Abstract
The highly organized pattern of acetylcholinesterase (AChE) molecules attached to the basal lamina of the neuromuscular junction (NMJ) suggests the existence of specific binding sites for their precise localization. To test this hypothesis we immunoaffinity purified quail globular and collagen-tailed AChE forms and determined their ability to attach to frog NMJs which had been pretreated with high-salt detergent buffers. The NMJs were visualized by labeling acetylcholine receptors (AChRs) with TRITC-α-bungarotoxin and AChE by indirect immunofluorescence; there was excellent correspondence (>97%) between the distribution of frog AChRs and AChE. Binding of the exogenous quail AChE was determined using a speciesspecific monoclonal antibody. When frog neuromuscular junctions were incubated with the globular G4/G2 quail AChE forms, there was no detectable binding above background levels, whereas when similar preparations were incubated with the collagen-tailed A12 AChE form >80% of the frog synaptic sites were also immunolabeled for quail AChE attached. Binding of the A12 quail AChE was blocked by heparin, yet could not be removed with high salt buffer containing detergent once attached. Similar results were obtained using empty myofiber basal lamina sheaths produced by mechanical or freeze-thaw damage. These experiments show that specific binding sites exist for collagen-tailed AChE molecules on the synaptic basal lamina of the vertebrate NMJ and suggest that these binding sites comprise a “molecular parking lot” in which the AChE molecules can be released, retained, and turned over.
Acetylcholinesterase (AChE),1 the enzyme responsible for hydrolyzing acetylcholine released at the neuromuscular junction, is localized in the synaptic cleft interposed between the nerve terminals and the postsynaptic membrane, where a substantial fraction of the enzyme is attached to the synaptic basal lamina (McMahan et al., 1978; for review see Massoulié et al., 1993). The distribution of AChE molecules on the synaptic basal lamina closely matches the distribution of nicotinic acetylcholine receptors (AChRs), as well as other molecules on the pre- and postsynaptic membranes, indicating a high degree of organization of the molecular components at the neuromuscular junction (reviewed in Hall and Sanes, 1993). Although most of the AChE expressed in muscle can be solubilized, the AChE molecules attached to the synaptic basal lamina in birds and mammals are not removed by high ionic strength buffers, detergents, or chaotropic agents (Rossi and Rotundo, 1993). On the other hand, the basal lamina–associated AChE can be detached using mixtures of collagenase and other proteases (Hall and Kelly, 1971; Betz and Sakmann, 1973) or highly purified collagenase (Rossi and Rotundo, 1993).
The junctional AChE molecules can be contributed either by the muscle fibers (Anglister and McMahan, 1985; De La Porte et al., 1986) or by the motoneurons (Anglister, 1991), with the former most likely contributing most of the basal lamina–associated enzyme. After denervation, there is a large decrease in the density of AChE molecules at the neuromuscular synapse which can be restored by electrical stimulation of the denervated muscles or by their reinnervation either at the original (Lømo and Slater, 1980) or at ectopic sites (Weinberg and Hall, 1979). Furthermore, regenerating myofibers re-accumulate basal lamina AChE at original synaptic sites in the absence of innervation (Anglister and McMahan, 1985). Altogether, these studies show that muscle fibers can produce synaptic AChE and, moreover, indicate that the information necessary for organizing AChE molecules on the synaptic basal lamina is associated either with the muscle or contained within the synaptic matrix itself.
In birds and mammals the predominant, if not exclusive, form of AChE attached to the synaptic basal lamina is the asymmetric A12 form of the enzyme consisting of three tetramers covalently linked to a three-stranded collagen-like tail (reviewed in Massoulié et al., 1993). The collagen-like tail is generally thought to be required for attachment of AChE to the extracellular matrix. The observation that only proteolysis using collagenase can remove the junctional AChE is consistent with this view and suggests that the AChE molecules become covalently attached to components of the specialized extracellular matrix at the neuromuscular synapse.
Tissue-cultured muscle cells express the same oligomeric forms of AChE as adult muscle including the globular monomeric (G), dimeric (G2), and tetrameric (G4) forms and the asymmetric collagen-tailed form (A12). When the myotubes differentiate into actively contracting myofibers they assemble the A12 collagen-tailed form which in turn becomes organized into clusters on the extracellular matrix overlying individual myonuclei (Rossi and Rotundo, 1992). These clustered AChE molecules behave very much like the AChE attached to the synaptic basal lamina in that they cannot be removed using high ionic strength buffers or detergents, yet can be detached using purified collagenase. When these forms are externalized at the surface plasma membrane they appear to transiently interact with heparan sulfate-like proteoglycans before attaching more permanently to the extracellular matrix (Rossi and Rotundo, 1996). Although these studies show that the A12 AChE form is selectively retained on the extracellular matrix in the vicinity of the myonuclei, they do not distinguish between several possible mechanisms of targeting including association with the extracellular matrix at the sites of externalization, or, more interestingly, preferential attachment to specific sites organized on the basal lamina.
In the present study we sought to determine whether specific attachment sites for the collagen-tailed AChE form were situated on the synaptic basal lamina of adult muscle. Using purified quail globular and collagen-tailed AChE forms, we demonstrate that only the collagen-tailed form of the enzyme can bind to the frog neuromuscular junction where it attaches to the extracellular matrix. These experiments show that specific sites exist for localizing AChE at the neuromuscular synapse and that the properties of these sites are shared across species as divergent as amphibians and birds. Furthermore, since AChE molecules can be turned over at the neuromuscular junction while maintaining their appropriate distributions (Kasprzak and Salpeter, 1985), our observations suggest that a “molecular parking lot” exists for the insertion and removal of this enzyme on the synaptic basal lamina.
Materials and Methods
Preparation of Tissues and Empty Basal Lamina Sheaths
Frogs (Rana pipiens) obtained from Hazen Co. (Alburg, VT) were housed at 20–22°C in the animal care facility at the Hebrew University Hadassah Medical School under veterinary supervision and fed twice weekly. Frozen sections of frog muscle, 30 μm thick, were cut from the anterior tibialis muscle, mounted on gelatin-coated slides, and stored frozen until used. Before extraction the slides were warmed to room temperature, and a 0.5-cm high by 1.2-cm diam plastic ring was placed around the sections and held in place with rubber cement. The plastic rings formed the wells in which the various extractions, incubations, and immunohistochemical procedures were performed.
