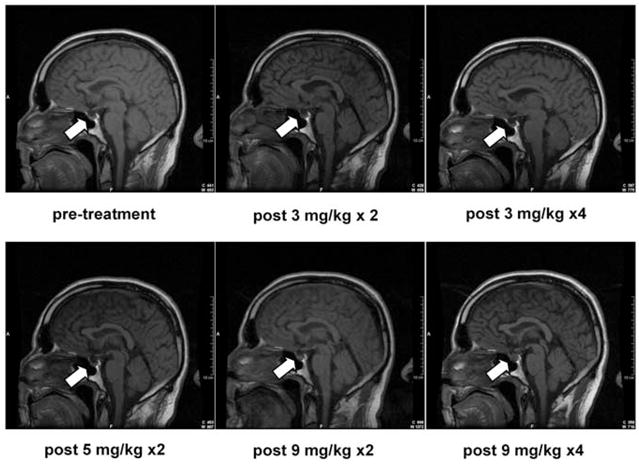
Patient 1 experienced enlargement of his pituitary gland and symptoms of hypopituitarism after receiving a maximal dose of 9 mg/kg of anti–CTLA-4 antibody. Eight of 46 patients (17%) experienced autoimmune hypophysitis.
TABLE 4.
The Role of High-dose Steroid Administration on Disease Recurrence After an Antitumor Response to CTLA-4 Blockade
| Protocol | Patients | Responders | +Steroids (Recurrences) | No Steroids (Recurrences) | P* |
|---|---|---|---|---|---|
| A. Antibody+peptide vaccine† | 56 | 7 | 2 (0) | 5 (2) | 1 |
| B. Antibody alone (dose escalation) | 46 | 5 | 3 (1) | 2 (0) | 1 |
| A+B | 102 | 12 | 5 (1) | 7 (2) | 1 |
Two-tailed, Fisher exact test.
These patients were treated in a previous study reported elsewhere.46
Pharmacokinetics
Peripheral blood samples were obtained immediately before antibody administration and 1 hour post-infusion from patients who began treatment at 3 mg/kg of anti–CTLA-4 antibody to analyze the effect of increasing antibody doses on both peak and trough plasma levels of antibody. Trough levels increased proportionally from below detection levels to 68.9 μg/mL with increasing doses and concentrations of anti–CTLA-4 antibody. Similarly, peak levels increased proportionally from 55.8 to 356.3 μg/mL with increasing doses and concentrations of antibody, though levels seemed to stabilize with repeated dosing at 9 mg/kg (Table 5). Plasma concentrations of anti–CTLA-4 antibody measured before and after doses of 3 mg/kg were comparable to peak and trough plasma concentrations from patients treated on previous Surgery Branch protocols where patients received antibody doses of 3 mg/kg.
TABLE 5.
Pharmacokinetics of Anti–CTLA-4 Antibody Administration
| Sample Timepoint | N | Antibody Concentration (μg/mL) |
|---|---|---|
| Pretreatment | 13 | BD |
| Post 3 mg/kg × 1 | 5 | 55.8 ± 6.1 |
| Pre 3 mg/kg × 2 | 3 | 17.4 ± 2.9 |
| Post 3 mg/kg × 2 | 2 | 116.2 ± 15.4 |
| Pre 5 mg/kg | 4 | 25.5 ± 1.3 |
| Post 5 mg/kg × 1 | 3 | 131.5 ± 66.8 |
| Pre 5 mg/kg × 2 | 4 | 29.6 ± 9.3 |
| Post 5 mg/kg × 2 | 3 | 193.1 ± 30.7 |
| Pre 9 mg/kg | 10 | 48.0 ± 4.9 |
| Post 9 mg/kg × 1 | 3 | 287.0 ± 109.8 |
| Pre 9 mg/kg × 2 | 5 | 60.0 ± 12.4 |
| Post 9 mg/kg × 2 | 3 | 366.4 ± 28.6 |
| Pre 9 mg/kg × 3 | 5 | 63.7 ± 8.6 |
| Post 9 mg/kg × 3 | 4 | 342.8 ± 68.2 |
| Pre 9 mg/kg × 4 | 2 | 68.9 ± 7.3 |
| Post 9 mg/kg × 4 | 3 | 356.3 ± 74.2 |
Reported as the mean concentration ± the standard error of the mean.
