Abstract
Early in S phase, the vacuole (lysosome) of Saccharomyces cerevisiae projects a stream of vesicles and membranous tubules into the bud where they fuse and establish the daughter vacuole. This inheritance reaction can be studied in vitro with isolated vacuoles. Rapid and efficient homotypic fusion between saltwashed vacuoles requires the addition of only two purified soluble proteins, Sec18p (NSF) and LMA1, a novel heterodimer with a thioredoxin subunit. We now report the identity of the second subunit of LMA1 as IB 2, a previously identified cytosolic inhibitor of vacuolar proteinase B. Both subunits are needed for efficient vacuole inheritance in vivo and for the LMA1 activity in cell extracts. Each subunit acts via a novel mechanism, as the thioredoxin subunit is not acting through redox chemistry and LMA1 is still needed for the fusion of vacuoles which do not contain proteinase B. Both Sec18p and LMA1 act at an early stage of the in vitro reaction. Though LMA1 does not stimulate Sec18p-mediated Sec17p release, LMA1 cannot fulfill its function before Sec18p. Upon Sec17p/Sec18p action, vacuoles become labile but are rapidly stabilized by LMA1. The action of LMA1 and Sec18p is thus coupled and ordered. These data establish LMA1 as a novel factor in trafficking of yeast vacuoles.
Cell proliferation depends on the inheritance of organelles, which are duplicated and segregated into daughter cells rather than synthesized de novo during cell division (reviewed in Warren and Wickner, 1996). Through cytological, genetic, and biochemical means, we have begun to study the inheritance of vacuoles (lysosomes) in Saccharomyces cerevisiae. In early S phase, the vacuole projects a stream of vesicles and membranous tubules, termed a segregation structure, into the bud (Weisman and Wickner, 1988; Gomes de Mesquita et al., 1991; Raymond et al., 1992). These vesicles fuse in the bud, forming the daughter cell vacuole. Vacuole inheritance is defective in vac mutants in which the growing bud receives little or no maternal vacuolar material (Weisman et al., 1990; Shaw and Wickner, 1991; Nicolson et al., 1995). This process can be studied in an in vitro vacuole inheritance assay. In the presence of ATP, cytosol, and physiological temperature, isolated vacuoles form segregation structures and undergo homotypic fusion (Conradt et al., 1992, 1994; Haas et al., 1994). The fusion can be monitored by fluorescence microscopy and by biochemical assays. This reaction is abolished when its components are prepared from vac mutants, establishing its authenticity (Haas et al., 1994; Nicolson et al., 1995). This in vitro vacuole inheritance reaction requires Sec18p and Sec17p (Haas and Wickner, 1996), Ypt7p (Haas et al., 1995), and a low molecular weight activity termed LMA1 (Xu and Wickner, 1996).
Like vacuole inheritance (Haas and Wickner, 1996), many vesicle trafficking and membrane fusion events employ N-ethylmaleimide–sensitive fusion protein (NSF)1, and soluble NSF attachment proteins (SNAPs) (Rothman, 1994). The homologues of NSF and α-SNAP in budding yeast are Sec18p and Sec17p, respectively (Wilson et al., 1989; Griff et al., 1992). NSF is a homotrimeric ATPase that requires SNAPs to bind to membranes at SNAP receptors, termed SNAREs (Söllner et al., 1993). NSF supports membrane trafficking events such as vesicular transport from the ER through the Golgi apparatus to the endosomes and plasma membrane, homotypic endosome fusion, and synaptic vesicle fusion to the plasma membrane (Rothman, 1994). Although homotypic ER fusion and nuclear envelope fusion require neither NSF nor SNAP, they require Cdc48, an NSF-like protein (Latterich et al., 1995). In yeast, Sec17p and Sec18p work together to promote the ATP-dependent release of Sec17p from the vacuole membrane at an early stage of the in vitro inheritance reaction (Mayer et al., 1996).
Vacuole inheritance also requires LMA1, a novel heterodimeric protein which contains thioredoxin (Xu and Wickner, 1996). Thioredoxin is a ubiquitous small protein (Holmgren, 1985) with a pair of cysteine within a highly conserved active site. These cysteine residues are reduced by an NADPH-dependent thioredoxin reductase and, in turn, reduce target proteins. Although thioredoxin function generally involves its redox properties, redox-independent roles are also known (Russel and Model, 1986; Huber et al., 1986; Tonissen et al., 1993). Deletion of both yeast thioredoxin genes obliterates LMA1 activity of the cytosol in vitro and causes a striking vacuole inheritance defect in vivo (Xu and Wickner, 1996).
We now report that the other subunit of LMA1 is IB 2, a previously described S. cerevisiae protein that can inhibit vacuolar proteinase B (Maier et al., 1979; Schu et al., 1991). Both components of LMA1 are required for efficient vacuole inheritance. LMA1 acts via a novel mechanism as, in this reaction, thioredoxin is not employing a redox mechanism and IB 2 is not acting via inhibition of proteinase B. LMA1 and Sec18p synergistically support fusion of saltwashed vacuoles and act in an ordered manner at an early stage of the in vitro vacuole inheritance reaction.
Materials and Methods
Reagents and Strains
Reagents were purchased or prepared as described (Xu and Wickner, 1996; Mayer et al., 1996; Haas and Wickner, 1996). Yeast strains were described in Xu and Wickner (1996) and Muller (1995). The strains YS18 (Mata ura3-5 his3-11 leu2-3,-112 canR) and YPS19 (Mata Δpbi2::URA3) were kindly provided by Dr. D.H. Wolf (Institut für Biochemie der Universitat Stuttgart, Germany).
In Vitro Homotypic Vacuole Fusion Assay
Preparation of cytosol, vacuoles, salt-washed vacuoles, and purification of the p10 subunit of LMA1 were as described (Xu and Wickner, 1996). For salt-washing, freshly isolated vacuoles were diluted to 0.3 mg/ml with 0% Ficoll buffer (Haas et al., 1994). Equal volumes of BJ3505 and DKY6281 vacuoles were mixed and KCl and KOAc added (from 3M stocks) to 100 mM and 50 mM, respectively. Aliquots of 200 μl were transferred to Eppendorf tubes, incubated 10 min at 30°C, chilled at 0°C for 2 min, and centrifuged (10,000 g, 75 s, 4°C). Supernatants were removed and pellets covered with 160 μl of cold 0% Ficoll buffer. LMA1 and Sec18p were largely removed by salt-washing.
The 30-μl in vitro vacuole fusion reaction (Haas et al., 1994) contains 4.8 μg of mixed, salt-washed vacuoles from BJ3505 and DKY6281, 20 mM Pipes-KOH, pH 6.8, 200 mM sorbitol, 150 mM KOAc, 5 mM MgCl2, an ATP regenerating system (1 mM ATP, 40 mM creatine phosphate, and 70 U/ml creatine phosphokinase), either 1 mg/ml cytosol (from yeast strain K91-1A), fractions from Sephacryl S100HR gel filtration or purified proteins as indicated, and 0.1× proteinase inhibitor cocktail (PIC; Xu and Wickner, 1996). Salt-washed vacuoles were used in all experiments. In vitro reactions were incubated at 25°C for 90–120 min and stopped by chilling on ice. Alkaline phosphatase activity was determined as described (Haas et al., 1994). 1 U of fusion activity corresponds to 1 μmol of p-nitrophenol produced at 25°C per minute per microgram of BJ 3505 vacuoles.