Empty basal lamina sheaths of cutaneous pectoris muscles were prepared as described in detail elsewhere (Anglister et al., 1994a ). Muscles were damaged by freezing or crushing and were denervated by excision and removal of a 1–2-cm segment of the second spinal nerve near the vertebral column. The frogs were X-irradiated once on each of the first three days following surgery to prevent regeneration of the muscle fibers from the remaining satellite cells. After allowing 5–6 wk for degeneration of the muscle fibers, the sheaths of the cutaneous pectoris muscles were dissected, pinned in small chambers, and extracted with several changes of borate extraction buffer (see below). All extractions and incubations were carried out in the 150-μl chamber volume. When necessary, the chambers were sealed by pressing a 2.5-cm diam glass coverslip onto the surface to prevent evaporation.
Isolation and Purification of Avian AChE Forms
The globular and collagen-tailed AChE forms were isolated from tissuecultured quail myotubes by preparative sucrose gradient sedimentation as previously described (Rotundo, 1984a ). The pooled fractions containing the G4 tetramers and the G2 dimers (globular forms) or A12 collagen- tailed AChE forms were diluted with borate buffer (see below) and passed through a 1-ml immunoaffinity column made by coupling the monoclonal anti-avian AChE antibody 1A2 (Rotundo, 1984b ) to CNBr activated Sepharose 2B (Pharmacia LKB Biotechnology, Piscataway, NJ) at a concentration of 1 mg IgG/ml swelled gel. After washing the column with additional buffer the enzyme was eluted with 2 mM triethanolamine, pH 11, with 1 M NaCl and 100-μl fractions collected in microfuge tubes containing 50 μl 0.5 M Tris, pH 7. The fractions containing the catalytically active AChE forms were pooled, concentrated, and stored frozen until used. The concentration of AChE protein was estimated by enzymatic assay using the turnover number for chicken AChE, 1.45 mmol/min/mg protein, determined by Vigny et al. (1978). The stock solutions of the A12 and G4/G2 were 97 ng/ml and 23 ng/ml, respectively.
Extraction of AChE from Tissue Sections and Transfer of Quail Enzyme
Tissue sections and empty basal lamina sheaths were pre-extracted with detergent-containing buffer before all experiments to remove muscle plasma membranes, soluble proteins, and/or cellular debris that could interfere with access to the synaptic basal lamina. All extractions were carried out in microwells using borate extraction buffer consisting of 20 mM sodium borate, pH 9.0, 5 mM EDTA, 1 M NaCl, 0.5% Triton X-100, and 5 mg/ml bovine serum albumin high salt buffer (HSB), unless otherwise indicated. In some experiments the salt concentration was reduced to 130 mM low salt buffer (LSB) and/or the Triton X-100 was eliminated. Because of the small size of the tissue sections, protease inhibitors were generally not included in the extraction buffers. However, the sections were always washed with several changes to dilute and remove the extracted proteins.
After extraction, the tissue sections or empty basal lamina sheaths were preincubated for 30 min in HSB containing 5 mg/ml each of bovine serum albumin, chicken ovalbumin, and gelatin (all from Sigma Chem. Co., St. Louis, MO) which have isoelectric points in the same range as avian AChE. The addition of this protein mixture was essential in order to reduce nonspecific binding of AChE to the tissues. The stock AChE solutions were diluted 1:50–1:20 in HSB containing the protein mix, to give 1–2 ng AChE/well final, and the NaCl concentration was adjusted to 0.5 M. This solution was placed in the wells, 100 μl/well, and the ionic strength lowered to 0.3 M stepwise over a period of 2 h to reduce the chances of the collagen-tailed AChE form aggregating which can occur at salt concentrations below 0.3 M. After adjustment to 0.3 M NaCl the sections were incubated overnight at room temperature on a rotary platform. The next day the NaCl concentration was lowered to 0.25–0.15 M NaCl to reduce detachment of bound AChE, the sections rinsed with LSB, and the samples prepared for immunofluorescence. This procedure was modified slightly for use with the empty basal lamina sheaths. For these thicker samples the amount of quail AChE was reduced by a half, the protein added directly to HSB adjusted to 0.3 M NaCl, and the incubations were performed for 2 d in the microchambers sealed with a glass coverslip.
The fraction of the quail AChE that bound to the tissue sections was determined biochemically by treating frozen sections of frog muscle mounted on glass slides with diisopropylfluorophosphate, to irreversibly inhibit AChE activity, followed by extensive washing to remove unreacted inhibitor. The sections were then incubated overnight with HSB containing the protein mixture as described above, either with or without A12 or G4/G2 AChE. The sections were rinsed to remove unbound enzyme, scraped into 100-μl HSB and sonicated to disperse the tissue. Aliquots of the tissue extract were assayed for AChE activity, together with samples of the AChE solution before incubation with the tissue sections, to determine the percent AChE bound. In both cases, only a very small percentage of the total AChE added actually bound to the tissue sections, 1.0% and 1.5% for the A12 and G4/G2 AChE forms, respectively. Although small amounts of both globular and collagen-tailed AChE binding could be detected biochemically, on the order of about 10 pg each, most of this probably reflects nonspecific binding since the available surface area of the junctional regions on these frozen sections is <0.1% of the total cross-sectional area. Therefore, in practice, it is not possible to estimate the extent of specific AChE binding in these experiments and for this reason we used immunofluorescence localization of the quail AChE.
