Abstract
Polarized cells such as epithelial cells and neurons have distinct endosomal compartments associated with different plasma membrane domains. The endosomes of the neuronal cell body and the basolateral cytoplasm of epithelial cells are thought to perform cellular “housekeeping” functions such as the uptake of nutrients and metabolites, while the endosomes in the apical cytoplasm or axons are thought to be specialized for the sorting and transcytosis of cell type–specific ligands and receptors. However, it is not known if nonpolarized cells such as fibroblasts contain a specialized endosomal compartment analogous to the specialized endosomes found in neurons and epithelia. We have expressed a protein that is normally found in the apical early endosomes of developing intestinal epithelial cells in normal rat kidney fibroblasts. This apical endosomal marker, called endotubin, is targeted to early endosomes in transfected fibroblasts, and is present in peripheral as well as perinuclear endosomes. The peripheral endosomes that contain endotubin appear to exclude transferrin, fluid phase markers, and the mannose-6-phosphate receptor, although in the perinuclear region colocalization of endotubin and these markers is present. In addition, endotubin positive structures do not tubulate in response to brefeldin A and instead redistribute to a diffuse perinuclear location. Since this endosomal compartment has many of the characteristics of an apical or axonal endosomal compartment, our results indicate that nonpolarized cells also contain a specialized early endosomal compartment.
Endocytosis is the process by which surface bound ligands and fluid phase macromolecules are internalized by eukaryotic cells. After endocytosis, internalized macromolecules must be sorted and targeted to their next cellular destination (Trowbridge et al., 1993). Sorting, recycling, and targeting are mediated by a series of morphologically and functionally heterogeneous membrane-bound compartments known collectively as endosomes (Geuze et al., 1984; Schmid et al., 1988; Dunn and Maxfield, 1992). Much of our understanding of endosomal dynamics has resulted from studies of nonpolarized cells. In these cells, sorting endosomes are located in the periphery of the cell and contain internalized ligands and receptors (Yamashiro et al., 1984; Dunn et al., 1989; Ghosh et al., 1994). Recycling endosomes are a pericentriolar network of tubules and vesicles that are distinct from the sorting endosomes and contain recycling receptors and lipids (Dunn et al., 1989; Mayor et al., 1993; Tooze and Hollinshead, 1991). Late endosomes contain lysosome-directed receptors and ligands, and are thought to mature from the sorting endosomes (Stoorvogel et al., 1991; Dunn and Maxfield, 1992).
The distribution of different types of endosomal compartments in polarized cells remains controversial. Distinct early endosomal populations in the apical and basolateral cytoplasm of epithelial cells have been identified in tracer studies (Bomsel et al., 1989; Parton et al., 1989; Fujita et al., 1990). Basolateral endosomes are involved in the uptake and recycling of receptors and ligands involved in cell maintenance, and are sometimes referred to as “housekeeping endosomes” (Kelly, 1993). In contrast, apical endosomes were thought to be involved in epithelial cell type–specific processes such as transcytosis and therefore specialized for epithelial cells (Simonoski et al., 1986; Barroso and Sztul, 1994). However, recent work in MadinDarby Canine Kidney (MDCK) cells and the intestinal cell line, Caco-2, has shown that some apical endosomes contain recycling transferrin (Apodaca et al., 1994; Hughson and Hopkins, 1990; Knight et al., 1995) and has led to the suggestion that no “specialized” apical endosomal compartment exists (Apodaca et al., 1994). However, the fact that some endosomes of the apical cytoplasm fail to label with internalized transferrin leaves open the possibility that portions of the apical endosomal compartment are unique in composition and function (Hughson and Hopkins, 1990; Knight et al., 1995). Also, we have previously identified a glycoprotein, called endotubin, that is highly enriched in the apical early endosomal tubules of epithelial cells of the neonatal rat ileum (Wilson et al., 1987) and serves as a marker for this specialized endosomal compartment. Neurons represent another type of polarized cell that contains different endosomal populations (Rodriquez-Boulan and Powell, 1992; Kelly, 1993). Endosomes of the cell body and dendrites perform housekeeping functions, whereas endosomes located in the axons are specialized for recycling of synaptic vesicle proteins (Parton et al., 1992; Cameron et al., 1991; Mundigli et al., 1993; Bonzelius et al., 1994). These endosomal populations contain different synaptic vesicle proteins and have been shown to have differing sensitivity to the fungal metabolite brefeldin A (BFA)1 (Mundigli et al., 1993). Therefore, it seems clear that polarized cells contain endosomal compartments that are functionally and biochemically distinct.
The question remains open, however, whether nonpolarized cells such as fibroblasts contain a specialized endosomal compartment comparable to the apical endosomes of polarized cells (Rodriguez-Boulan and Powell, 1992; Matter and Mellman, 1994). Progress on this question has been hampered by a lack of morphological or biochemical markers for these membranes. Because endotubin is found in a specialized endosomal compartment in epithelial cells, we wished to determine if it would be targeted to an endosomal compartment when expressed in nonpolarized cells. Expression of the cDNA encoding endotubin in normal rat kidney (NRK) fibroblasts results in targeting of endotubin to an early endosomal compartment. This result indicates that this protein has the molecular signals to be targeted to endosomes, even in nonpolarized cells. This endosomal compartment has many of the characteristics of an apical or axonal endosomal compartment, suggesting that nonpolarized cells contain a specialized early endosomal compartment analogous to the apical or axonal endosomes seen in polarized cells.
Materials and Methods
Cell Culture and Transfection
NRK cells were obtained from the Amer. Type Tissue Culture association (Rockville, MD) and were maintained in DMEM (GIBCO-BRL, Gaithersburg, MD) supplemented with 5% FBS, glutamine, and 100 U/ml penicillin and 100 μg/ml streptomycin under 5% CO2 at 37°C. The full-length cDNA encoding endotubin (Speelman et al., 1995) was subcloned into the PCB6 expression vector which contains the cytomegalovirus immediate early promoter and the G418 resistance gene. Cells were plated in 10-cm dishes and transfected by CaPO4 precipitation under 3% CO2 (Chen and Okayama, 1987) using 10 μg of plasmid DNA per plate. After transfection, cells were selected for 2 wk in the presence of 500 μg/ml G418 (GIBCO-BRL). Experiments were done using pooled clones of resistant colonies. All cells were maintained in medium containing 500 μg/ml G418.
Immunofluorescent Labeling
For immunofluorescent labeling of neonatal rat intestine, ileum from 12-dold neonatal rats was fixed for 1.5 h in paraformaldehyde-lysine-periodate fixative (Mclean and Nakane, 1974), dehydrated, and embedded in paraffin. Sections on slides were deparaffinized, rehydrated, and blocked for 30 min at room temperature with PBS containing 10% goat serum. After blocking, sections were incubated with primary antibodies for 2 h at room temperature. Anti-endotubin monoclonal antibody (Wilson et al., 1987) was used as a cell culture supernatant diluted 1:1 with blocking buffer. Polyclonal anti-lgp120 antibody was generously provided by Dr. William Dunn (University of Florida, Gainesville, FL; Dunn, 1990) and was diluted 1:100 in blocking buffer. Secondary antibodies conjugated with fluoroscein isothiocyanate or Texas red (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) were diluted 1:200 in blocking buffer, and incubations were carried out for 30 min at room temperature. After washing, slides were mounted with Moviol (Aldrich Chem. Co., Milwaukee, WI).