Gel Filtration
Cytosol (stored at −80°C) was thawed and mixed with PIC and ATP to final concentrations of 30× and 0.1 mM, respectively, and then incubated on ice for 20 min followed by centrifugation (14,000 g, 15 min, 4°C). A 200-μl aliquot of clarified cytosol was applied to a Sephacryl S100HR column (1 cm × 26 cm) equilibrated in buffer C (20 mM TrisCl, pH 8.3, 5 mM Mg(OAc)2, and 0.1 mM MnCl2). This buffer has been optimized in Mn concentration for LMA1 purification. The column was eluted (12 ml/cm2/h, 300 μl fractions) with buffer C at 4°C.
Microscopy
Yeast vacuoles were visualized with the vital fluorophores fluorescein-5isothiocyanate (FITC, Molecular Probes, Inc., Eugene, OR) or FM 4-64 as described (Vida and Emr, 1995; Xu and Wickner, 1996).
Other Methods
Protein concentration was determined with a protein reagent (Bio-Rad Laboratories, MA) with BSA as a standard. SDS-PAGE and “High Tris” SDS-PAGE (Xu and Wickner, 1996) and affinity purification of IgG and immunoblot analysis (Mayer et al., 1996; Haas and Wickner, 1996) have been described. Thioredoxin reductase was purified from baker's yeast as described (Gonzales et al., 1970). Reductase activity was determined with the 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) assay described in Luthman and Holmgren (1982).
Results
The vacuole inheritance reaction can be assayed in vitro by incubating isolated, salt-washed vacuoles with ATP and cytosol at physiological temperatures. The reaction is performed with vacuoles purified from two yeast strains (Haas et al., 1994). One strain has normal vacuolar proteases but a deletion in the PHO8 gene encoding the vacuole proalkaline phosphatase. The other strain has a normal PHO8 gene but lacks vacuolar proteases A and B which are needed for processing the catalytically inactive pro-alkaline phosphatase to its mature, active form. Upon fusion of vacuoles isolated from these strains, the proteases from one fusion partner cleave the pro-alkaline phosphatase from the other to produce the catalytically active enzyme, allowing quantitative assay of vacuole fusion.
The Small Subunit of LMA1
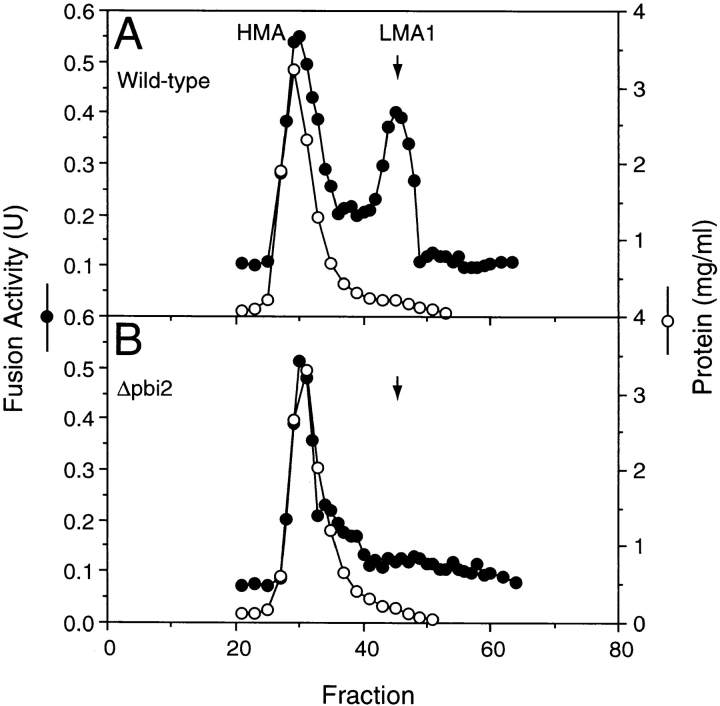
We have previously reported the purification of a low molecular weight activity, termed LMA1, which supports the in vitro reaction (Xu and Wickner, 1996). LMA1 is a 23-kD heterodimer with a thioredoxin subunit of 12 kD and a smaller subunit termed p10. Edman degradation of the purified p10 subunit showed that it has the sequence ThrLysAsnPheIleValThrLeuLysLysAsnThrProAspValGluAlaLysLys which is identical to a portion of the reported sequence of yeast proteinase yscB inhibitor IB 2, encoded by the PBI2 gene (Schu et al., 1991). Antibody prepared to a peptide from the COOH terminus of this protein specifically decorated the p10 subunit of LMA1 (data not shown), confirming its identification from the NH2-terminal sequence. To determine whether IB 2 is a functional component of LMA1, cytosols were prepared from a pbi2 null mutant and its corresponding wild-type strain. Cytosolic components were resolved by Sephacryl S100HR chromatography and fractions were assayed for their ability to replace cytosol in the in vitro reaction of vacuole fusion. The wild-type cytosol showed both the previously reported (Xu and Wickner, 1996) high molecular weight activity peak, termed HMA, and LMA1 (Fig. 1 A). Deletion of the PBI2 gene left the HMA peak while obliterating activity from the LMA1 region (Fig. 1 B). The identity of the LMA1 peak from wildtype cytosol was confirmed by immunoblot with anti-TRX1p peptide antiserum, whereas monomeric thioredoxin, recovered from more included fractions of pbi2Δ cytosol (data not shown), had no activity in our assay (Fig. 1 B). Deletion of the PBI2 gene had no effect on the cellular content of thioredoxin (data not shown). Thus IB 2 is clearly an essential component of LMA1. To test whether IB 2 plays a role in vacuole inheritance in vivo, the PBI2 deletion mutant and wild-type cells were examined by fluorescence microscopy. The PBI2 deletion mutant showed a clear vac phenotype of buds which frequently had no vacuole (Fig. 2 B). While almost all wild-type cells (Fig. 2 A) with a bud diameter of at least half the maternal cell diameter had inherited a bud vacuole, 34% of PBI2 deletion cells had no vacuole in their buds (Fig. 2 B and Table I). Taken together, these in vitro and in vivo results demonstrate that IB 2, previously identified as a cytosolic inhibitor of vacuolar proteinase B, is a novel factor required for efficient vacuole inheritance in yeast.
Figure 1.
Deletion of PBI2 abolishes LMA1 activity. Yeast cytosols were prepared from PBI2 (A) and Δpbi2 (B) strains (Schu et al., 1991) and fractionated by Sephacryl S100HR gel filtration as described in Materials and Methods. Aliquots (4 μl) were assayed for protein concentration (open circles) and fusion activity (closed circles) as described in Materials and Methods. LMA2 is for unknown reasons not present in the PBI2 wild-type strain background.
Figure 2.