Immunofluorescence Localization of AChE and Labeling of AChR
After incubation with purified AChE forms, the tissue samples were washed with several chamber volumes of 10 mM PBS, pH 7.4, containing 10% horse serum (PBS/HS), to remove unbound enzyme and saturate nonspecific binding sites on the tissue sections. The sections were incubated with PBS/HS containing 20 μg/ml 1A2 anti-avian AChE monoclonal antibody for 60 min followed by three washes of PBS/HS over a 30– 60-min period. The second antibody was fluorescein-conjugated rabbit anti–mouse IgG (Cappel Laboratories, Malvern, PA) used at a concentration of 10 μg/ml in PBS/HS, for 60 min. After washing with additional PBS/HS, samples were rinsed with PBS alone, fixed with 4% paraformaldehyde in PBS, and mounted in 90% glycerol/10% 100 mM bicarbonate buffer, pH 9.5, containing 1 mg/ml phenylenediamine (Sigma) to reduce photobleaching. In some experiments 1 μg/ml TRITC-conjugated α-bungarotoxin (αBtx) (Molecular Probes, Inc., Eugene, OR) was included together with the first antibody to label the frog nicotinic acetylcholine receptors, and the frog AChE was localized using either a cross-reacting polyclonal antibody generated against the enzyme isolated from the electric ray (antibody R80, a generous gift from Dr. P. Taylor, U.C. San Diego, La Jolla, CA) or by immunocytochemistry following the procedure of Karnovsky (1964).
Quantitation of AChR and AChE Colocalization
The frog neuromuscular junction consists of several nerve terminal branches coursing in parallel to the long axis of the muscle fiber. On cross sections of muscle fibers these synaptic gutters appear as a series of several peripherally localized clusters of AChR and AChE molecules (see Figs. 2 and 3). To determine the extent of colocalization, all TRITC-αBtx labeled AChR clusters were counted on three randomly chosen cross sections of frog muscle per slide. The fraction of TRITC-αBtx labeled AChR clusters that were also positive for fluorescein-labeled AChE was then determined and expressed as percent of total. At least three slides per group were quantitated and the results expressed as the mean percentage ± SEM.
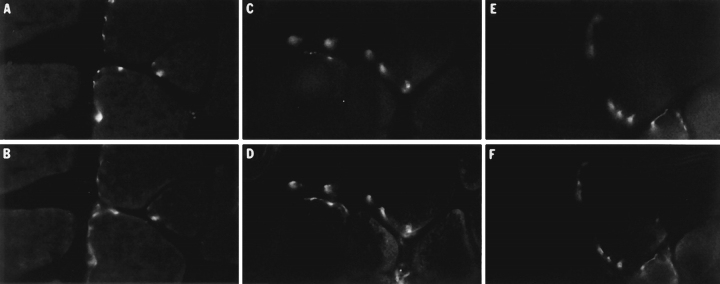
Figure 2.
Localization of AChR and frog AChE at the frog neuromuscular junction; effects of extraction procedures. Frozen sections of anterior tibialis muscle were extracted for 90 min at room temperature in either frog Ringer's solution, LSB, or HSB before localizing sites of AChR accumulation using TRITC-αBtx and frog AChE using the polyclonal antibody R80. Upper panels show the distribution of AChR and the lower panels show the corresponding distribution of frog AChE in the same fields. (A–B) Muscle sections incubated in frog Ringer's solution; (C–D) sections extracted with LSB; and (E–F) sections extracted with HSB. In all cases there was a high degree of correspondence between the sites of AChR and AChE localization, regardless of whether the sections were extracted with high ionic strength buffers and/or detergents, indicating that the sites of AChE attachment are preserved during extraction and can still be identified using AChR as a marker.
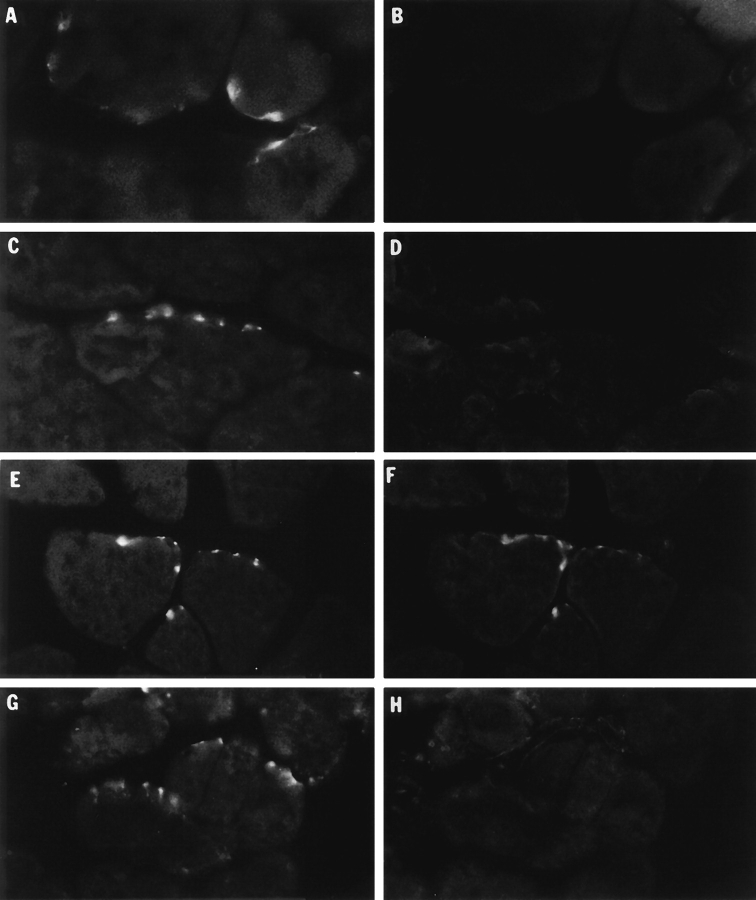
Figure 3.
Binding of collagen-tailed quail AChE molecules to frog muscle and colocalization with AChR at the neuromuscular junction. Frozen sections of frog anterior tibialis muscle were incubated overnight with HSB containing either no exogenous quail enzyme (control, panels A–B), avian G4/G2 AChE (C–D), avian A12 AChE (E–F), or avian A12 AChE plus 1 mg/ml heparin (G–H) as described in the text. After incubation the sections were rinsed with PBS and the quail AChE molecules localized by indirect immunofluorescence. Sites of nerve muscle contact were visualized using TRITC-αBtx. Left panels, distribution of AChR at sites of nerve-muscle contact. Right panels, corresponding fields showing the distribution of quail AChE molecules. Only the A12 AChE form bound to the tissue sections, where it colocalized with frog AChR, indicating that this form of the enzyme binds specifically to the neuromuscular junction. In contrast, there is little or no binding of the globular G4/G2 forms under the same conditions.