For immunofluorescent labeling of transfected cells, cells were plated on coverslips and grown for 2 d. Before all experimental manipulations, cells were treated overnight with 0.01 M butyric acid and, just before the experiment, incubated for 1 h at 37°C in 10 μg/ml cycloheximide to deplete endotubin from the biosynthetic pathway. Unless otherwise noted, cells were fixed in paraformaldehyde-lysine-periodate for 20 min. After fixation, cells were incubated in 1 mg/ml NaBH4, blocked and permeabilized in PBS containing 10% goat serum, 0.05% saponin (blocking buffer), and incubated with specific antibodies as described above. Polyclonal antiTGN38/41 antibody was generously provided by Dr. Kathryn Howell (University of Colorado, Denver, CO, Luzio et al., 1990) and was diluted 1:1,000. For immunofluorescent labeling with anti-TGN 38/41, cells were fixed for 5 min in methanol and then incubated with antibodies as described above. Polyclonal antibody against the mannose-6-phosphate receptor was generously provided by Dr. William Brown (Cornell University, Ithaca, NY) and was diluted 1:1,000 in blocking buffer.
For cell surface labeling, cells were fixed and blocked as above, but saponin was omitted from blocking buffer and the first antibody incubation. After incubation with the monoclonal antibody and secondary antibody conjugated to Texas red, the cells were permeabilized with 0.05% saponin and incubated again with the monoclonal antibody in blocking buffer followed by secondary antibody conjugated to FITC.
Uptake Studies
For ricin uptake, transfected cells were incubated for 30 min at 4°C in 100 μg/ml ricin120-FITC (Sigma Chem. Co., St. Louis, MO) in serum-free medium. Cells were warmed to 37°C for 2–20 min, placed back on ice, rinsed with 0.1 M lactose to remove surface bound ligand, fixed on ice, and blocked as described above. For transferrin uptake, cells were incubated in serum-free medium for 1 h at 37°C, and then transferred to medium containing 1 mg/ml ovalbumin, 100 μg/ml iron saturated human transferrin (Polysciences Inc., Warrington, PA) for 1 h at 4°C, and then warmed for 2 or 60 min to 37°C. After the 2-min incubation, cells were washed at 4°C in buffer containing 25.5 mM citric acid, 24.5 mM sodium citrate, 280 mM sucrose, pH 4.6, to remove surface bound transferrin. Cells were fixed on ice and processed as described above. Anti–human transferrin antibody (Boehringer Mannheim Biochemicals, Indianapolis, IN) was diluted 1:200 in blocking buffer. For ovalbumin uptake experiments, ovalbumin conjugated to Texas red (Molecular Probes, Eugene, OR) was reconstituted in media at 600 μg/ml, centrifuged for 5 min at 14,000 g and placed on the cells for 1 h at 37°C. Cells were rinsed in PBS and fixed in methanol for 5 min followed by processing for immunofluorescence.
Brefeldin A Treatment.
Cells were incubated in transferrin for 60 min at 37°C as described above. Brefeldin A (BFA, Epicentre Technologies, Madison, WI) was added to 1 or 5 μg/ml and the cells were incubated for 10 min at 37°C followed by fixation and processing for immunofluorescence with anti-transferrin and anti-endotubin antibodies.
Potassium Depletion.
Potassium depletion was carried out as described (Larkin et al., 1983). Briefly, cells were washed three times in buffer containing 140 mM NaCl, 20 mM Hepes, pH 7.4, 1 mM CaCl2, 1 mM MgCl, 1 mg/ml D-glucose (buffer A) at 37°C, followed by 5 min at 37°C in buffer A diluted 1:1, followed by three more rinses in buffer A at 37°C and an additional 15-min incubation at 37°C. Cells were then incubated with 100 μg/ ml ricin-FITC in buffer A for 30 min at 4°C and then warmed to 37°C for 2–20 min followed by washing with 0.1 M lactose at 4°C, fixation, and processing for immunofluorescence.
Confocal Microscopy
Fluorescent images were obtained using a Leica TCS 4D laser scanning confocal microscope (Arizona Research Laboratory, Division of Biotechnology, University of Arizona, Tucson, AZ) using a 100×, NA 1.3 oil immersion objective. Simultaneous two channel recording was performed with a pinhole size of 90 μm using excitation wavelengths of 488/588 nm, a 510/580 double dichroic mirror, and a 515–545 band pass FITC filter together with a 590-nm-long pass filter. All figures are derived from a single optical section which is estimated to be 0.35 μm in thickness. Images were processed and merged using Adobe Photoshop software and printed using a Codonics NP1600 dye sublimation printer.
To quantitate the proportions of peripheral endosomes containing endotubin immunoreactivity and the different internalized tracers or ligands, immunofluorescent images were merged and the peripheral cytoplasm more than 5 μm from the nucleus was divided into sections. Endosomes were scored for the presence of endotubin staining, tracer (ricin or transferrin) staining, or colocalization of fluorescent staining. A minimum of 400 endosomes were scored for each time point.
Western Blotting
Cells were treated overnight with 0.01 M butyric acid and then scraped into buffer containing 0.25 M sucrose, 10 mM Hepes, pH 7.4, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 0.25 M phenylmethylsulfonyl fluoride. The cells were homogenized with a Teflon pestle and breakage was monitored by phase contrast microscopy. The homogenate was spun at 500 g for 5 min and the resulting supernatant respun for 1 h at 100,000 g. The crude membrane pellet was solubilized by boiling in SDS-PAGE sample buffer, separated by SDS-PAGE, and electroblotted to nitrocellulose. Blots were blocked using 5% milk in Tris buffered saline containing 0.1% Tween 20 and then incubated with the monoclonal antibody followed by anti–mouse antibody conjugated to alkaline phosphatase (Sigma). Blots were developed using Western blue alkaline phosphatase substrate (Promega, Madison, WI).