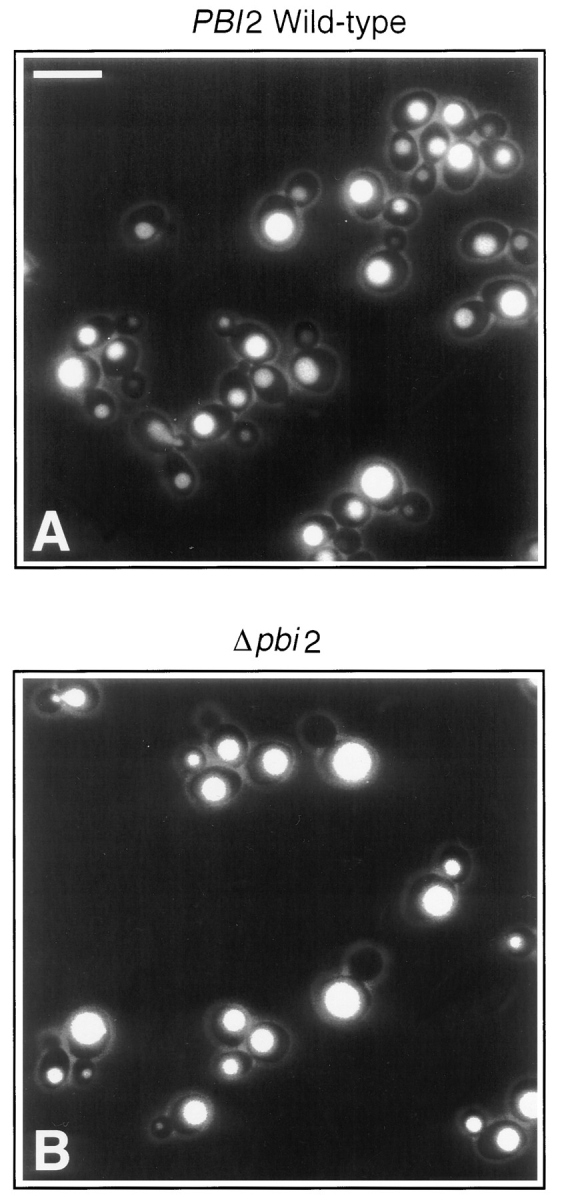
Vacuole inheritance deficiency in Δpbi2 cells. Wildtype (A) or Δpbi2 (B) yeast were grown in YCM medium (Rogers and Bussey, 1978) to early log phase. Cells from 1 ml of the culture were harvested (3,000 g, 5 min, 4°C) and resuspended in 1 ml YCM with 10 μl of 4 mg/ml FITC (Molecular Probes Inc., Eugene, OR). Cells were incubated at 37°C for 10 min, collected by centrifugation, resuspended in 50 μl of YCM, and examined by fluorescence microscopy. Bar, 4 μm.
Table I.
IB 2 Is Needed for Efficient Vacuole Inheritance In Vivo
| Strain | Cells with Bud diameter/ Cell diameter > 0.5 | Bud with no vacuoles | Percentage | |||
|---|---|---|---|---|---|---|
| % | ||||||
| PIB2 | 110 | 3 | 2.7 | |||
| Δpib2 | 127 | 44 | 34.6 |
PB12 and Δpbi2 cells were grown and harvested and examined by fluorescence microscopy as described in the legend to Fig. 2. Photos were taken from random fluorescent fields and the diameters of cells and buds were measured.
Mechanism of LMA1 Action
To understand how LMA1 participates in the in vitro reaction, we tested whether it requires the redox activity of its thioredoxin subunit or the proteinase B inhibitor activity of its IB 2 subunit. Yeast thioredoxin reductase was assayed and purified from baker's yeast as described (Gonzales et al., 1970; Luthman and Holmgren, 1982). No stimulation of fusion was observed when both NADPH and purified thioredoxin reductase were added to fusion reactions supported by LMA1 (Fig. 3 A). To further examine the role of the thioredoxin redox activity in vacuole fusion, we used a mutant form of Trx1p in which both cysteinyl residues at its active site were replaced by serines (Muller, 1995). The redox activity of Trx1p is completely abolished by this means. The genes for mutant and wild-type forms of TRX1 were transformed into the trx1, trx2 double deletion strain and cytosols were prepared. As reported (Xu and Wickner, 1996), the fusion activity of TRX1,TRX2 wildtype cytosol (Fig. 3 B a) is resolved into HMA, LMA1, and LMA2 by Sephacryl S100HR gel filtration and the LMA1 peak is selectively lost in the trx1,trx2 double deletion strain (Fig. 3 B b). As expected, the LMA1 peak is restored when the wild-type TRX1p is expressed from a plasmid in the trx1,trx2 double deletion strain (Fig. 3 B c). Strikingly, the LMA1 peak is also restored in this trx1,trx2 double deletion strain by a plasmid which expresses the double Cys to Ser mutant form of Trx1p (Fig. 3 B d). Furthermore, the vac phenotype of the trx1,trx2 double deletion strain was rescued in vivo by introducing this double Cys to Ser mutant form of Trx1p (data not shown). These data demonstrate that the function of thioredoxin in vacuole inheritance and fusion does not employ a cyclic reduction-oxidation mechanism.
Figure 3.
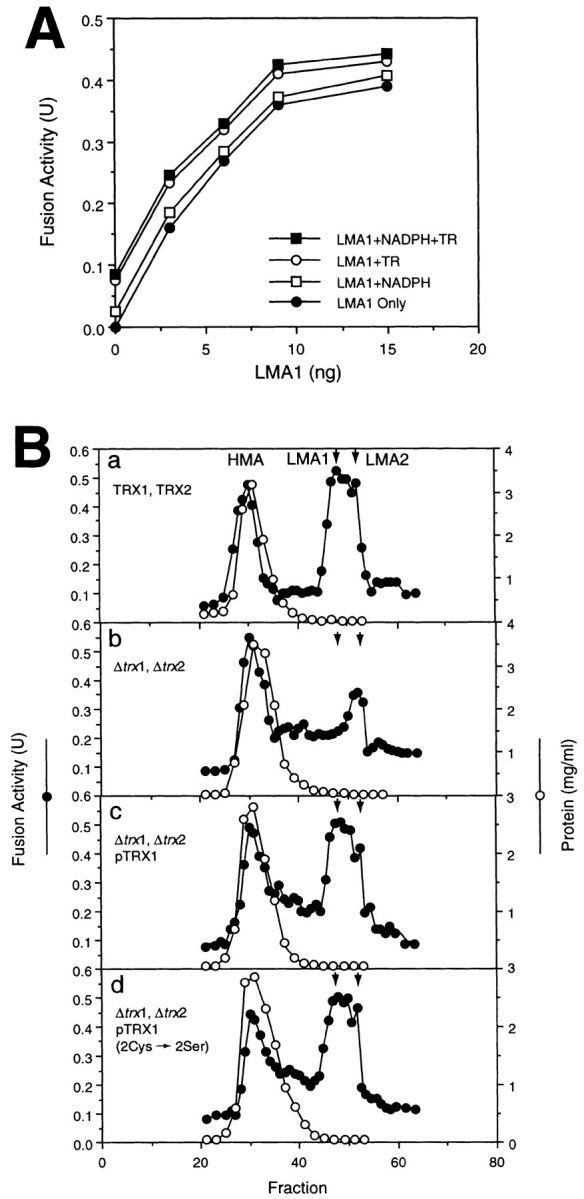
Redox-independent fusion activity of LMA1. (A) NADPH and thioredoxin reductase (TR) do not stimulate vacuole fusion supported by LMA1. Fusion reactions contained the indicated amount of LMA1 alone (•), LMA1 and NADPH (0.5 mM final conc.) (□), LMA1 and TR (0.1 mg/ml, final conc.) (○), or LMA1 and both NADPH and TR (▪). (B) Expression of the double Cys to Ser mutant form of Trx1p restores the LMA1 activity peak in Δtrx1, Δtrx2 cell extracts. Cytosols were prepared from the indicated strains (Muller, 1995). Sephacryl S100HR gel filtration was performed and fractions tested in the in vitro inheritance assay as described in Materials and Methods. LMA1 and LMA2 peaks are indicated with arrows.