Results
Distribution of Nuclei at the Frog Neuromuscular Junction
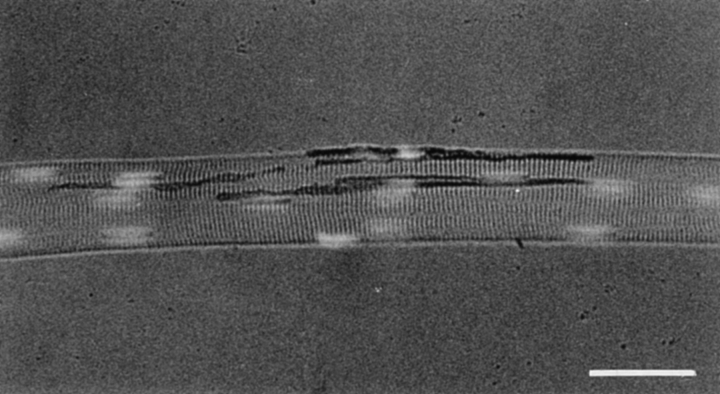
In both mammals and birds there is an accumulation of nuclei in the innervated regions of the muscle fibers that express higher levels of AChR (Merlie and Sanes, 1985; Klarsfeld et al., 1991; Simon et al., 1992) and AChE (Jasmin et al., 1993; Michel et al., 1995; Legay et al., 1995) transcripts. To determine the numbers and distribution of myonuclei at frog neuromuscular junctions, teased fibers from the cutaneous pectoris muscles were stained histochemically for AChE (Karnovsky, 1964) and the nuclei labeled with HOECHST 33342 (Fig. 1). The average neuromuscular junction in the cutaneous pectoris muscle is located along a 340 ± 50 μm (mean ± SEM, n = 24) long central segment of the myofiber and has about 3–4 nuclei distributed along its length. These myonuclei are typically elongated, sausage-shaped, organelles that can be easily distinguished from the rounded nuclei in the Schwann cells, fibroblasts, and satellite cells. The nuclei within the innervated region of the fibers were counted and compared to the distribution of nuclei within the same length of non-innervated regions in the same fibers. In these fibers the ratio of junctional to extrajunctional nuclei was 1.09 ± 0.04 (mean ± SEM; n = 24). Thus, in contrast to higher vertebrates where a dozen or more disc-shaped nuclei can be distributed immediately beneath the sites of nerve-muscle contact, at the frog neuromuscular junction there does not appear to be such an accumulation of myonuclei. These observations indicate that, in frog, the AChE molecules that are destined to become incorporated at the neuromuscular junction must move long distances following translation before their extracellular attachment at precise and orderly locations on the synaptic basal lamina of the elongated junction.
Figure 1.
Distribution of muscle nuclei at the frog neuromuscular junction. Frog cutaneous pectoris muscles were pinned at resting length in Silgard-coated dishes and the neuromuscular junctions visualized by histochemically staining for AChE followed by 60 min incubation in frog Ringer's containing 100 U/ml of collagenase (Sigma type IV) to facilitate teasing the fibers free of fibroblasts and Schwann cells. After fixation with 4% paraformaldehyde in PBS and staining of nuclei with HOECHST 33342, the fibers were teased and mounted in glycerol/phenylenediamine. The myonuclei in frog muscle have the typical elongated shape characteristic of myonuclei in other species, and can be easily distinguished from the rounded nuclei in the Schwann cells and fibroblasts. In contrast to mammals and birds, there is no accumulation of nuclei at the frog neuromuscular junction. Bar, 50 μm.
Attachment of Acetylcholinesterase to the Frog Neuromuscular Junction
This initial study was performed to determine the extractability of frog AChE and the extent to which the AChR and AChE remained colocalized following incubation with extraction buffers. Frog muscles were pretreated so that synaptic sites would retain the putative “association sites” for AChE while releasing at least some of the frog AChE from those sites. We have previously shown that AChE is poorly extracted from quail and rat neuromuscular junctions even using high ionic strength buffers, with or without detergents (Rossi and Rotundo, 1993). Similar extraction procedures were applied to frog muscles, and the extent to which the AChR and AChE clusters remained colocalized under the different conditions was examined as follows. Frozen sections of anterior tibialis muscle were incubated with frog Ringer's solution, LSB, or HSB, with or without detergent, for either 90 min or overnight. The distribution of AChR and AChE were examined by incubating the sections with TRITC-αBtx to label AChR and by indirect immunofluorescence to label AChE (Fig. 2). The different extractions had only a small effect on the intensity of AChR and AChE staining, indicating that under the conditions of this study most of these molecules remained attached to the frog muscle frozen sections throughout the incubation period. These results, presented in Table I, indicate that the distribution of AChR clusters accurately matches the distribution of AChE molecules even after extensive extraction with high salt and detergent containing buffers. Similar results were obtained using whole mounts of cutaneous pectoris muscle (data not shown).
Table I.
Colocalization of AChR and AChE after Different Extraction Procedures
| Extraction buffer | ||||||
|---|---|---|---|---|---|---|
| 1st extraction | Overnight extraction | Percent colocalization of AChE and AChR clusters | ||||
| Ringer's | − | 93.0 ± 3.4 | ||||
| HSB + TX-100 | Ringer's | 95.6 ± 2.2 | ||||
| HSB + TX-100 | HSB + TX-100 | 97.1 ± 1.6 | ||||
| Minus Detergent | Plus Detergent | |||||
| Ringer's | − | 94.6 ± 3.4 | 97.6 ± 2.3 | |||
| LSB | − | 98.3 ± 1.6 | 98.6 ± 1.3 | |||
| HSB | − | 99.5 ± 0.5 | 98.6 ± 1.5 | |||
Frozen sections of frog anterior tibialis muscle were extracted with either frog Ringer's solution, HSB plus detergent, or LSB plus detergent for 90 min at room temperature and kept in frog Ringer's solution or same extraction buffer overnight before incubation with TRITC-αBTX to label AChR and Karnovsky reaction or anti-frog AChE antibody R80 to localize AChE. Other samples were extracted using buffers plus or minus detergent and labeled thereafter. The colocalization of AChR and AChE clusters exceeded 90% under the different extraction conditions, and their normal distribution persisted after overnight incubation. All values are the mean ± SEM of at least three slides per group.