Results
Endotubin Expression in NRK Cells
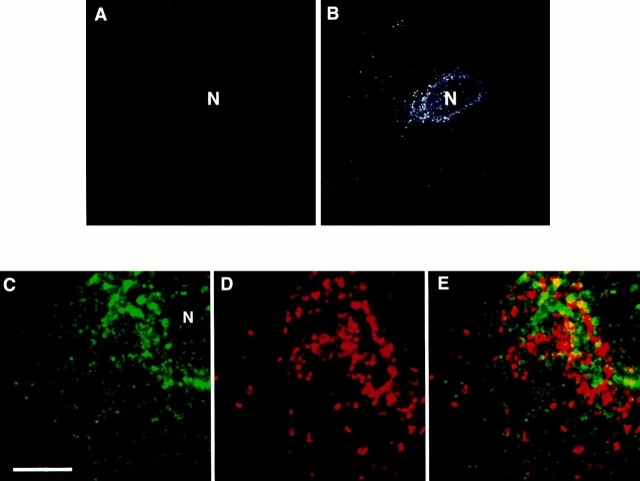
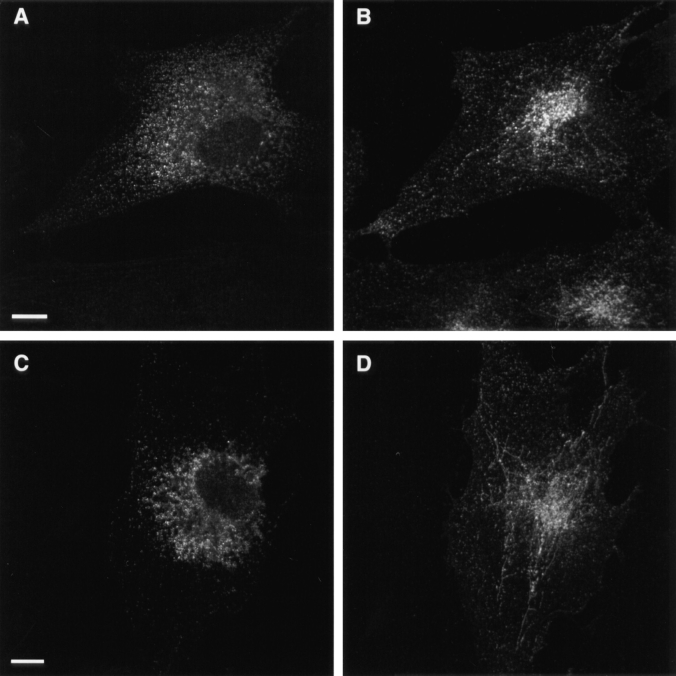
In the epithelial cells of the neonatal rat ileum, endotubin is present in an apical endocytic complex of tubules and vesicles and is excluded from the large supranuclear lysosomal vacuole (Fig. 1; Cornell and Padykula, 1969; Wilson et al., 1987). To determine the distribution of endotubin in nonpolarized fibroblasts after transfection with the endotubin cDNA, we analyzed both the cell surface and subcellular staining patterns. Many proteins that are markers of intracellular membranes are present in small amounts on the plasma membrane (Lippincott-Schwartz and Fambrough, 1987; Reaves et al., 1993), and, in neonatal rat ileum, some endotubin is detected on the apical plasma membrane (Wilson et al., 1987). To determine if endotubin was present on the plasma membrane of NRK cells, unpermeabilized cells were stained using either a monoclonal antibody (Fig. 2 A) or a polyclonal antibody (not shown) against the ectoplasmic domain of the protein. After binding of antibody to the cell surface, the cells were permeabilized and re-incubated with antibody to label intracellular populations of endotubin and to ensure that the cells stained were expressing endotubin at significant levels (Fig. 2 B). These experiments showed that endotubin was targeted almost exclusively to intracellular compartments, as fluorescence was barely detected on the cell surface. To determine if endotubin is targeted to lysosomes in NRK cells, we performed double label immunofluorescence with antibodies against both endotubin and the lysosomal membrane protein lgp120 (Lewis et al., 1985; Dunn, 1990). Although endotubin is normally excluded from the lysosomes of intestinal epithelial cells, if NRK cells lacked the correct endosomal compartment for endotubin targeting, it might be targeted to lysosomes for degradation. Endotubin staining was distributed throughout the cytoplasm in small punctate spots, with larger, brightly stained spots concentrated in the perinuclear region (Fig. 2 C). The lysosomal staining was present in large spots both in the perinuclear region and in the peripheral cytoplasm (Fig. 2 D). Merging of these images showed that most of the endotubin and lgp 120 staining was distinct, although there may be some colocalization in the perinuclear region (Fig. 2 E). These results indicate that endotubin is not being targeted to lysosomes by default, and is instead targeted to another intracellular compartment.
Figure 1.
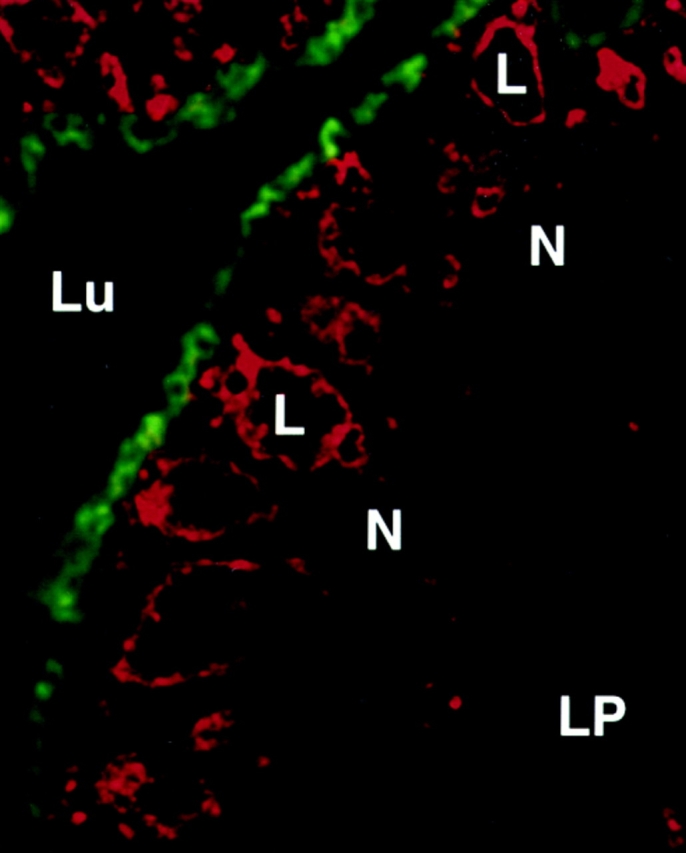
Distribution of endotubin and lgp120 staining in neonatal rat intestinal epithelial cells. Sections of neonatal rat ileum were stained with anti-endotubin and anti-lgp120 to identify the apical endosomal compartment and the lysosomal complex. Endotubin staining (green fluorescence) is concentrated in the apical pole of the cell, separated from the lysosomal complex (red fluorescence). Lu, intestinal lumen; L, giant lysosome; N, nucleus; LP, lamina propria.
Figure 2.
Distribution of endotubin staining in transfected NRK cells. There is little expression of endotubin on the cell surface, as endotubin staining is not detected on nonpermeabilized cells (A), even though intracellular expression is high (B). In C and D, and E, NRK cells transfected with endotubin cDNA were double labeled with antibodies against endotubin (C) and lgp120 (D). The merged image (E) shows the distinct staining patterns, although there may be some colocalization in the perinuclear region (arrows). N, nucleus. Bar equals 5 μm.
We next compared the size of the protein expressed in NRK cells with the protein found in neonatal rat intestine. In the neonatal rat intestine, endotubin is synthesized as a high molecular weight precursor of approximately 140 kD that is cleaved and glycosylated to a 55–60-kD form (Allen, K.A, K.E. Gokay, B.A. Speelman, and J.M. Wilson, manuscript submitted for publication). To determine if endotubin is processed in a similar fashion in NRK cells, we performed Western blot analysis on membranes isolated from transfected NRK cells. Western blotting with the monoclonal antibody showed that, in these cells, the expressed protein is also 55–60 kD, indicating that synthesis and processing in NRK cells is similar to that found in intestinal epithelial cells (Fig. 3).
Figure 3.