Since LMA1 does not act via redox, we asked whether it acts by inhibiting vacuolar proteinase B, the purported activity of its other subunit. Sec18p is one of the two necessary soluble factors, as shown below (Fig. 5), and functions with LMA1 to support vacuole fusion and is also protease sensitive. We therefore checked the stability of Sec18p during the fusion reaction in the presence, or absence, of LMA1. The amount of soluble and membrane-bound fulllength Sec18p remained unchanged (Fig. 4 A), indicating that LMA1 is not acting to prevent Sec18p degradation. To determine whether LMA1 functions in vacuole fusion via inhibition of proteinase B cleavage of some other, unidentified vacuole protein, we prepared vacuoles from the strain BJ3505 (Moehle et al., 1986) in which both proteinase A and B genes are deleted and examined the fusion of these vacuoles by fluorescence microscopy. While clustering of these vacuoles was promoted by Sec18p (Mayer and Wickner, 1997), the fusion of these protease-deficient vacuoles still requires both LMA1 and Sec18p (Fig. 4 B). Measurements of all vacuole diameters in random fields showed average diameters of 0.58 μm (0.25 SEM), 0.73 μm (0.24), 0.66 μm (0.22), or 1.29 μm (0.21) after incubation with ATP only, LMA1 only, Sec18p only, or LMA1 and Sec18p, respectively. Thus, LMA1 does not support vacuole fusion via a proteinase B inhibitor activity of its IB 2 subunit. The function of LMA1 in vacuole fusion must employ novel mechanisms which are unrelated to the established functions of its subunits in other cellular processes.
Figure 5.
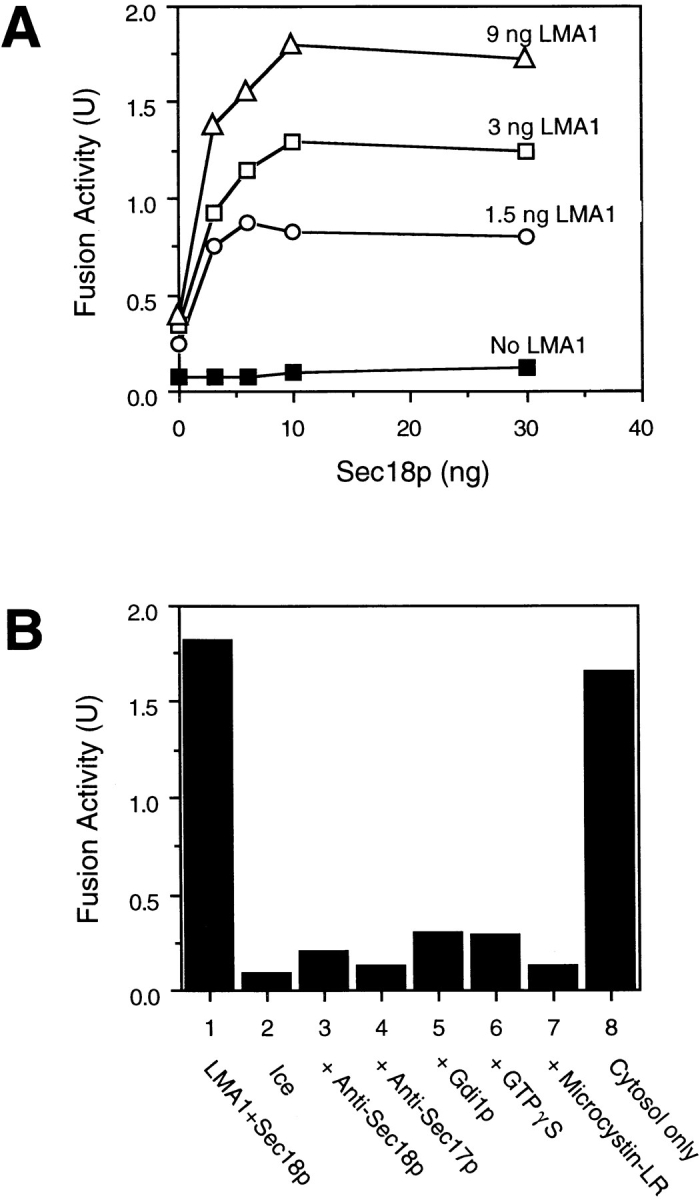
Sec18p and LMA1 synergistically support fusion of salt-washed vacuoles. (A) Fusion reactions (30 μl) contained ATP and the indicated amounts of Sec18p and LMA1. Reactions were incubated at 25°C for 100 min. (B) Fusion reactions containing 9 ng LMA1 and 10 ng Sec18p (lanes 1–7) or 1 mg/ml cytosol (lane 8) were incubated at 25°C or on ice for 100 min. Affinitypurified antibodies against either Sec18p (1.5 μg, lane 3) or Sec17p (1 μg, lane 4) or the fusion inhibitors Gdi1p (2 μg, lane 5), GTPγS (1 mM final conc., lane 6), or Microcystin-LR (10 μM final conc., lane 7) were added to reactions before starting the incubation. The reactions were stopped by chilling on ice and fusion activity was measured via alkaline phosphatase activity.
Figure 4.