Binding of Quail Collagen-tailed AChE Molecules to the Frog Neuromuscular Junction
Newly synthesized A12 AChE molecules undergo transient electrostatic interactions with components of the extracellular matrix before becoming more permanently attached (Rossi and Rotundo, 1996). The initial interactions are blocked in the presence of heparin, whereas once the A12 molecules are attached, heparin cannot remove them. These observations suggest that specific attachment sites for the A12 AChE form exist on the extracellular matrix and that the availability of such sites at the neuromuscular junctions could determine the distribution of AChE on the synaptic basal lamina.
To determine whether exogenous quail AChE could attach to specific sites on frog muscle, purified globular G4/G2 or collagen-tailed A12 forms, prepared from tissue-cultured quail myotubes as described in Materials and Methods, were incubated with high salt-detergent extracted sections of frog muscle under conditions where the ionic strength of the medium could be gradually reduced. After washing the chambers to remove unbound enzyme, the sections were incubated with TRITC-αBtx to localize AChRs and mAb 1A2 to localize quail AChE (Fig. 3). The monoclonal anti-AChE antibody used in these experiments is species-specific and does not recognize the frog enzyme (Fig. 3, A and B). When frog muscle was incubated with the globular G4/G2 AChE forms there was little or no binding of the enzyme molecules to sites of nervemuscle contact represented by the clusters of AChR accumulation (Fig. 3, C and D). In contrast, when the frog muscle sections were incubated with purified A12 AChE most sites of nerve-muscle contact also had quail AChE attached (Fig. 3, E and F). This attachment was specific in that inclusion of heparin in the incubation medium prevented attachment of the A12 (Fig. 3, G and H), just as it prevented attachment of the newly synthesized AChE to cell surface clusters on tissue-cultured myotubes (Rossi and Rotundo, 1996). These results are presented quantitatively in Fig. 4 showing that more than 85% of the sites of frog nerve-muscle contact were also positive for quail AChE, while inclusion of heparin reduced binding to <15%.
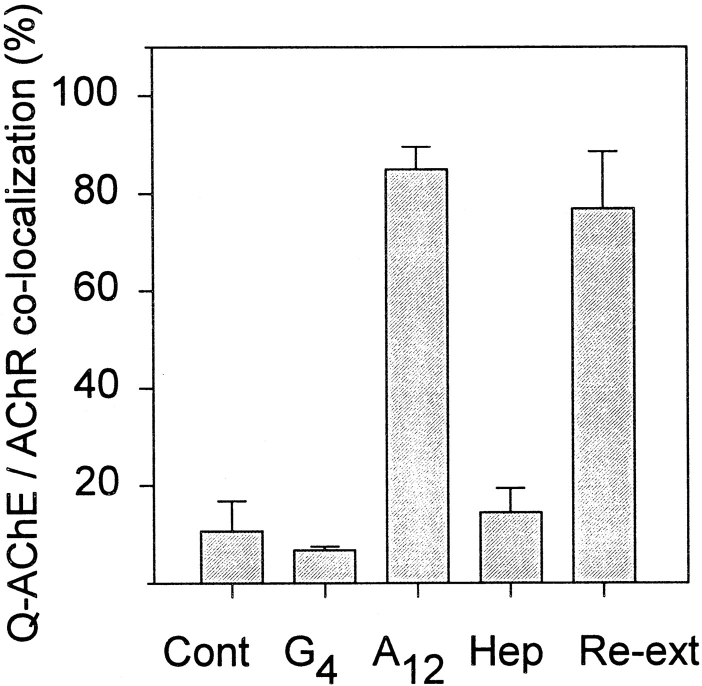
Figure 4.
Quail AChE binding to frog neuromuscular junctions. The number of frog neuromuscular contacts, visualized using TRITC-αBtx, that had bound avian AChE labeled with mAb 1A2 (shown in Fig. 3), were determined by counting all sites of αBtx binding on three frozen sections of muscle per slide, with the total from three slides averaged per group. (Cont) Control muscle sections incubated in the absence of quail AChE; (G4/G2) sections incubated with purified quail globular AChE forms; (A12) sections incubated with quail collagen-tailed AChE; (Hep) sections incubated with A12 AChE in the presence of 1 mg/ml heparin; and (Re-ext) sections incubated with A12 AChE followed by re-extraction using HSB. When incubated with the A12 form, 85 ± 5% of the frog AChR sites showed quail AChE binding, whereas only 7 ± 1% of the sites had quail AChE bound when incubated with the globular AChE forms. Addition of heparin to the incubation mixture reduced the A12 binding to 14 ± 5%. Once the quail AChE was bound to the frog neuromuscular junctions, it remained tightly attached since it could not be re- extracted with HSB 77 ± 12%.
Interestingly, re-extraction of the frog muscle sections incubated with A12 AChE using high salt and detergent containing buffers did not remove the quail AChE (Fig. 4). This result is similar to observations made both on adult neuromuscular junctions and on tissue-cultured myotubes, indicating that once the avian A12 AChE molecules are attached, high ionic strength buffers cannot dissociate them from the extracellular matrix (Rossi and Rotundo, 1993, 1996).