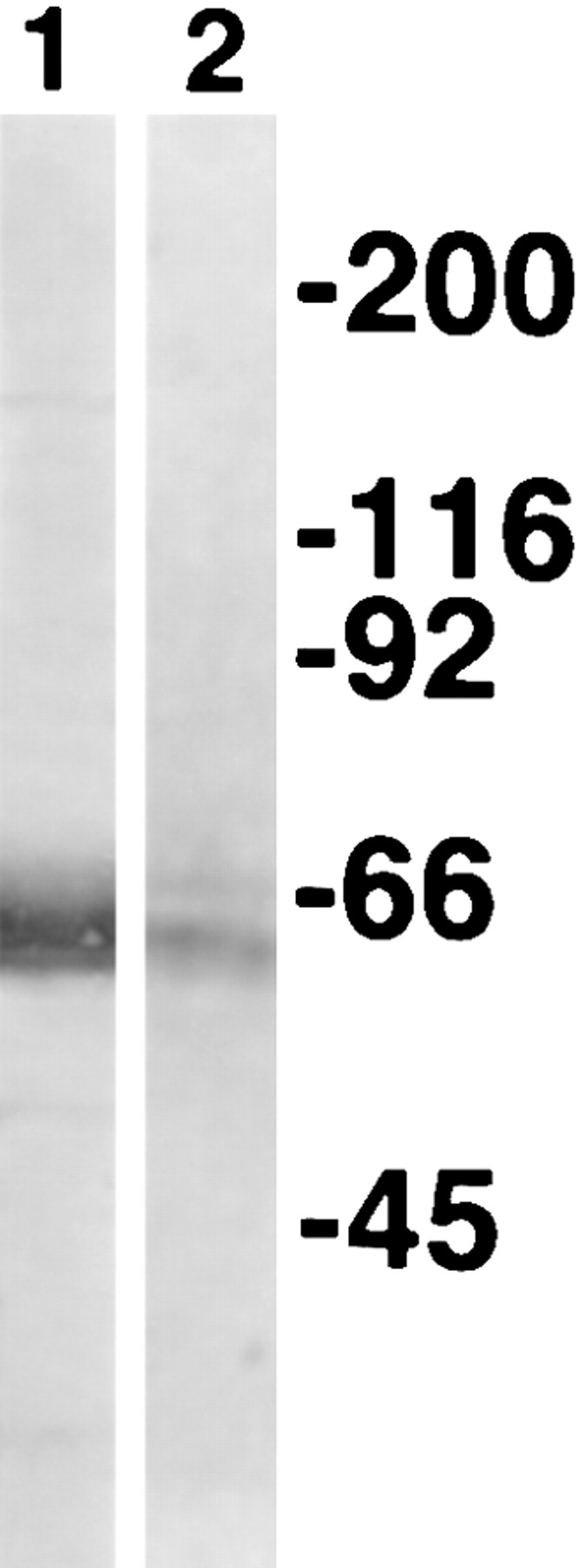
Western blot of endotubin in NRK cells and neonatal rat intestine. Western blots of membrane from rat intestinal epithelial cells (lane 1) and transfected cells (lane 2) were incubated with monoclonal antibody against endotubin. The molecular weight of endotubin is the same in NRK cells as in neonatal rat intestine. The second band seen in lane 2 is likely due to differences in glycosylation in NRK cells.
Endotubin Is Present in Early Endosomes of NRK Cells
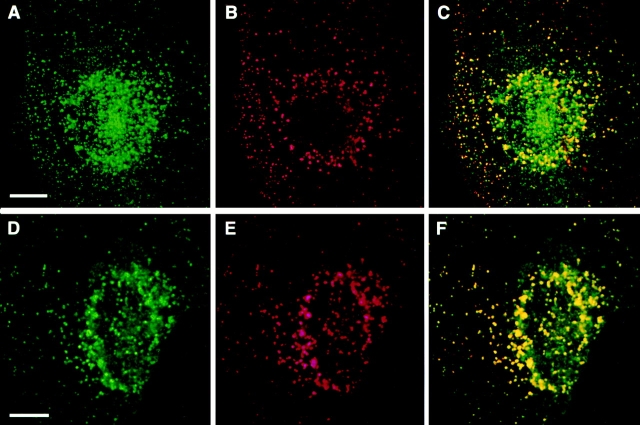
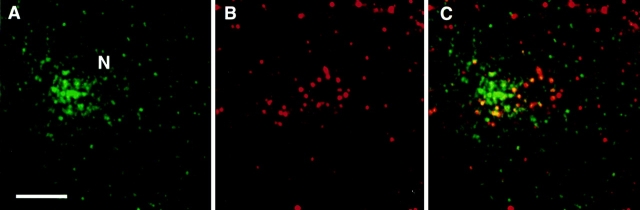
As mentioned above, endotubin is present in the apical endosomes of ileal epithelial cells, and short incubation with exogenous tracer labels these tubules and vesicles (Gonnella and Neutra, 1984; Wilson et al., 1987), indicating that these endosomes are early in the endocytic pathway. To determine if endotubin was being targeted to an early endosomal compartment in NRK cells, transfected cells were incubated with fluorescent ricin for 2 min at 37°C, processed for immunofluorescence using the monoclonal antibody against endotubin, and imaged using confocal microscopy. After 2 min of ricin uptake, many stained spots ricin-FITC and endotubin staining were seen to colocalize in the periphery of the cell (Fig. 4, A–C). There were also many spots that contained endotubin staining that did not contain ricin staining, and some ricin positive spots that did not stain for endotubin. The colocalization seen after 2 min of uptake was predominantly in the periphery of the cell, although some colocalization was evident in the perinuclear area. After 20 min of ricin uptake, there was extensive colocalization of ricin and endotubin fluorescence in endosomes in the periphery as well as in the perinuclear region of the cell (Fig. 4, D–F). When the endosomes in the periphery of the cell were counted to determine the proportions that contained endotubin staining alone, endotubin and ricin colocalization, or ricin staining alone, it was found that the proportion of endosomes containing both labels increased between 2 and 20 min of uptake (Table I). These results may indicate that this compartment continues to fill with tracer during this time and that exit from the compartment is not as rapid as entry into these endosomes.
Figure 4.
Endotubin is present in early endosomes. Cells were incubated with ricin-FITC for 30 min at 4°C and then warmed to 37°C for 2 min (A–C) or 20 min (D–F). After warming, cells were returned to 4°C and washed with 0.1 M lactose to remove surface bound ricin, fixed, and stained with antibody against endotubin. Endotubin staining (A and D) is present in the periphery and perinuclear region, and ricin (B) is present in the periphery. Colocalization in peripheral endosomes is evident by the presence of yellow spots in the merged image (C). After 20 min of ricin uptake, ricin is present in both the cell periphery and in the perinuclear region (E), and the merged image shows colocalization in both areas (F). Bar equals 5 μm.
Table I.
Proportions of Peripheral Endosomes Containing Endocytosed Tracer and/or Endotubin Immunostaining
| 2 min ricin | 20 min ricin uptake | |||
| uptake (575 endosomes) | (535 endosomes) | |||
| Ricin alone | 2.4 | 3.7 | ||
| Endotubin alone | 66.9 | 48.2 | ||
| Ricin plus endotubin | 30.6 | 48.0 | ||
| 2 min transferrin uptake | 1 h transferrin uptake | |||
| (669 endosomes) | (578 endosomes) | |||
| Transferrin alone | 55.6 | 41.7 | ||
| Endotubin alone | 37.4 | 47.6 | ||
| Transferrin plus endotubin | 7.0 | 10.7 |
Ricin and Endotubin Colocalization Is Not Due to Nonclathrin Mediated Endocytosis
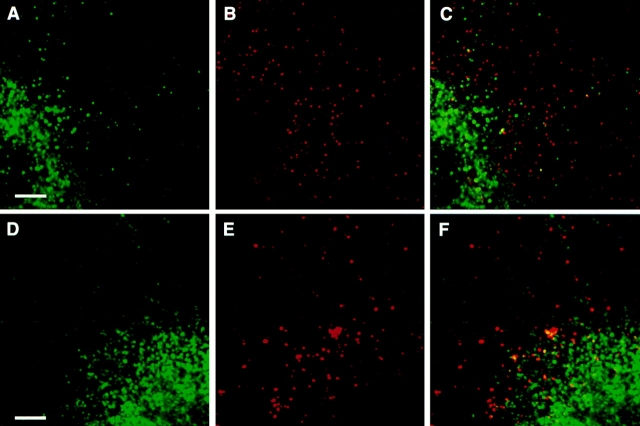
Ricin can be taken up by both clathrin-mediated and nonclathrin-mediated endocytosis (Moya et al., 1985), and these pathways can be distinguished by depleting intracellular K+ before tracer internalization (Larkin et al., 1983; Moya et al., 1985; Hansen et al., 1993). K+ depletion has been shown to cause disassembly of clathrin lattices and inhibition of clathrin-mediated endocytosis (Larkin et al., 1983; Hansen et al., 1993). Therefore, to examine if the colocalization of endotubin and ricin could be explained by uptake via nonclathrin-mediated endocytosis, the ricin uptake experiments were repeated using K+ depleted cells. When the cells were K+ depleted and allowed to take up ricin for 2 min at 37°C followed by fixation and immunolabeling using anti-endotubin antibodies, no colocalization of the ricin and endotubin staining was observed (Fig. 5, A–C). Even after 20 min of ricin uptake, no colocalization of ricin and endotubin was seen (Fig. 5, D–F), suggesting that endotubin was not targeted to an endosomal compartment associated with the nonclathrin-mediated pathway. Interestingly, there was a redistribution of much of the endotubin staining to a diffuse perinuclear location, similar to that seen after brefeldin A treatment (see below).