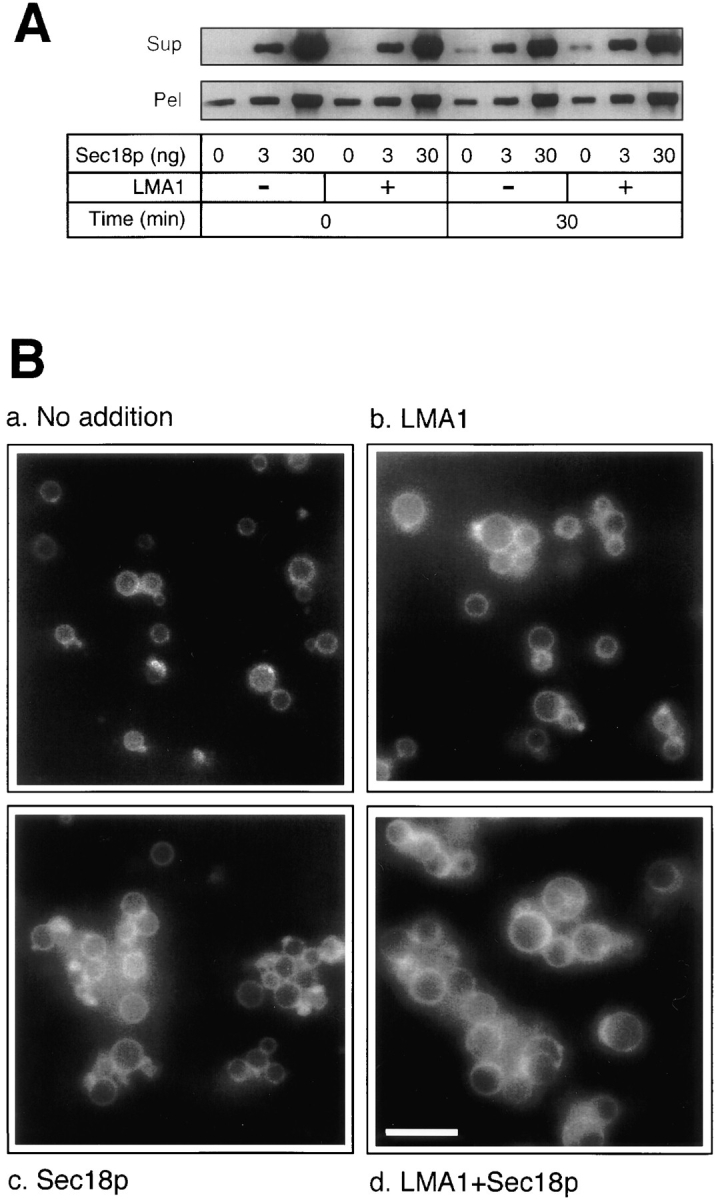
LMA1 does not support vacuole fusion via inhibition of proteinase B. (A) LMA1 does not act to prevent Sec18p degradation. Fusion reactions (30 μl) containing 0, 3, or 30 ng of Sec18p in the absence or presence of 9 ng of LMA1 were incubated at 25°C for 0 or 30 min and centrifuged (10,000 g, 5 min, 4°C). Supernatants (SUP) were recovered. Pellets (PEL) were suspended in 300 μl of 0% Ficoll buffer (10 mM Pipes-KOH, pH 6.8, 200 mM sorbitol) containing 3× PIC, 1 mM PMSF, and 5 mM EDTA and centrifuged as before. The pellets were resuspended in 25 μl of 0% Ficoll buffer and transferred to fresh 1.5 ml Eppendorf tubes. Sample buffer containing 1 mM PMSF and 5 mM EDTA (Mayer et al., 1996) was added before SDS-PAGE. Immunoblot and probing with anti-Sec18p antibody were performed as described (Mayer et al., 1996). (B) LMA1 and Sec18p support fusion of vacuoles which completely lack proteinases A and B. Fusion reactions contained 4.8 μg of only BJ3505 vacuoles with (a) no added pure proteins, (b) 9 ng LMA1, (c) 10 ng Sec18p, or (d) both LMA1 and Sec18p. The vacuoles were labeled with the lipophilic styryl dye FM 4-64 (Vida and Emr, 1995) and incubated at 25°C for 2 h before microscopy. Bar, 4 μm.
Purified LMA1 and Sec18p Synergistically Support In Vitro Fusion of Salt-washed Vacuoles
Both LMA1 and Sec18p can support vacuole fusion (Xu and Wickner, 1996; Haas and Wickner, 1996), yet the degree of requirement for each of these pure proteins varies among fresh vacuole preparations (Xu, Z., A. Mayer, E. Muller, and W. Wickner, unpublished observations). We find a consistent requirement for both proteins when the vacuoles have been salt-washed. Thus, little fusion occurred with limiting concentrations of Sec18p or LMA1 alone (Fig. 5 A), but full activity was obtained when both proteins were added. Furthermore, the reaction supported by these two pure proteins occurred to the same extent as seen with cytosol (Fig. 5 B) and was still sensitive to Gdi1p, GTPγS, and microcystin-LR, known inhibitors of later stages of the reaction (Conradt et al., 1994; Haas et al., 1994, 1995). Our isolated vacuole preparations have variable amounts of Sec18p and LMA1 that are largely removed or inactivated during a salt-wash procedure, and these two pure proteins constitute a minimal set of soluble proteins needed for reconstitution of the fusion reaction.
LMA1 Action Follows that of Sec18p and Sec17p Proteins
Previous studies (Conradt et al., 1994) have established that our in vitro reaction of vacuole inheritance occurs in defined stages in an obligatory sequence. To establish the stage(s) where Sec18p and LMA1 act, vacuoles were incubated with ATP, LMA1, and Sec18p. At the indicated times (Fig. 6 A), vacuoles were collected by centrifugation and resuspended in either the same, complete reaction mix or in buffer and ATP alone before resuming the second incubation. To control for fusion that occurred at each time of centrifugation, an aliquot was also transferred to ice. After a 30-min incubation, the reisolated vacuoles can undergo fusion without added LMA1 or Sec18p in the second incubation (Fig. 6 A). As expected, the reaction became resistant to anti-Sec18p antibodies in the second incubation but was still sensitive to GTPγS and Microcystin LR (Fig. 6 B). Thus, LMA1 and Sec18p participate at an early stage of the reaction.
Figure 6.
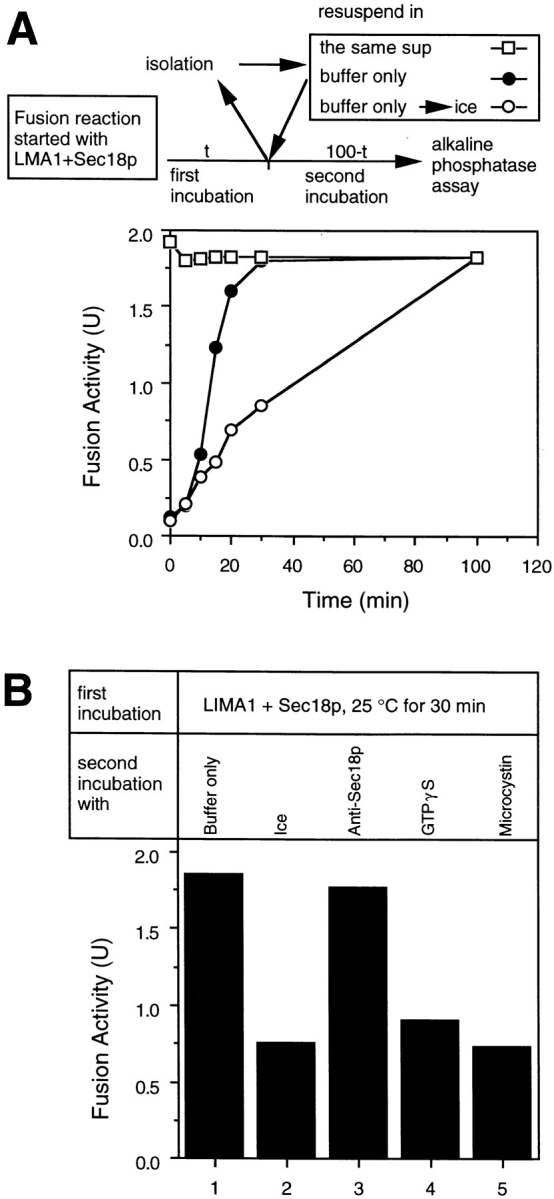
LMA1 and Sec18p act at an early stage of vacuole fusion. (A) Fusion reactions with LMA1 (9 ng) and Sec18p (10 ng) were incubated at 25°C. At the indicated times, vacuoles were collected (10,000 g, 45 s, 4°C) and resuspended in the same supernatant (□) or in 30 ml of reaction buffer (20 mM pipes-KOH, pH 6.8, 0.2 M sorbitol, 150 mM KOAc, 5 mM MgCl2, 0.1 × PIC, and the ATP regenerating system) (• and ○). The reactions were left at 25°C (□ and •) or transferred to ice (○) and incubation continued to 100 min (total). (B) Fusion reactions containing LMA1 (9 ng) and Sec18p (10 ng) were incubated at 30°C for 30 min followed by centrifugation (10,000 g, 45 s, 4°C). The reisolated vacuoles were then resuspended in 30 ml of reaction buffer (lanes 1 and 2) with 1.5 μg anti-Sec18p (lane 3), 1 mM GTPγS (lane 4), or 10 μM Microcystin-LR (lane 5). The reactions were further incubated at 25°C (lanes 1, 3, to 5) or on ice (lane 2) for another 70 min followed by alkaline phosphatase assay.