The Collagen-tailed AChE Molecules Attach to Specific Sites on the Synaptic Basal Lamina
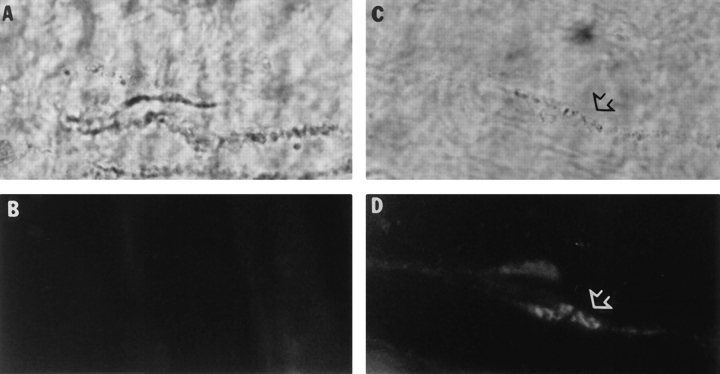
The attachment of quail AChE to the frog neuromuscular junction could occur by direct interactions with components of the extracellular matrix or with molecules localized on the postsynaptic membrane or on the nerve terminal (or some combination). To distinguish between these possibilities, empty basal lamina sheaths were prepared by denervating the frog cutaneous pectoris muscles and destroying the muscle fibers, followed by X-irradiation to prevent fiber regeneration (Anglister et al., 1994a ). After allowing 5–6 wk for the damaged fibers and nerve terminals to completely degenerate and be removed by phagocytosis, the empty basal lamina sheaths were dissected, pinned out in Silgard microwells, and extracted with HSB. The empty basal lamina sheaths were incubated in buffers containing either the globular G4/G2 or collagen-tailed A12 AChE forms for 48 h, washed, and stained for quail AChE using mAb 1A2 and frog AChE using enzyme histochemistry (Fig. 5). Only the A12 AChE bound to the empty basal lamina sheaths where it was localized along portions of the synaptic gutters in a pattern identical to the native frog enzyme molecules. These results indicate that the A12 AChE molecules are binding to specific components of the synaptic basal lamina, and moreover, that this component(s) is highly organized along the region of the basal lamina contacted by the nerve terminals. Furthermore, all the components of the reaction responsible for binding the collagen-tailed AChE to the extracellular matrix must belong to the extracellular matrix itself or to the purified enzyme, since it was possible to transplant avian A12 AChE to the empty basal lamina sheaths.
Figure 5.
Binding sites for AChE are regularly arrayed along the synaptic basal lamina. Empty frog basal lamina sheaths were incubated with either the G4/G2 (A and B), or A12 (C and D) quail AChE and the neuromuscular junctions localized histochemically (A and C). The quail enzyme was labeled by indirect immunofluorescence using mAb 1A2 (B and D). Only the A12 collagen-tailed AChE enzyme bound to the neuromuscular junction where it colocalized with the previously bound frog AChE.
Discussion
The multiple oligomeric forms of AChE expressed in nerves and muscle have been extensively studied and much is known about their structure, synthesis, assembly, and regulation at the cellular and molecular level. However, the mechanisms involved in localizing the newly synthesized AChE molecules at cholinergic synapses are still not well understood. In particular, the unique association of the collagen-tailed A12 AChE form with the specialized extracellular matrix interposed between the motor nerve terminals and the skeletal muscle fibers, requires that a precise targeting mechanism exist to insure the appropriate distribution and density of enzyme molecules at the neuromuscular junction as well as providing a means for their periodic replacement.
The major events during the synthesis and assembly of the oligomeric AChE forms in skeletal muscle are well documented (for review see Massoulié et al., 1993). The catalytic subunits consist of single polypeptide chains that are cotranslationally glycosylated in the rough endoplasmic reticulum where they are assembled into dimers and tetramers. After vesicular transport into the Golgi apparatus a subset of the oligomers are attached to the noncatalytic subunit, the three-stranded collagen-like tail, via disulfide bonds to complete the hetero-oligomeric form consisting of twelve catalytic subunits of ∼65–100 kD each and three noncatalytic polypeptide chains of ∼55 kD each for a total M r of ∼1.1–1.4 million and a length of ∼50 nm depending upon the species. The newly assembled A12 forms are then transported to the cell surface and externalized via fusion of the transport vesicles. In tissue cultured skeletal muscle, the A12 form remains associated with the cell surface, whereas in tissue-cultured neurons the A12 form appears to be mostly, if not entirely, secreted. Thus, the fate of the newly synthesized A12 AChE, i.e., whether it is released vs retained, depends upon the cell type in which it was synthesized and the molecular composition of its cell surface components. In tissue-cultured myotubes, the A12 AChE molecules preferentially accumulate in cell surface clusters in the vicinity of the myonuclei around which they were synthesized (Rossi and Rotundo, 1992).
In vivo, the problem of targeting the A12 AChE to the mature neuromuscular junction is more complex due to the intricate architecture of the synaptic region and the requirement that the AChE molecules lie precisely between the neurotransmitter release sites on the nerve terminals and the array of nicotinic acetylcholine receptors on the postsynaptic membrane. Although there is a higher level of AChE mRNA, as well as protein expression in innervated regions of skeletal muscle fibers (Jasmin et al., 1993), which in turn can increase localized secretion of the enzyme, this alone is not sufficient to insure correct organization of the AChE molecules on the synaptic basal lamina since many of the areas of high AChE density can be tens of micrometers from the nearest nucleus (Fig. 1). Thus, secretion followed by limited diffusion of the protein must account, at least in part, for the distribution of AChE molecules along the synaptic basal lamina.
While limited diffusion can account for the dispersion of A12 AChE molecules from sites of secretion on the synaptic sarcolemma, it cannot account for the highly organized pattern of matrix-associated AChE interposed between the nerve terminals and the postsynaptic membrane. Within a species, the density of AChE molecules at the neuromuscular junctions is remarkably constant from one area of nerve contact to another (Anglister et al., 1994b , and references therein). Together, these observations imply that some molecular component(s) of the synaptic basal lamina is already organized and available for the attachment of the A12 AChE. That this is indeed the case is suggested by the experiments presented in Figs. 3–5 showing that only the A12 AChE form can bind to the neuromuscular junctions where it colocalizes with AChRs.