Figure 5.
K+ depletion inhibits ricin and endotubin colocalization. Cells were depleted of intracellular potassium and incubated with ricin as described in Fig. 4. After 2 min of uptake (A–C), endotubin staining (A) remains punctate in the periphery, but some diffuse perinuclear staining is evident. Ricin fluorescence (B) is distributed in a punctate pattern in the cell periphery, but there is no colocalization of endotubin and ricin (C). After 20 min of ricin uptake (D–F), endotubin staining is predominantly perinuclear (D). Ricin fluorescence remains punctate in the cell periphery (E), and there is no colocalization with endotubin staining (F). Bar equals 5 μm.
Localization of Endotubin and Transferrin
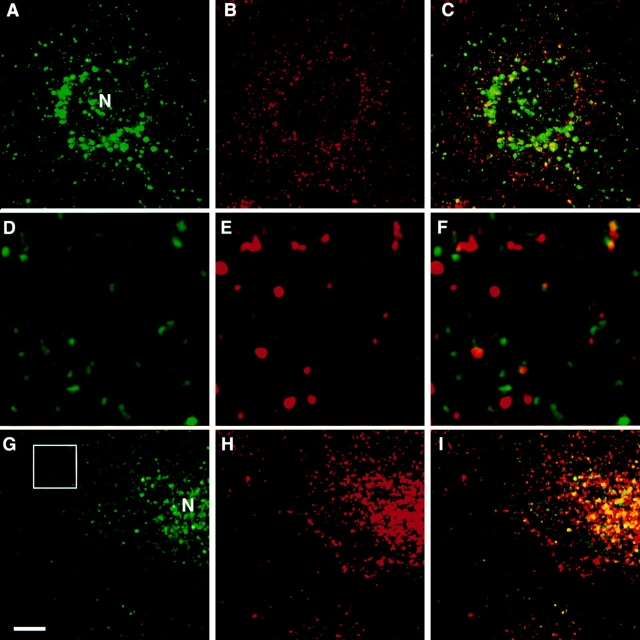
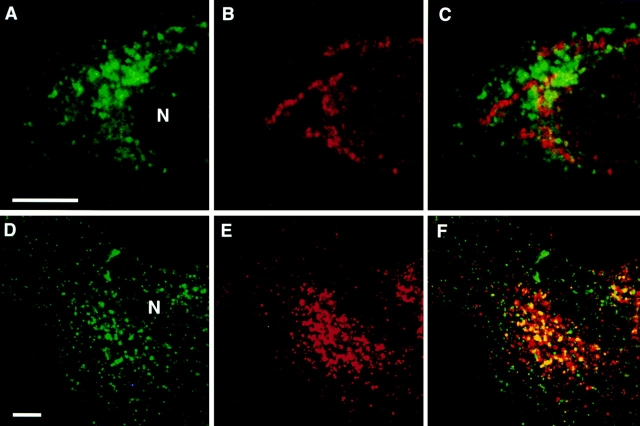
Ricin serves as a general marker of the endocytic complex and can label early and late endosomes, as well as lysosomes. We next wanted to determine if transferrin, a marker of basolateral and recycling endosomes, colocalized with endotubin. Transferrin has been used in many studies of endosomal dynamics and is considered a classical marker of the early endosomal compartment (Hopkins, 1983; Schmid et al., 1988). In epithelial cells, transferrin is internalized at the basolateral plasma membrane to basolateral endosomes before recycling to the basolateral plasma membrane. However, an endosomal compartment in the apical cytoplasm has also been implicated in the recycling of transferrin to the basolateral surface of epithelial cells (Hughson and Hopkins, 1990; Apodaca et al., 1994; Knight et al., 1995). To further characterize the endosomal population that contains endotubin, cells were incubated with iron-saturated transferrin and processed for double label immunofluorescence with anti-endotubin and antitransferrin antibodies. After 2 min of uptake, endotubin and transferrin staining was present in small, punctate dots throughout the cells. However, there was little colocalization of endotubin and transferrin immunostaining (Fig. 6, A–C). Quantitation of endosomes containing either or both markers indicated that there was a much lower proportion of endosomes with transferrin and endotubin colocalization compared to the proportion of endosomes with endotubin and ricin colocalization after 2 min of ricin uptake (Table I). In these experiments, 2 min of uptake will label early sorting endosomes; however, it is possible that this short pulse may not label all sorting endosomes through which transferrin would normally traffic. To saturate the transferrin endocytic pathway, cells were incubated for 1 h in the continuous presence of transferrin. Under these conditions, both the sorting and recycling endosomes will be labeled (Hopkins, 1983; Dunn et al., 1989). After 1 h of continuous transferrin uptake, colocalization of transferrin and endotubin in the peripheral endosomes remained low (Fig. 6, D–F and Table I); however, there was colocalization of these markers in the perinuclear region (Fig. 6, G–I).
Figure 6.
Comparison of the distribution of endotubin and transferrin containing endosomes. Cells were incubated with transferrin for 2 min (A–C) or 1 h (D–I), fixed, and double labeled with anti-endotubin (A, D, and G) and anti-transferrin (B, E, and H) antibodies. After 2 min of uptake, endotubin (A) and transferrin (B) staining is present in distinct compartments, and the merged image shows little colocalization (C). After 1 h of continuous uptake, transferrin is present in both the peripheral endosomes and the perinuclear region (G). The area within the white square of G is enlarged in D–F. Endotubin staining (D) remains distinct from transferrin staining (E), with little colocalization in the merged image (F). There is colocalization of endotubin and transferrin staining in the perinuclear region (I). N, nucleus. Bar equals 5 μm.
Uptake of Fluid Phase Markers
Both ricin and transferrin bind to the cell surface and are taken up by adsorptive endocytosis. Fluid phase markers will also label endocytic structures; however, tubular apical endosomes in neonatal, intestine and MDCK cells remain largely unlabeled after uptake of these markers (Gonnella and Neutra, 1984; Barroso and Sztul, 1994; Apodaca et al., 1994). To determine if the endotubin containing compartment could be labeled by molecules taken up by fluid phase endocytosis, we incubated transfected cells with ovalbumin conjugated to Texas red for 1 h at 37°C to label the endocytic pathway followed by anti- endotubin staining. After this incubation, the labels were in a distinct punctate pattern in the periphery of the cell (Fig. 7, A–C); although there was some colocalization of endotubin staining and ovalbumin in the perinuclear region. These results indicate that peripheral endosomes have similar properties to the tubular apical endosomes of epithelial cells whereas the perinuclear endosomes are more like a late endosomal compartment.
Figure 7.
Uptake of fluid phase markers. Cells were incubated with ovalbumin-Texas red for 1 h and 37°C (B) followed by fixation and staining for endotubin (A). Staining in the cell periphery remains distinct, however, there is colocalization in larger endosomes in the perinuclear region (yellow, C). Bar equals 5 μm. N, nucleus.