BD indicates below detection.
Phenotypic Changes
Using flow cytometry, pretreatment and posttreatment peripheral blood samples were analyzed for changes in surface expression of T-cell activation markers (Table 6). Samples were obtained immediately before each course (3 weeks after the prior course of treatment) to analyze the effect of increasing antibody doses on the activation of T cells. Peripheral blood was stained for both CD3 and CD4 surface expression. Patients for whom a pretreatment sample and at least one posttreatment course sample was available were used for analysis, including 3 of the 5 responders. There were highly significant increases in the proportions of CD3+CD4+ and CD3+CD4− cells that expressed the T-cell activation marker HLA-DR after all doses of anti–CTLA-4 antibody. CD25 was less frequently expressed on CD4+ cells after 5 and 9 mg/kg doses were given. At every dose of antibody, there were decreases in CD69 expression and increases in CD45RO+ expression on both CD4+ and CD8+ cells (all P<0.1, see Table 6).
TABLE 6.
Flow Cytometric Analysis of Selected T-cell Surface Markers in Peripheral Blood of Patients Receiving Escalating Doses of Anti–CTLA-4 Antibody
| Mean % Change in Positive Cells | |||
|---|---|---|---|
| 0 to 3 mg/kg | 0 to 5 mg/kg | 0 to 9 mg/kg | |
| N | 18 | 24 | 12 |
| CD25+ (% CD3+CD4+) | − 0.5 | − 4.7 | − 6.8 |
| P | 0.8 | 0.006 | 0.03 |
| HLA-DR+ (% CD3+CD4+) | +10.1 | +10.7 | +13.2 |
| P | 0.0002 | <0.0001 | 0.0002 |
| HLA-DR+ (%CD3+CD4−) | +11.0 | +8.5 | +11.5 |
| P | 0.01 | 0.002 | 0.003 |
| CD69+ (% CD3+CD4+) | − 11.2 | − 5.6 | − 10.5 |
| P | 0.04 | 0.1 | 0.1 |
| CD69+ (%CD3+CD4−) | − 12.8 | − 11.4 | − 18.6 |
| P | 0.01 | 0.004 | 0.007 |
| CD45RO+ (% CD3+CD4+) | +3.9 | +5.7 | +9.8 |
| P | 0.02 | 0.0005 | 0.007 |
| CD45RO+ (%CD3+CD4−) | +6.0 | +4.2 | +7.0 |
| P | 0.03 | 0.06 | 0.04 |
Two-tailed P value using paired t test.
DISCUSSION
In our past studies,46,48 the administration of anti–CTLA-4 antibody seemed to release an “immunologic brake” on both CD4+ and CD8+ T cells, tipping the homeostatic balance towards lymphocyte effector function. This was reflected in an up-regulation of T-cell surface markers of activation, in clinical responses, and toxicities thought to be attributed to autoimmunity. Flow cytometric analysis of peripheral blood from patients receiving CTLA-4 blockade revealed significant increases in the T-cell surface markers of activation (HLA-DR) and memory (CD45RO).46,48
In the current study, we again demonstrated significant increases in the expression of these T-cell activation and memory markers on both CD4+ and CD8+ T cells in the peripheral blood of patients who were treated with escalating doses of anti–CTLA-4 antibody. With the caveat that samples from different studies were analyzed at different times, the changes in phenotypic expression were similar in type and magnitude when the previous low-dose study was compared with the current dose-escalation study. Within the current study, there was often no clear effect of increasing antibody dose on levels of activation marker expression, with the exceptions of CD45RO expression on CD4+ cells (showing increases of +3.9% to +9.8% with increasing doses of antibody) and CD25 on CD4+ cells (showing decreases of 0.5% to 6.8% with increasing doses of antibody). The significance of decreased expression of CD69 on lymphocytes is unclear, though the window in which this phenotypic activation marker may be up-regulated could have been missed at the time of sample procurement.50,51
The objective clinical response rate in 56 HLA-A*0201–positive patients treated with ≤ 3 mg/kg of anti–CTLA-4 antibody plus peptide vaccination in our previous trials was 12.5%,46 similar to the response rate of 11% in the 46 HLA-A*0201–negative patients given escalating doses ≥ 3 mg/kg in the present study. The current study design not only increased the dose of antibody, but also the average duration of treatment, yet there did not seem to be a clear impact on the magnitude of T-cell activation by phenotypic criteria nor an increase in response rate.