Although the molecule(s) to which the A12 AChE bind have not yet been identified, there is strong indirect evidence to suggest that it may be a heparan sulfate-like proteoglycan localized in the extracellular matrix. Early studies on the structure and membrane association of AChE from Torpedo electric organs showed that the collagentailed, but not the globular forms, could associate with fragments of the extracellular matrix isolated by sucrose gradient centrifugation (Lwebuga-Mukasa et al., 1976). The collagenic tail of AChE contains a heparin-binding domain (Deprez and Inestrosa, 1995) and can specifically associate with heparan sulfate glycosaminoglycans (Bon et al., 1978; Vigny et al., 1983; Brandan et al., 1985). A specific heparan sulfate proteoglycan is highly concentrated at the vertebrate neuromuscular junction (Anderson and Fambrough, 1983; Bayne et al., 1984; Sanes et al., 1986; Swenarchuk et al., 1990), where its distribution correlates very closely with AChE. Whether AChE attaches only to this sulfated proteoglycan or to other molecules with similar sulfated carbohydrates remains to be determined.
Based upon the above observations and our present studies, a plausible mechanism for localizing AChE on the synaptic basal lamina would be that the newly synthesized A12 AChE molecules, preferentially expressed in innervated regions of the fibers, would be secreted into the space between the sarcolemma and the overlying synaptic basal lamina. The space would afford limited diffusion of these large molecules until they could interact electrostatically with the appropriate heparan sulfate proteoglycans. This interaction would be transient (Rossi and Rotundo, 1996) until additional events resulted in a more permanent form of attachment, such as formation of covalent bonds with neighboring molecules or additional intrachain disulfide bonds within the collagen-like tail subunits (Krejci et al., 1991). The A12 AChE would then remain attached to the synaptic basal lamina until it was detached via some as yet unknown, but possibly enzymatic, mechanism. To date, the only available quantitative data on turnover of AChE at the neuromuscular junction indicates that it has a relatively long half life of ∼20 d and is removed by a process that exhibits first order decay kinetics (Kasprzak and Salpeter, 1985). A specific attachment site on the synaptic basal lamina would therefore be the molecular equivalent of a space in a “parking lot,” whereby the newly synthesized collagen-tailed AChE molecules could be inserted and removed in the region between the nerve terminals and the acetylcholine receptors, as necessary. Furthermore, these studies also emphasize the fact that, despite differences in the underlying cellular and molecular mechanisms regulating AChE biogenesis between amphibians and aves, similar mechanisms determine the ultimate numbers and patterns of distribution of AChE molecules at the neuromuscular synapse.
Acknowledgments
We would like to thank Rachel Cohen for excellent technical assistance, Drs. Kenneth J. Muller and Israel Silman for their critical reviews of the manuscript, and Dr. Palmer Taylor for the anti-AChE antibody R80.
Abbreviations used in this paper
- AChE
acetylcholinesterase
- AChR
acetylcholine receptors
- Btx
α-bungarotoxin
- HSB
high salt buffer
- LSB
low salt buffer
Footnotes
This research was supported by research grants from the National Institutes of Health to R.L. Rotundo and from the Israel Academy of ScienceCharles H. Revson Foundation to L. Anglister. These experiments were carried out at the Hebrew University Hadassah Medical School while R.L. Rotundo was Hebrew University Visiting Professor of Medicine and Science.
Please address all correspondence to Richard L. Rotundo, Department of Cell Biology and Anatomy (R-124), University of Miami School of Medicine, 1600 N.W. 10th Avenue, Miami, Florida 33136. Tel: (305) 547-6940. Fax: (305) 545-7166. E-mail: rrotundo@molbio.med.miami.edu
References
- Anderson MJ, Fambrough DM. Aggregates of acetylcholine receptors are associated with plaques of a basal lamina heparan sulfate proteoglycan on the surface of skeletal muscle fibers. J Cell Biol. 1983;97:1396–1411. doi: 10.1083/jcb.97.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglister L. Acetylcholinesterase from the nerve terminal accumulates on the synaptic basal lamina of the myofiber. J Cell Biol. 1991;115:755–764. doi: 10.1083/jcb.115.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglister L, McMahan UJ. Basal lamina directs acetylcholinesterase accumulation at synaptic sites in regenerating muscle. J Cell Biol. 1985;101:735–743. doi: 10.1083/jcb.101.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglister L, Haesaert B, McMahan UJ. Globular and asymmetric acetylcholinesterase in the synaptic basal lamina of skeletal muscle. J Cell Biol. 1994a;125:183–196. doi: 10.1083/jcb.125.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglister L, Stiles JR, Salpeter MM. Acetylcholinesterase density and turnover number at frog neuromuscular junctions, with modeling of their role in synaptic function. Neuron. 1994b;12:783–794. doi: 10.1016/0896-6273(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Bayne EK, Anderson MJ, Fambrough DM. Extracellular matrix organization in developing muscle: correlation with acetylcholine receptor aggregates. J Cell Biol. 1984;99:1486–1501. doi: 10.1083/jcb.99.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W, Sakmann B. Effects of proteolytic enzymes on function and structure of frog neuromuscular junctions. J Physiol (Lond) 1973;230:673–688. doi: 10.1113/jphysiol.1973.sp010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon S, Cartaud J, Massoulié J. The dependence of acetylcholinesterase aggregation at low ionic strength upon a polyanionic component. Eur J Biochem. 1978;85:1–14. doi: 10.1111/j.1432-1033.1978.tb12207.x. [DOI] [PubMed] [Google Scholar]