Endotubin Containing Endosomes Do Not Tubulate in Response to BFA
Endosomes in NRK cells have been shown to be sensitive to the fungal metabolite BFA, forming long membrane tubules after incubation with this drug (Lippincott-Schwartz et al., 1991; Wood et al., 1991). To compare the effect of BFA on the endotubin containing endosomal compartment with the transferrin containing endosomal compartment, cells were incubated with transferrin for 1 h at 37°C and then exposed to 1 or 5 μg/ml BFA for 10 min at 37°C followed by fixation and double label immunofluorescence. Under these conditions, the differences between the transferrin and endotubin containing endosomes were striking (Fig. 8). At 1 μg/ml BFA, the transferrin containing endosomes had started to tubulate. However, the endotubin containing endosomes do not tubulate, but the strong perinuclear staining has disappeared and staining was present in a diffuse punctate pattern throughout the cell (Fig. 8, A–B). In the presence of 5 μg/ml BFA, the transferrin containing endosomes were even more extensively tubulated (Fig. 8 D). The endotubin staining was also redistributed, but to a predominantly perinuclear localization (Fig. 8 C). Under these conditions, there was no colocalization of the transferrin and endotubin immunostaining.
Figure 8.
Brefeldin A causes tubulation of transferrin containing endosomes but not endotubin containing endosomes. Cells were incubated with transferrin for 1 h at 37°C and then for 10 min at 37°C with 1 μg/ml (A–B) or 5 μg/ml BFA (C–D). In 1 μg/ml BFA, endotubin containing endosomes form a diffuse punctate pattern throughout the cell (A) whereas the transferrin containing endosomes have begun to tubulate (B). In 5 μg/ml BFA, the endotubin staining is concentrated in the perinuclear region (C), but the transferrin containing endosomes are more extensively tubulated (D). Bar equals 5 μm.
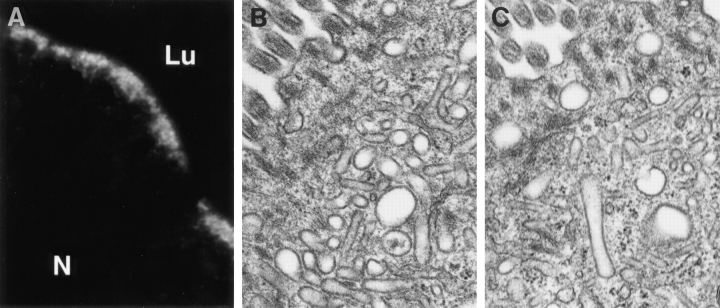
Because the endotubin containing compartment of NRK cells did not tubulate in response to BFA, we next wished to determine if the apical endosomes of neonatal rat intestine were affected by BFA. Incubation of explants of ileum in 10 μg/ml BFA for 15 min did not change the distribution of endotubin staining (Fig. 9 A). However, because of the intensity of the endotubin staining, it was possible that we would not detect any tubulation if it were present. To rule this out, we determined if tubulation of these endosomes could be detected at the ultrastructural level. Intestinal explants were incubated in BFA and processed for electron microscopy. The apical endosomes from neonatal intestine normally have a tubular-vesicular morphology (Wilson et al., 1987; Fig. 9 B), and this morphology was unaltered by BFA treatment (Fig. 9 C), suggesting that, at least in these cells, apical endosomes are not affected by BFA.
Figure 9.
Brefeldin A does not cause tubulation of apical endosomes of developing rat intestine. Explants of neonatal rat intestine were incubated for 15 min at 37°C in 10 μg/ml BFA, fixed, and processed for immunofluorescence with anti-endotubin antibodies (A) or electron microscopy (C). There was no redistribution of endotubin staining after BFA treatment (compare with Fig. 1). Electron microscopy of control incubated (B) and BFA-treated (C) intestine shows the tubular vesicular morphology of these endosomes was unaffected by BFA treatment. N, nucleus.
Comparison of Endotubin Distribution with Other Organelle Markers
To further characterize the compartment that contained endotubin, cells were double-labeled with antibodies against endotubin and the trans-Golgi network marker TGN38/41 (Luzio et al., 1990) or the mannose-6-phosphate receptor (M6PR). TGN38/41 staining was present in a cisternal pattern of the perinuclear area and was distinct from the perinuclear endotubin staining (Fig. 10, A–C). The M6PR is concentrated in the trans-Golgi network and late endosomes (Pfeffer, 1987; Braulke et al., 1987; Geuze et al., 1988); however, it is also present on the cell surface and throughout the endocytic pathway (Duncan and Kornfeld, 1988; Jin et al., 1989). When cells were double-labeled for endotubin and the M6PR, the staining of these markers was distinct in the periphery of the cell (Fig. 10, D–F). Some colocalization of these markers was evident in the perinuclear region, but distinct staining was also present. These results, together with the results of the TGN38/41 staining and previous studies of M6PR in NRK cells (Wood et al., 1991), suggest that endotubin and the M6PR that are colocalized represent a late endosomal compartment.
Figure 10.
Immunostaining with antibodies against the trans-Golgi network and mannose-6-phosphate receptor. Cells were double labeled to localize endotubin (A) and TGN38/41 (B). Merging of the images (C) shows no colocalization of these markers. Staining for endotubin (D) and M6PR (E) results in some colocalization in the perinuclear region (C). However, staining in the periphery is distinct. Bar equals 5 μm. N, nucleus.
One possible explanation for the different characteristics of the peripheral endotubin containing compartment is that expression of this protein induced the formation of a new endosomal compartment in NRK cells. Although we cannot rule this out, the pattern and extent of uptake of exogenous tracers was indistinguishable between expressing and non-expressing cells, suggesting that no change in endosomal populations had occurred.
Discussion
In this study, we have expressed the apical endosomal protein endotubin (Wilson et al., 1987) in a fibroblast cell line to determine if this protein could be targeted to an endosomal compartment in nonpolarized cells. We found that endotubin is targeted both to an early endosomal compartment in the periphery of NRK fibroblasts and a perinuclear endosomal compartment. The peripheral endosomes are distinct from transferrin and M6PR containing peripheral endosomes, although the perinuclear endosomes may contain both markers. However, unlike transferrin containing endosomes, the endotubin containing endosomes do not tubulate in response to BFA and instead redistribute to a diffuse perinuclear location. Also, like the tubular apical endosomes of the neonatal rat ileum and MDCK cells (Gonnella and Neutra, 1984; Barroso and Sztul, 1994; Apodaca et al., 1994), the endotubin containing peripheral endosomes failed to label with fluid phase markers. Table II lists the characteristics of early endosomes in polarized and nonpolarized cells. As illustrated in this table, endotubin containing peripheral endosomes demonstrate many characteristics of an apical or axonal endosomal compartment. In contrast, the perinuclear endosomes that contain endotubin, M6PR, transferrin, and ovalbumin may represent a common recycling compartment. Alternatively, endotubin may be present in both a transferrin containing recycling compartment and a late endosomal compartment labeled by fluid phase markers and the M6PR.
Together, our results are consistent with the idea that there are multiple populations of early endosomes in nonpolarized cells and that endosomes analogous to the specialized apical or axonal endosomes exist in nonpolarized cells (Rodriquez-Boulan and Powell, 1992; Kelly, 1993; Matter and Mellman, 1994). Although we cannot rule out that expression of endotubin has induced a novel compartment in these cells, these results nevertheless indicate that nonpolarized cells contain the sorting machinery necessary for targeting endotubin to a specialized compartment. In addition, these results are also consistent with recent studies showing that post-Golgi signals and machinery for sorting and targeting of apical and basolateral proteins in nonpolarized cells are similar to those in polarized cells (Musch et al., 1996; Yoshimori et al., 1996), suggesting that membrane trafficking pathways in polarized and nonpolarized cells have many elements in common.