To determine whether Sec18p and LMA1 function in an obligatory sequence, we first tested whether LMA1 stimulated the Sec17p release reaction, driven by Sec18p and ATP. Vacuoles were incubated with either no addition, LMA1, Sec18p, or both proteins for various times, then reisolated and assayed by immunoblot for bound Sec17p. LMA1 does not stimulate Sec17p release in our assay (Fig. 7 A). We further examined Sec17p release from fresh vacuoles (not salt-washed) prepared from a trx1, trx2 double deletion strain and a pbi2 null strain and found that neither LMA1 subunit is required to stimulate Sec17p release (data not shown). We then determined whether LMA1 action can be completed before that of Sec18p. Vacuoles were incubated with ATP and LMA1 alone for 30 min, after which time LMA1 can normally be removed without loss of subsequent reaction (Fig. 6). After reisolation, vacuoles still required both LMA1 and Sec18p for fusion (Fig. 7 B). Thus, the requirement for LMA1 cannot be satisfied before the Sec18p-catalyzed release of Sec17p.
Figure 7.
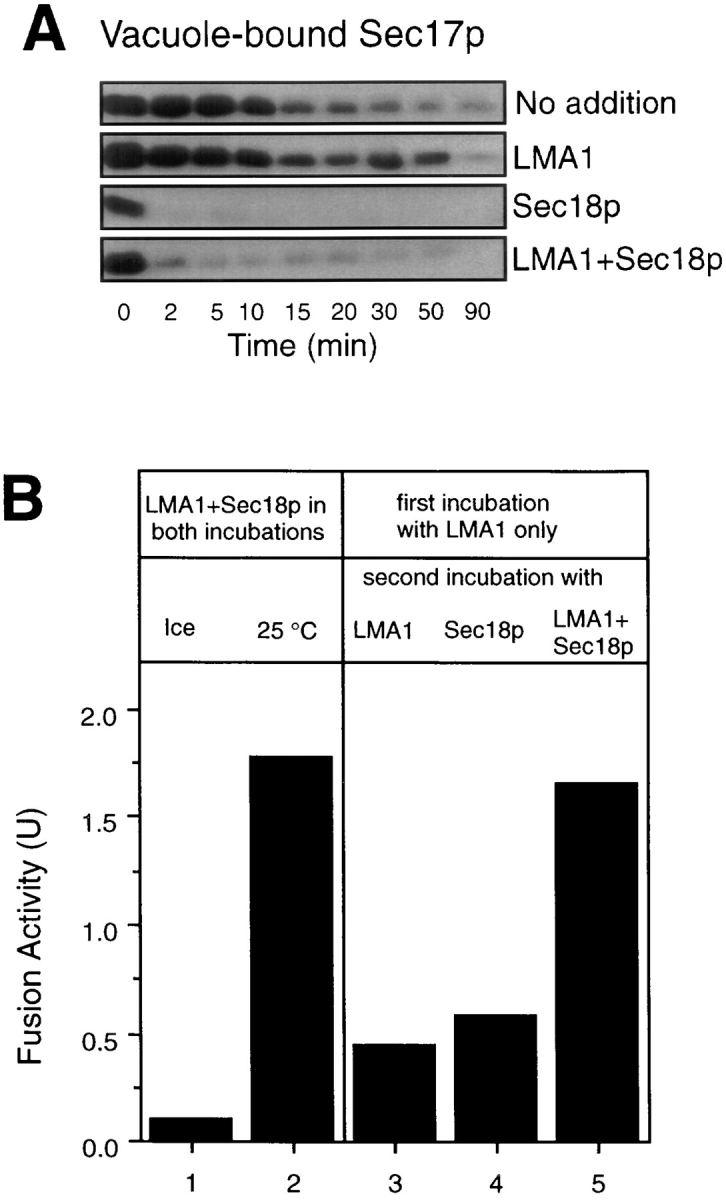
LMA1 cannot complete its function before Sec18p. (A) LMA1 does not stimulate Sec17p release. Fusion reactions of 5× the normal scale (150 μl) contained ATP and either no added proteins, 45 ng LMA1, 50 ng Sec18p, or both LMA1 and Sec18p. At indicated times, the reactions were transferred to ice and vacuoles were collected (10,000 g, 5 min, 4°C) for measuring Sec17p release as described (Mayer et al., 1996). (B) LMA1 cannot complete its action before Sec18p. Fusion reactions with 9 ng LMA1 began either with (lanes 1 and 2) or without (lanes 3–5) 10 ng Sec18p. The reactions were incubated at 25°C (lanes 2–5) or on ice (lane 1) for 30 min and vacuoles were then collected (10,000 g, 45 s, 4°C). Vacuoles were resuspended in reaction buffer containing 9 ng LMA1 (lane 3), 10 ng Sec18p (lane 4), or both LMA1 and Sec18p (lanes 1, 2, and 5) for a second incubation either at 25°C (lanes 2–5) or on ice (lane 1) for 70 min. Fusion was measured via alkaline phosphatase activity assay.
LMA1 cannot act long after Sec18p-mediated Sec17p release, as this reaction creates a labile state of the vacuoles in which the vacuoles remain intact (data not shown) but lose competence for the reaction which leads to fusion (Fig. 8 A). Vacuoles were pre-incubated with ATP alone or with ATP and either Sec18p, antibody to Sec18p, or LMA1. After various incubation times, vacuoles were collected and resuspended in reaction buffer with ATP and both LMA1 and Sec18p for a second incubation. In agreement with earlier observations (Conradt et al., 1994), the competence of vacuoles to undergo fusion was lost during pre-incubation in the absence of LMA1 (Fig. 8 A) or cytosol (data not shown); the latter may contain additional stabilizing factors as well as LMA1. This lability was exacerbated by added Sec18p and partially relieved by an antibody to Sec18p and thus was largely due to Sec18p action. This lability limits the interval after Sec18p-mediated Sec17p release in which LMA1 can act. Can the Sec18p/ Sec17p reaction be separated from the LMA1 requirement, despite their close kinetic relationship? We incubated vacuoles with Sec18p for varying intervals, mixed them with an antibody to Sec17p, and added LMA1 (Fig. 8 B). Upon addition at 0 time, the antibody effectively blocked the reaction as there had been insufficient time for Sec17p release (Mayer et al., 1996). If the vacuoles were instead incubated for a few minutes before addition of antibody to Sec17p, the release reaction occurred with little vacuole inactivation and LMA1, when added, “captured” the activated vacuoles for the subsequent fusion reaction. After longer incubations, however, the labile vacuoles (Fig. 8 A) became inactive. This delay in addition of LMA1 resulted in loss of subsequent fusion capacity (Fig. 8 B). Thus LMA1 must act during, or shortly after, the Sec18p/ 17p reaction to permit the later steps of the reaction.