On the basis of our early observation that toxicities possibly attributable to autoimmunity significantly correlated with response in melanoma patients treated with anti–CTLA-4 antibody,46 we hypothesized that raising blood levels of the antibody may increase the incidence of autoimmunity. In the current trial, plasma concentrations increased proportionally with increasing antibody doses, and patients were exposed to the antibody over a longer period of time and at higher plasma concentrations than in previous trials. In some patients, this increase was measured at over 3 times the average plasma concentration measured in patients who previously received a maximum of 3 mg/kg.46
Sixteen of 46 (35%) patients experienced grade III/IV autoimmunity, compared with 14 of 56 (25%) patients who received 3 mg/kg of antibody plus a peptide vaccine (P = 0.38) in an earlier study.46 Including grade I/II autoimmunity, patients on dose escalation experienced a 59% incidence of autoimmunity (27/46) compared with 30% (17/56) in the previous study (P = 0.005). Thus, there was a trend toward increased rates of autoimmunity with the current dosing regimen. Furthermore, in the current study, all 16 cases experiencing grade III/IV autoimmune events occurred at either 5 or 9 mg/kg. Therefore, most patients experienced a severe autoimmune toxicity at a higher dose than their initial dose. However, many of these patients received doses of anti–CTLA-4 antibody every 3 weeks over a period of months, so it may be that prolonged exposure to the antibody was related to autoimmune toxicity rather than the actual dose administered. The average number of doses received per patient was 4.6 ± 0.3 (over approximately 14 wk) compared with 3.7 ± 0.3 doses (over approximately 11 weeks) in the previous study of 56 patients. Alternatively, the autoimmune effects experienced may have been initiated at either lower doses or earlier time points, and not fully clinically manifested until the patients had already escalated dose levels or received additional antibody doses.
In our previous studies, enteritis (particularly colitis) was the most common toxicity (57%, 8/14) and it consistently presented with diarrhea.46 Limiting oral intake and treatment with high-dose steroids successfully controlled the diarrhea in the great majority of patients. In this study, grade III diarrhea/colitis was present in 31% of patients (5/16) with grade III/IV autoimmune toxicity; however, hypophysitis emerged as the most common grade III/IV autoimmune event (44%, 7/16). This toxicity was experienced at dose concentrations of 9 mg/kg in 6 of 7 patients. When compared to one of 56 patients with hypophysitis in the previous study at doses ≤ 3 mg/kg, the difference was statistically significant (P = 0.01). None of the 36 patients who received anti–CTLA-4 antibody in conjunction with IL-2 experienced hypophysitis.47 Our experience detecting and treating this toxicity has evolved, therefore, it is possible that mild cases in earlier studies may not have been diagnosed. Given the small numbers involved in this subgroup analysis, further studies will be necessary to elucidate the relationship between hypophysitis and either higher dose levels or increased time of exposure to anti–CTLA-4 antibody. Those patients who experienced hypophysitis (n = 7) averaged 5.8 ± 0.8 doses, or about 17 weeks of treatment, compared with 4.3 ± 0.4 doses, or about 13 weeks of treatment in patients who did not experience hypophysitis (n = 38, 2-tailed P = 0.1). The one patient, who experienced hypophysitis in the 56 previous patients treated at 3 mg/kg received 5 doses at 3 mg/kg, compared with an average of 3.6 doses received by the other 55 patients on that study, a difference of 4 weeks of treatment.
Therefore, we conclude that CTLA-4 blockade with an anti–CTLA-4 antibody can cause the durable regression of metastatic melanoma, but find that the administration of escalating doses of antibody, whereas possibly increasing autoimmune toxicity, does not increase the overall objective clinical response rate. Further studies are underway to determine the impact of adding concurrent vaccination to CTLA-4 blockade in patients with melanoma and to investigate the efficacy of CTLA-4 blockade in patients with other tumor histologies.