In polarized cells, specialized endosomes have been suggested to have a variety of functions. Specialized endosomes of epithelial cells may be involved in the transcytosis of polymeric IgA, IgG, or growth factors (Abrahamson and Rodewald, 1980; Simonoski et al., 1987; Barroso and Sztul, 1994), and the insertion of water channels into the apical plasma membrane in response to stimulation by vasopressin (Brown and Sabolic, 1993). In addition, specialized endosomes in neurons may be important in the sorting of synaptic vesicle proteins after a cycle of exo- endocytosis at the synapse (Heuser, 1989; Linstedt and Kelly, 1991; Sulzer and Holtzman, 1989). In nonpolarized cells, a novel endosomal compartment is involved in the association of MHCII with peptide antigen for presentation by B lymphocytes (Amigorena et al., 1994; West et al., 1994) and may mediate the cycling of the glucose transporter GLUT4 to the adipose cell surface in response to insulin (Slot et al., 1991 a,b; James and Piper, 1994). In addition, different endosomal populations have been identified in the macropinocytic and clathrin-mediated pathways in A431 cells (Hewlett et al., 1994). It is not yet known what role specialized endosomes might play in fibroblasts. However, polarized insertion of recycling receptors into the leading lamellae of fibroblasts has been reported (Hopkins et al., 1994), and disruption of endocytosis in fibroblasts inhibits the development of a polarized morphology (Altankov and Grinnell, 1993).
The biochemical composition of specialized endosomes is still largely unresolved. In epithelial cells, the early endosomes in the apical and basolateral portions of the cell share small GTP-binding proteins (Bucci et al., 1994), but are not able to fuse together in in vitro fusion assays (Bomsel et al., 1990), evidence of their distinct compositions. In neurons, endosomes located in the dendrites and cell body contain the transferrin receptor whereas endosomes located in the axons exclude this receptor (Cameron et al., 1991; Mundigli et al., 1993), and immunostaining of synaptic vesicle proteins showed that different populations of vesicles contain synaptophysin, synaptotagmin, and SV2 (Mundigli et al., 1993). It is of interest to note that when the synaptic vesicle proteins synaptophysin and SV2 are expressed in CHO fibroblasts, synaptophysin colocalizes with transferrin immunoreactivity (Cameron et al., 1991; Feany et al., 1993) but SV2 did not colocalize with transferrin or other intracellular markers (Feany et al., 1993). It may be that in CHO fibroblasts, SV2 was targeted to a compartment similar to the endotubin containing endosomal compartment described in this study.
In MDCK cells, the fungal metabolite brefeldin A has been shown to cause tubulation of transferrin and IgA containing endosomes (Hunziker et al., 1991). BFA inhibited transepithelial transport of polymeric immunoglobulin A, and may have effects at both the level of sorting from the basolateral endosome or exit from the apical endosome (Hunziker et al., 1991; Barroso and Sztul, 1994). In neurons, BFA caused tubulation of transferrin and synaptotagmin containing endosomes, but endosomes of the axon failed to tubulate (Mundigli et al., 1993). Our results indicate that, in fibroblasts, BFA has differential effects on transferrin containing endosomes and endotubin containing endosomes, but both compartments undergo a redistribution after BFA treatment. These results suggest that both populations of endosomes contain coat proteins that are sensitive to BFA treatment and may explain the effect of this drug at both the basolateral and apical points in the transcytotic pathway in epithelial cells.
The redistribution of the endotubin staining to a diffuse, perinuclear localization after BFA treatment strongly resembled the redistribution of endotubin seen after K+ depletion. BFA is known to prevent the binding of coat proteins ARF, AP-1, and B-COP to Golgi membranes (Donaldson et al., 1992 a,b; Helms and Rothman, 1992; Traub et al., 1993), and members of the COP-1 and ARF families have been shown to bind to endosomal membranes in a BFA dependent fashion (Whitney et al., 1995). In addition, endosomes have been shown to contain clathrin-coated membranes (Stoorvogel et al., 1996). Since K+ depletion has been shown to affect the ability of clathrin coats to bind to membrane (Larkin et al., 1983; Hansen et al., 1993), it is possible that the endotubin containing endosomes contain a clathrin coat that is sensitive to BFA, but this sensitivity is not manifested by tubulation of the membranes.
Table 2.
Characteristics of Early Endosomes in Polarized and Nonpolarized Cells
| BFA sensitive | Tf/Trf-R | M6PR | Fluid phase markers | |||||
|---|---|---|---|---|---|---|---|---|
| Apical/axonal | − | + and − | − | − | ||||
| Basolateral/dendritic | + | + | + | + | ||||
| Fibroblasts/previous work | + | + | + | + | ||||
| Fibroblasts/endotubin | ||||||||
| containing endosomes | − | − | − | − | ||||
| (this study) |
For specific references, see text.
Acknowledgments
We would like to thank Dr. William Dunn for providing the anti-lysosomal glycoprotein antibody, Dr. Kathryn Howell for providing the antiTGN38/41 antibody, and Dr. William Brown for providing the anti-mannose-6-phosphate receptor antibody. We also thank Drs. Anne Pollack, Alison Adams, Carol Gregorio and Paul St. John for their comments on the manuscript.
This work was supported by National Institutes of Health grant DK43329 (to J.M. Wilson).
Abbreviations used in this paper
- BFA
brefeldin A
- lgp
lysosomal glycoprotein
- M6PR
mannose-6-phosphate receptor
- NRK
normal rat kidney
Footnotes
This manuscript is dedicated to the memory of Dr. Barry King.