Figure 8.
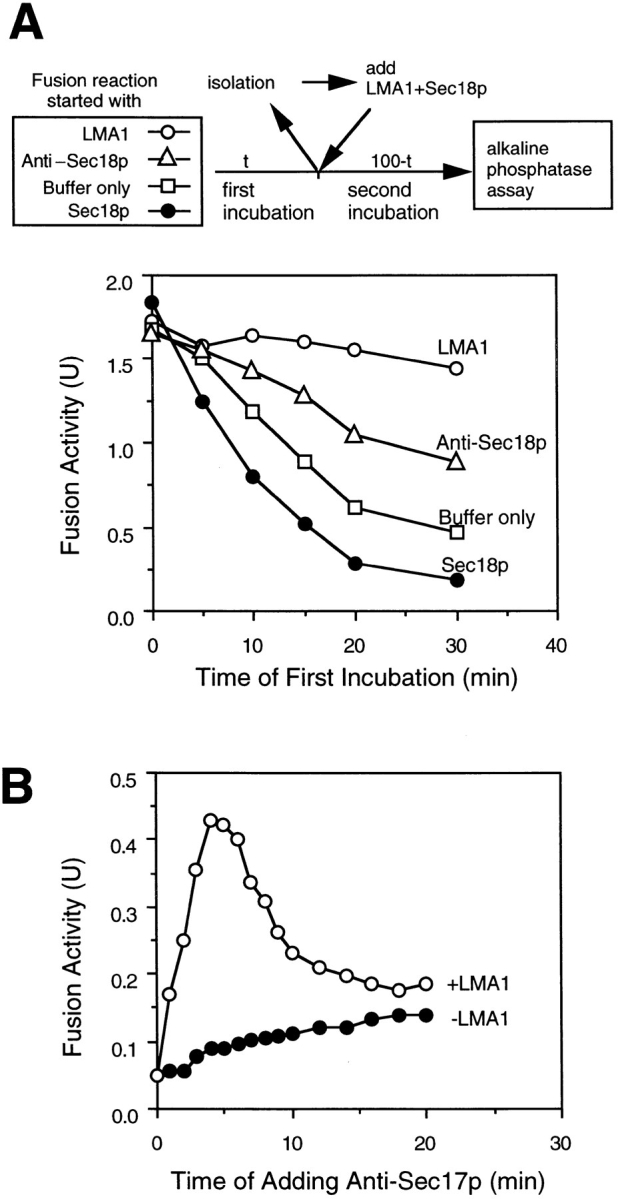
Vacuoles rendered labile by Sec18p activity are stabilized by LMA1. (A) Vacuoles become labile after Sec18p action. Fusion reactions (30 μl) containing ATP plus buffer only (□), 9 ng LMA1 (○), 10 ng Sec18p (•), or 1 μg anti-Sec18p (Δ) were incubated at 25°C. At indicated times, vacuoles were isolated and fresh LMA1 (9 ng), Sec18p (10 ng), and ATP were added. The reactions were continued at 25°C for 100 min (total) and alkaline phosphatase activity was assayed. (B) Fusion reactions containing ATP and 10 ng Sec18p were incubated at 25°C. At the indicated times, 1.5 μg anti-Sec17p antibody was added to the reactions either with 9 ng LMA1 (○), or without (•). The incubations were continued at 25°C for 100 min (total) and alkaline phosphatase activity was measured.
Discussion
Our in vitro reaction measures the last step of inheritance, the fusion of vacuoles, but relies on a sequence of coupled steps which represents earlier stages in the pathway as well. Thus, the first step of inheritance to be observed in vivo, the creation of a segregation structure which projects into the bud, is also seen in our in vitro reaction (Conradt et al., 1992) and requires the vac-encoded proteins (Conradt et al., 1992; Haas et al., 1994; Nicolson et al., 1995). Recent studies (Fanton, V., and W. Wickner, unpublished) indicate that structure formation requires Sec17 and Sec18 proteins, further connecting it to the early stages of the in vitro reaction as assayed biochemically (Mayer et al., 1996). Analysis of the in vitro reaction thus offers insight into several stages of the organelle inheritance process.
Analysis of this inheritance reaction initially established four subreactions which occur in an obligate order: (I) A brief salt-dependent step, (II) a cytosol-dependent step, (III) an ATP-dependent step, and (IV) a step sensitive to microcystin-LR (Conradt et al., 1994). These reactions culminate in the fusion of vacuoles and mixing of contents, assayed by proenzyme maturation. Subsequent studies have identified some of the proteins which support these reactions. These include Sec17p (α-SNAP) and Sec18p (NSF) (Haas and Wickner, 1996), LMA1 (Xu and Wickner, 1996), and the Ras-like GTPase Ypt7p (Haas et al., 1995). As isolated, the vacuoles bear on their surface many or, in some vacuole preparations, all of the requisite proteins. Nevertheless, after “salt-wash,” both Sec18p and LMA1 are needed to reconstitute the cytosol requirement. Like the earlier findings with cytosol, our current studies show that these proteins act at an early stage. LMA1 stabilizes the reaction competence of vacuoles and is required during or immediately after the Sec18p-mediated release of Sec17p. Docking also requires prior Sec17p release (Mayer et al., 1997) and is followed by the Stage IV (Conradt et al., 1994) microcystin LR and GTPγS-sensitive fusion reaction.
Despite the increasing resolution of this reaction into an ordered series of steps, the catalytic roles of the relevant proteins are not known. LMA1, the focus of the current report, was detected in a general scheme of cytosol fractionation and purified to homogeneity (Xu and Wickner, 1996). Each subunit, thioredoxin and IB 2, is needed for normal vacuole inheritance in vivo (Fig. 2 and Table I; also, Xu and Wickner, 1996). However, LMA1 acts neither in a typical thioredoxin redox reaction (Fig. 3) nor solely to inhibit proteinase B (Fig. 4). Thioredoxin has previously been shown to be required in a nonredox fashion in T7 replication (Huber et al., 1986) where it confers processivity on the polymerase, in filamentous phage assembly (Russel and Model, 1986), and in the mammalian early pregnancy factor system (Tonissen et al., 1993). It is also reasonable that the IB 2 subunit has a role in addition to that of proteinase B inhibitor, since IB 2 is cytosolic and proteinase B is soluble in the vacuole lumen and thus these two proteins may only meet infrequently under normal growth conditions. Though our current studies indicate that LMA1 may stabilize the activity of vacuoles in early reaction steps, the LMA1 requirement cannot be satisfied before Sec18p action. LMA1 acts during or immediately after the Sec17p/Sec18p reaction; its relation to vacuole docking and to the Ypt7p-dependent events is still to be determined. Nevertheless, the observation of its coupling to the action of the yeast homologues of NSF and α-SNAP, which are required for almost every step of membrane trafficking, suggests that LMA1 or its functional equivalents may also have a necessary role in other intracellular trafficking events. A detailed comparison of homotypic fusion during vacuole inheritance and the heterotypic fusion events during inter-organelle vesicular traffic will require identification of any SNARE proteins which support vacuole fusion.