References
- 1.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 3.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 4.Chambers CA, Kuhns MS, Egen JG, et al. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 5.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 6.Koulova L, Clark EA, Shu G, et al. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991;173:759–762. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linsley PS, Brady W, Urnes M, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 10.Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 11.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis SJ, Ikemizu S, Evans EJ, et al. The nature of molecular recognition by T cells. Nat Immunol. 2003;4:217–224. doi: 10.1038/ni0303-217. [DOI] [PubMed] [Google Scholar]
- 14.Birebent B, Lorho R, Lechartier H, et al. Suppressive properties of human CD4+CD25+ regulatory T cells are dependent on CTLA-4 expression. Eur J Immunol. 2004;34:3485–3496. doi: 10.1002/eji.200324632. [DOI] [PubMed] [Google Scholar]
- 15.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 16.Lindsten T, Lee KP, Harris ES, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151:3489–3499. [PubMed] [Google Scholar]
- 17.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 19.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 20.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 21.Ariyan C, Salvalaggio P, Fecteau S, et al. Cutting edge: transplantation tolerance through enhanced CTLA-4 expression. J Immunol. 2003;171:5673–5677. doi: 10.4049/jimmunol.171.11.5673. [DOI] [PubMed] [Google Scholar]
- 22.Greenwald RJ, Latchman YE, Sharpe AH. Negative co-receptors on lymphocytes. Curr Opin Immunol. 2002;14:391–396. doi: 10.1016/s0952-7915(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz AA, Sullivan TJ, Krummel MF, et al. Specific blockade of CTLA-4/B7 interactions results in exacerbated clinical and histologic disease in an actively-induced model of experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73:57–62. doi: 10.1016/s0165-5728(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 24.Luhder F, Hoglund P, Allison JP, et al. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrin PJ, Maldonado JH, Davis TA, et al. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 26.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Elsas A, Sutmuller RP, Hurwitz AA, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristiansen OP, Larsen ZM, Pociot F. CTLA-4 in autoimmune diseases—a general susceptibility gene to autoimmunity? Genes Immun. 2000;1:170–184. doi: 10.1038/sj.gene.6363655. [DOI] [PubMed] [Google Scholar]
- 29.Uchikoshi F, Yang ZD, Rostami S, et al. Prevention of autoimmune recurrence and rejection by adenovirus-mediated CTLA4Ig gene transfer to the pancreatic graft in BB rat. Diabetes. 1999;48:652–657. doi: 10.2337/diabetes.48.3.652. [DOI] [PubMed] [Google Scholar]
- 30.Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63:3281–3288. [PubMed] [Google Scholar]
- 31.Espenschied J, Lamont J, Longmate J, et al. CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J Immunol. 2003;170:3401–407. doi: 10.4049/jimmunol.170.6.3401. [DOI] [PubMed] [Google Scholar]
- 32.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 33.Hurwitz AA, Yu TF, Leach DR, et al. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 36.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang YF, Zou JP, Mu J, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036–4041. [PubMed] [Google Scholar]
- 39.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 40.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho WY, Blattman JN, Dossett ML, et al. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 44.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 45.Abrams SI. Role of anti-CTLA-4 therapies in the treatment of cancer. Curr Opin Mol Ther. 2004;6:71–77. [PubMed] [Google Scholar]
- 46.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with CTLA-4 blockade and IL-2: a phase I/II study. Ann Surg Oncol. 2005 doi: 10.1245/ASO.2005.03.536. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 50.Arva E, Andersson B. Kinetics of cytokine release and expression of lymphocyte cell-surface activation markers after in vitro stimulation of human peripheral blood mononuclear cells with Streptococcus pneumoniae. Scand J Immunol. 1999;49:237–243. doi: 10.1046/j.1365-3083.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- 51.Reddy M, Eirikis E, Davis C, et al. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J Immunol Methods. 2004;293:127–142. doi: 10.1016/j.jim.2004.07.006. [DOI] [PubMed] [Google Scholar]