References
- Abrahamson DR, Rodewald R. Evidence for the sorting of endocytic vesicle contents during the receptor-mediated transport of IgG across the newborn rat intestine. J Cell Biol. 1981;91:270–280. doi: 10.1083/jcb.91.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altankov G, Grinnell F. Depletion of intracellular potassium disrupts coated pits and reversibly inhibits cell polarization during fibroblast spreading. J Cell Biol. 1993;120:1449–1459. doi: 10.1083/jcb.120.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature (Lond) 1994;369:113–126. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso M, Sztul ES. Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G protein sensitive passage through the apical endosome. J Cell Biol. 1994;124:83–100. doi: 10.1083/jcb.124.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. doi: 10.1016/0092-8674(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Prydz K, Parton RG, Gruenberg J, Simons K. Endocytosis in filter-grown Madin-Darby Canine Kidney cells. J Cell Biol. 1989;109:3243–3258. doi: 10.1083/jcb.109.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzelius F, Herman GA, Cardone MH, Mostov KE, Kelly RB. The polymeric immunoglobulin receptor accumulates in specialized endosomes but not synaptic vesicles within the neurites of transfected neuroendocrine PC12 cells. J Cell Biol. 1994;127:1603–1616. doi: 10.1083/jcb.127.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braulke T, Gartung C, Hasilik A, von Figura K. Is movement of mannose-6-phosphate specific receptor triggered by binding of lysosomal enzymes? . J Cell Biol. 1987;104:1735–1742. doi: 10.1083/jcb.104.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D, Sabolic I. Endosomal pathways for water channel and proton pump recycling in kidney epithelial cells. J Cell Sci Suppl. 1993;17:49–59. doi: 10.1242/jcs.1993.supplement_17.8. [DOI] [PubMed] [Google Scholar]
- Bucci C, Wandinger-Ness A, Lutcke A, Chiariello M, Bruni CB, Zerial M. Rab5a is a common component of the apical and basolateral endocytic machinery in polarized epithelial cells. Proc Natl Acad Sci USA. 1994;91:5061–5065. doi: 10.1073/pnas.91.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron PL, Sudhof TC, Jahn R, De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Okayama H. High efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell R, Padykula HA. A cytological study of intestinal absorption in the suckling rat. Am J Anat. 1969;125:291–361. doi: 10.1002/aja.1001250304. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Cassel D, Kahn RA, Klausner RD. a. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein B-COP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalyzed exchange of guanine nucleotide onto ARF protein. Nature (Lond) 1992b;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Duncan JR, Kornfeld S. Intracellular movement of two mannose-6phosphate receptors: return to the Golgi apparatus. J Cell Biol. 1988;106:617–628. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, Maxfield FR. Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J Cell Biol. 1992;117:301–310. doi: 10.1083/jcb.117.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally directed ligands in an early sorting endosome. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WA. Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J Cell Biol. 1990;110:1935–1945. doi: 10.1083/jcb.110.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Yee AG, Delvy ML, Buckley KM. The synaptic vesicle proteins SV2, synaptotagmin and synaptophysin are sorted to separate cellular compartments in CHO fibroblasts. J Cell Biol. 1993;123:575–584. doi: 10.1083/jcb.123.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Reinhart F, Neutra MR. Convergence of apical and basolateral endocytic pathways at the late endosome in intestinal absorptive cells of the suckling rat ileum. J Cell Sci. 1990;97:385–394. doi: 10.1242/jcs.97.2.385. [DOI] [PubMed] [Google Scholar]
- Geuze HJ, Slot JW, Ger, Strous JAM, Peppard J, von Figura K, Hasilik A, Schwartz AL. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984;37:195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Geuze HJ, Stoorvogel W, Strous GJ, Slot J, Bleekemolen JE, Mellman I. Sorting of mannose-6-phosphate receptor and lysosomal membrane proteins in endocytic vesicles. J Cell Biol. 1988;107:2491–2501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh RN, Gelman DL, Maxfield FR. Quantification of low density lipoprotein and transferrin endocytic sorting in HEp2 cells using confocal microscopy. J Cell Sci. 1994;107:2177–2189. doi: 10.1242/jcs.107.8.2177. [DOI] [PubMed] [Google Scholar]
- Gonnella PA, Neutra MR. Membrane-bound and fluid-phase macromolecules enter separate prelysosomal compartments in absorptive cells of suckling rat ileum. J Cell Biol. 1984;99:909–917. doi: 10.1083/jcb.99.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SH, Sandvig K, van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993;121(1):61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JB, Rothman JE. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature (Lond) 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Heuser J. The role of coated vesicles in recycling of synaptic vesicle membrane. Cell Biol Int Rep. 1989;13:1063–1076. doi: 10.1016/0309-1651(89)90020-9. [DOI] [PubMed] [Google Scholar]
- Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins CR. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983;35:321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Strickland DK, Trowbridge IS. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson EJ, Hopkins CR. Endocytic pathways in polarized Caco-2 cells: identification of an endosomal compartment accessible from both apical and basolateral surfaces. J Cell Biol. 1990;110:337–348. doi: 10.1083/jcb.110.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Whitney JA, Mellman I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell. 1991;67:617–627. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- James DE, Piper RC. Insulin resistance, diabetes, and the insulinregulated trafficking of GLUT-4. J Cell Biol. 1994;126:1123–1126. doi: 10.1083/jcb.126.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Sahagian GG, Snider MD. Transport of surface mannose6-phosphate receptor to the Golgi complex in cultured human hepatocytes. J Biol Chem. 1989;264:7675–7680. [PubMed] [Google Scholar]
- Kelly RB. A question of endosomes. Nature (Lond) 1993;364:487–488. doi: 10.1038/364487a0. [DOI] [PubMed] [Google Scholar]
- Knight A, Hughson E, Hopkins CR, Cutler DF. Membrane protein trafficking through the common apical endosome compartment of polarized Caco-2 cells. Mol Biol Cell. 1995;6:597–610. doi: 10.1091/mbc.6.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JM, Brown MS, Goldstein JL, Anderson RGW. Depletion of intracellular potassium arrests coated pit formation and receptor- mediated endocytosis in fibroblasts. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Lewis V, Green SA, Marsh M, Vihko P, Helenius A, Mellman I. Lysosomal membrane glycoproteins. J Cell Biol. 1985;100:1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Kelly R. Synaptophysin is sorted from endocytic markers in neuroendocrine PC12 cells but not transfected fibroblasts. Neuron. 1991;7:309–317. doi: 10.1016/0896-6273(91)90269-6. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Fambrough DM. Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1988;49:669–677. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Brake B, Banting G, Howell KE, Braghetta P, Stanley KK. TGN38: identification, sequencing, and expression of an integral membrane protein of the trans-Golgi network. Biochem J. 1990;270:97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative: a new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Moya M, Dautry-Varsat A, Goud B, Louvard D, Boquet P. Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diptheria toxin but not that of ricin toxin. J Cell Biol. 1985;101:548–559. doi: 10.1083/jcb.101.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundigli O, Matteoli M, Daniell L, Thomas-Reetz A, Metcalf A, Jahn R, De Camilli P. Synaptic vesicle proteins and early endosomes in cultured hippocampal neurons: differential effects of brefeldin A in axons and dendrites. J Cell Biol. 1993;122:1207–1221. doi: 10.1083/jcb.122.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch A, Xu H, Shields D, Rodriguez-Boulan E. Transport of vesicular stomatitis virus G protein to the cell surface is signal mediated in polarized and nonpolarized cells. J Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. The endosomal concentration of a mannose-6-phosphate receptor is unchanged in the absence of ligand. J Cell Biol. 1987;105:229–234. doi: 10.1083/jcb.105.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Prydz K, Bomsel M, Simons K, Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby Canine Kidney Cell in late endosomes. J Cell Biol. 1989;109:3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B, Horn M, Banting G. TGN38/41 recycles between the cell surface and the TGN: brefeldin A affects its rate of return to the TGN. Mol Biol Cell. 1993;4:93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriquez-Boulan E, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–27. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Schmid SL, Fuchs R, Male P, Mellman I. Two distinct sub-populations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988;52:73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Simonoski K, Gonnella P, Bernanke J, Owen L, Neutra M, Murphy R. Uptake and transepithelial transport of nerve growth factor in suckling rat ileum. J Cell Biol. 1986;103:1979–1990. doi: 10.1083/jcb.103.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, James DE, Lienhard GE. a. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci USA. 1991;88:7815–7819. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. b. Immunolocalization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speelman BA, Allen K, Grounds TL, Neutra MR, Kirchhausen T, Wilson JM. Molecular characterization of an apical early endosomal glycoprotein from developing rat intestinal epithelial cells. J Biol Chem. 1995;270:1583–1588. doi: 10.1074/jbc.270.4.1583. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrincoated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Strous GJ, Geuze HJ, Schwartz AL. Late endosomes derive from early endosomes by maturation. Cell. 1991;65:417–427. doi: 10.1016/0092-8674(91)90459-c. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Holtzman E. Acidification and endosome-like compartments in the presynaptic terminals of frog retinal photoreceptors. J Neurocytol. 1989;18:529–540. doi: 10.1007/BF01474548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M. Tubular early endosomal networks in AtT20 and other cells. J Cell Biol. 1991;115:635–653. doi: 10.1083/jcb.115.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- West MA, Lucocq JM, Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature (Lond) 1994;369:147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis T, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Whitney JA, Neutra MR. Identification of an endosomal antigen specific to absorptive cells of suckling rat ileum. J Cell Biol. 1987;105:691–703. doi: 10.1083/jcb.105.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SA, Park JE, Brown WJ. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]