Further understanding of the mechanism of vacuole inheritance will require a full resolution of the proteins which support the process. This in turn raises the question of developing assays for these components. Some assays will arise from a continued definition of subreactions, such as searching for the early salt-modulated factor, the components needed for Sec17p release, the agents of docking, or the functional receptors for vacuole association of LMA1, Sec17p, Sec18p, and Ypt7p. Antibodies from the burgeoning yeast community may provide a second source of assays, just as they have allowed us to establish the roles of several components. A third source of assays is the growing collection of vac mutants (Wang et al., 1996) which may allow in vitro complementation. Continued fractionation of the cytosol will also yield components, from the HMA and LMA2 activities (Slusarewicz, P., Z. Xu, and A. Haas, unpublished) to kinases and phosphatases which may regulate inheritance components (Conradt et al., 1992, 1994). Finally, with as many individual components as possible in hand, the vacuole membrane will have to be solubilized and reconstituted into proteoliposomes which are competent for the individual subreactions.
Acknowledgments
We thank Dr. Dieter H. Wolf for generously providing yeast strains, Drs. Albert Haas, Pawel Slusarewicz, Robert Matts, and Charles Barlowe for discussions, and Marilyn Leonard and Barbara Atherton for expert assistance.
This work was supported by a grant from NIGMS.
Abbreviations used in this paper
- NSF
N-ethylmaleimide–sensitive fusion protein
- SNAP
soluble NSF attachment protein
Footnotes
Address all correspondence to W. Wickner, Department of Biochemistry, Dartmouth Medical School, 7200 Vail Building, Hanover, NH 03755-3844. Tel.: (603) 650-1701. Fax: (603) 650-1353.
References
- Conradt B, Shaw J, Vida T, Emr S, Wickner W. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. . J Cell Biol. 1992;119:1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt B, Haas A, Wickner W. Determination of four biochemically distinct, sequential stages during vacuole inheritance in vitro. J Cell Biol. 1994;126:99–110. doi: 10.1083/jcb.126.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes de Mesquita DS, ten Hoopen R, Woldringh CL. Vacuolar segregation to the bud of Saccharomyces cerevisiae: an analysis of morphology and timing in the cell cycle. J Gen Microbiol. 1991;137:2447–2457. doi: 10.1099/00221287-137-10-2447. [DOI] [PubMed] [Google Scholar]
- Griff IC, Schekman R, Rothman JE, Kaiser CA. The yeast SEC17 gene product is functionally equivalent to mammalian α-SNAP protein. J Biol Chem. 1992;267:12106–12115. [PubMed] [Google Scholar]
- Haas A, Wickner W. Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF) EMBO (Eur Mol Biol Organ) J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiaeis required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO (Eur Mol Biol Organ) J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Huber HE, Russel M, Model P, Richardson CC. Interaction of mutant thioredoxins of Escherichia coliwith Gene 5 protein of Phage T7. The redox capacity of thioredoxin is not required for stimulation of DNA polymerase activity. J Biol Chem. 1986;261:15006–15012. [PubMed] [Google Scholar]
- Latterich M, Fröhlich K-U, Schekman R. Membrane fusion and the cell cycle: cdc48 participates in the fusion of ER membranes. Cell. 1995;82:885–894. doi: 10.1016/0092-8674(95)90268-6. [DOI] [PubMed] [Google Scholar]
- Maier K, Muller H, Holzer H. Purification and molecular characterization of two inhibitors of yeast proteinase B. J Biol Chem. 1979;254:8491–8497. [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Moehle CM, Ayanardi MW, Kolodny MR, Park FJ, Jones EW. Proteinase B of Saccharomyces cerevisiae: isolation and regulation of the PRB1 structure gene. Genetics. 1986;115:255–263. doi: 10.1093/genetics/115.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller EGD. A redox-dependent function of thioredoxin is necessary to sustain a rapid rate of DNA synthesis. Arch Biochem Biophys. 1995;318:356–361. doi: 10.1006/abbi.1995.1240. [DOI] [PubMed] [Google Scholar]
- McConnell S, Yaffe M. Intermediate filament formation by a yeast protein essential for organelle inheritance. Science (Wash DC) 1993;260:687–689. doi: 10.1126/science.8480179. [DOI] [PubMed] [Google Scholar]
- Nicolson TA, Weisman LS, Payne GS, Wickner W. A truncated form of the Pho80 cyclin redirects the Pho85 kinase to disrupt vacuole inheritance in S. cerevisiae. . J Cell Biol. 1995;130:835–845. doi: 10.1083/jcb.130.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CK, Roberts CJ, Moore KE, Howald I, Stevens TH. Biogenesis of the vacuole in Saccharomyces cerevisiae. . Intern Rev Cytol. 1992;139:59–120. doi: 10.1016/s0074-7696(08)61410-2. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature (Lond) 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Russel M, Model P. The role of thioredoxin in filamentous phage assembly. Construction, isolation, and characterization of mutant thioredoxins. J Biol Chem. 1986;261:14997–15005. [PubMed] [Google Scholar]
- Schu P, Suarez P, Rendueles, Wolf DH. The proteinase yscB inhibitor (PBI2) gene of yeast and studies on the function of its protein product. Eur J Biochem. 1991;197:1–7. doi: 10.1111/j.1432-1033.1991.tb15874.x. [DOI] [PubMed] [Google Scholar]
- Shaw J, Wickner W. vac2: a yeast mutant which distinguishes vacuole segregation from Golgi-to-vacuole protein targeting. EMBO (Eur Mol Biol Organ) J. 1991;10:1741–1748. doi: 10.1002/j.1460-2075.1991.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature (Lond) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Tonnissen K, Wells J, Cock I, Perkins A, Orozco C, Clarke F. Site-directed mutagenesis of human thioredoxin. J Biol Chem. 1993;268:22485–22489. [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-X, Zhao H, Harding TM, Gomes de Mesquita DS, Woldringh CL, Klionsky DJ, Munn AL, Weisman LS. Multiple classes of yeast mutants are defective in vacuole partitioning yet target vacuole proteins correctly. Mol Biol Cell. 1996;7:1375–1389. doi: 10.1091/mbc.7.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G, Wickner W. Organelle inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- Weisman LS, Wickner W. Intervacuole exchange in the yeast zygote defines a new pathway in organelle communication. Science (Wash DC) 1988;241:589–591. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- Weisman L, Emr SD, Wickner W. Mutants of Saccharomyces cerevisiaethat block intervacuole vesicular traffic and vacuole division and segregation. Proc Natl Acad Sci USA. 1990;87:1076–1080. doi: 10.1073/pnas.87.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Wilcox CA, Flynn GC, Chen E, Kuang W-J, Henzel WJ, Block MR, Ullrich E, Rothman JE. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature (Lond) 1989;339:355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wickner W. Thioredoxin is required for vacuole inheritance in Saccharomyces cerevisiae. . J Cell Biol. 1996;132:787–794. doi: 10.1083/jcb.132.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]