- Brandan E, Maldonado M, Garrido J, Inestrosa N. Anchorage of collagen-tailed acetylcholinesterase to the extracellular matrix is mediated by heparan sulfate proteoglycan. J Cell Biol. 1985;101:985–992. doi: 10.1083/jcb.101.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Porte, S., F.M. Vallette, J. Grassi, M. Vigny, and J. Koenig. Presynaptic or postsynaptic origin of acetylcholinesterase at the neuromuscular junction? An immunological study in heterologous nerve-muscle cultures. Dev Biol. 1986;116:69–77. doi: 10.1016/0012-1606(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Deprez PN, Inestrosa NC. Two heparin-binding domains are present on the collagenic tail of asymmetric acetylcholinesterase. J Biol Chem. 1995;27:11043–11046. doi: 10.1074/jbc.270.19.11043. [DOI] [PubMed] [Google Scholar]
- Hall ZW, Kelly RB. Enzymatic detachment of endplate acetylcholinesterase from muscle. Nature (New Biol) 1971;232:62–63. doi: 10.1038/newbio232062a0. [DOI] [PubMed] [Google Scholar]
- Hall, Z.W., and J.R. Sanes. 1993. Synaptic structure and development: the neuromuscular junction. Cell. 72/Neuron. 10 (Suppl.):99–121. [DOI] [PubMed]
- Jasmin BJ, Lee RK, Rotundo RL. Compartmentalization of acetylcholinesterase mRNA and enzyme at the vertebrate neuromuscular junction. Neuron. 1993;11:467–477. doi: 10.1016/0896-6273(93)90151-g. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. The localization of cholinesterase activity in rat cardiac muscle by electron microscopy. J Cell Biol. 1964;23:217–232. doi: 10.1083/jcb.23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzak H, Salpeter MM. Recovery of acetylcholinesterase at intact neuromuscular junctions after in vivo inactivation with diisopropylfluorophosphate. J Neurosci. 1985;5:951–955. doi: 10.1523/JNEUROSCI.05-04-00951.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A, Bessereau JL, Salmon AM, Triller A, Babinet C, Changeux J-P. An acetylcholine receptor α-subunit promoter confering preferential synaptic expression in muscle of transgenic mice. EMBO (Eur Mol Biol Organ) J. 1991;10:625–632. doi: 10.1002/j.1460-2075.1991.tb07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci E, Coussen F, Duval N, Chatel J-M, Legay C, Puype M, Vandekerckhove J, Cartaud J, Bon S, Massoulié J. Primary structure of a collagenic tail peptide of Torpedo acetylcholinesterase: co-expression with catalytic subunit induces the production of collagen-tailed forms in transfected cells. EMBO (Eur Mol Biol Organ) J. 1991;5:1285–1293. doi: 10.1002/j.1460-2075.1991.tb08070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legay C, Huchet M, Massoulié J, Changeux J-P. Developmental regulation of acetylcholinesterase transcripts in the mouse diaphragm: alternative splicing and focalization. Eur J Neurosci. 1995;7:1803–1809. doi: 10.1111/j.1460-9568.1995.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Lømo T, Slater CR. Control of junctional acetylcholinesterase by neural and muscular influences in the rat. J Physiol (Lond) 1980;303:191–202. doi: 10.1113/jphysiol.1980.sp013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwebuga-Mukasa JS, Lappi S, Taylor P. Molecular forms of acetylcholinesterase from Torpedo californica: their relationship to synaptic membranes. Biochemistry. 1976;15:1425–1434. doi: 10.1021/bi00652a012. [DOI] [PubMed] [Google Scholar]
- Massoulié J, Pezzementi L, Bon S, Krejci E, Vallette FM. Molecular and cellular biology of cholinesterases. Prog Neurobiol. 1993;41:31–91. doi: 10.1016/0301-0082(93)90040-y. [DOI] [PubMed] [Google Scholar]
- McMahan UJ, Sanes JR, Marshall LM. Cholinesterase is associated with the basal lamina at the neuromuscular junction. Nature (Lond) 1978;271:172–174. doi: 10.1038/271172a0. [DOI] [PubMed] [Google Scholar]
- Merlie JP, Sanes JR. Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature (Lond) 1985;317:66–68. doi: 10.1038/317066a0. [DOI] [PubMed] [Google Scholar]
- Michel RN, Vu CQ, Tetzlaff W, Jasmin BJ. Neural regulation of acetylcholinesterase mRNAs at mammalian neuromuscular synapses. J Cell Biol. 1995;127:1061–1069. doi: 10.1083/jcb.127.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SG, Rotundo RL. Cell surface acetylcholinesterase molecules on multinucleated myotubes are clustered over the nucleus of origin. J Cell Biol. 1992;119:1657–1667. doi: 10.1083/jcb.119.6.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SG, Rotundo RL. Localization of “non-extractable” acetylcholinesterase to the vertebrate neuromuscular junction. J Biol Chem. 1993;268:19152–19159. [PubMed] [Google Scholar]
- Rossi SG, Rotundo RL. Transient interactions between collagentailed acetylcholinesterase and sulfated proteoglycans prior to immobilization on the extracellular matrix. J Biol Chem. 1996;271:1979–1987. doi: 10.1074/jbc.271.4.1979. [DOI] [PubMed] [Google Scholar]
- Rotundo RL. Asymmetric acetylcholinesterase is assembled in the Golgi apparatus. Proc Natl Acad Sci USA. 1984a;81:479–483. doi: 10.1073/pnas.81.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo RL. Purification and properties of the hydrophobic, membrane bound form of acetylcholinesterase from chicken brain: evidence for two distinct polypeptide chains. J Biol Chem. 1984b;259:13186–13194. [PubMed] [Google Scholar]
- Sanes JR, Schachner M, Covault J. Expression of several adhesion macromolecules in embryonic, adult and denervated adult skeletal muscle. J Cell Biol. 1986;102:420–431. doi: 10.1083/jcb.102.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, Hoppe P, Burden SJ. Spatial restriction of AChR gene expression to subsynaptic nuclei. Dev. 1992;114:545–553. doi: 10.1242/dev.114.3.545. [DOI] [PubMed] [Google Scholar]
- Swenarchuk LE, Champaneria S, Anderson MJ. Induction of a specialized muscle basal lamina at chimeric synapses in culture. Dev. 1990;110:51–61. doi: 10.1242/dev.110.1.51. [DOI] [PubMed] [Google Scholar]
- Vigny M, Bon S, Massoulié J, Leterrier F. Active-site catalytic efficiency of acetylcholinesterase molecular forms in Electrophorus, Torpedo, rat, and chicken. Eur J Biochem. 1978;85:317–323. doi: 10.1111/j.1432-1033.1978.tb12241.x. [DOI] [PubMed] [Google Scholar]
- Vigny M, Martin GR, Grotendorst GR. Interactions of asymmetric forms of acetylcholinesterase with basement membrane components. J Biol Chem. 1983;258:8794–8798. [PubMed] [Google Scholar]
- Weinberg CB, Hall ZW. Junctional form of acetylcholinesterase restored at nerve-free endplates. Dev Biol. 1979;68:631–635. doi: 10.1016/0012-1606(79)90233-1. [DOI] [PubMed] [Google Scholar]