Abstract
In vivo treatment of mice with the natural killer T (NKT) cell ligand, α-galactosylceramide (αGalCer), ameliorates autoimmune diabetes and experimental autoimmune encephalomyelitis (EAE) by shifting pathogenic Th1-type immune responses to nonpathogenic Th2-type responses. In the current study, in vivo activation of NKT cells in adult NZB/W mice by multiple injections of αGalCer induced an abnormal Th1-type immune response as compared with the Th2-type response observed in nonautoimmune C57BL/6 mice. This resulted in decreased serum levels of IgE, increased levels of IgG2a and IgG2a anti–double-stranded DNA (anti-dsDNA) Ab’s, and exacerbated lupus. Conversely, treatment of NZB/W mice with blocking anti-CD1d mAb augmented Th2-type responses, increased serum levels of IgE, decreased levels of IgG2a and IgG2a anti-dsDNA Ab’s, and ameliorated lupus. While total CD4+ T cells markedly augmented in vitro IgM anti-dsDNA Ab secretion by splenic B cells, the non–CD1d-reactive (CD1d-αGalCer tetramer-negative) CD4+ T cells (accounting for 95% of all CD4+ T cells) failed to augment Ab secretion. The CD1d-reactive tetramer-positive CD4+ T cells augmented anti-dsDNA Ab secretion about tenfold. In conclusion, activation of NKT cells augments Th1-type immune responses and autoantibody secretion that contribute to lupus development in adult NZB/W mice, and anti-CD1d mAb might be useful for treating lupus.
Introduction
Systemic lupus erythematosus is an autoimmune disease characterized by antinuclear autoantibodies and multiorgan tissue injury, including immune complex glomerulonephritis (1–4). There are several murine models of lupus, including some induced by the injection of cells or antigens into nonautoimmune mice (5–8). Others are hereditary, and the mice develop lupus spontaneously as they age (9–13). Hereditary lupus in female NZB/W F1 hybrid mice is characterized by lethal immune complex glomerulonephritis, and IgG2a anti–double-stranded DNA (anti-dsDNA) Ab’s have been reported to play a pathogenic role in glomerular injury (4, 14). Lupus in NZB/W mice closely resembles lupus in humans with severe glomerulonephritis (1, 15).
CD4 T cells play an important role in augmenting autoantibody secretion by autoreactive B cells in NZB/W mice, since anti-CD4 mAb therapy markedly ameliorates lupus in these mice and reduces serum levels of IgG anti-dsDNA Ab’s (16). Autoreactive CD4 T cells in murine lupus have been shown to recognize nucleosomes and also peptides derived from anti-DNA Ab’s (17–20). Recently, we have reported that CD1d-reactive transgenic CD4 T cells induced lupus in BALB/c nu/nu recipients (8). CD1d-reactive CD4 T cells have also been reported to contribute to the pathogenesis of lupus in NZB/W mice (20). Studies of the role of T cell–derived cytokines in NZB/W lupus indicate that the Th1 cytokine IFN-γ plays an important role in the development of disease as judged by the marked reduction of disease activity by anti–IFN-γ therapy and worsening of the disease by administration of IFN-γ (21, 22). IFN-γ is thought to facilitate the switch from IgM to IgG2a pathogenic autoantibodiess in NZB/W mice at about 6 months of age (9, 23), since this cytokine promotes isotype switching of activated B cells to IgG2a, whereas IL-4 promotes isotype switching to IgG1 and IgE (24, 25).
Natural killer T cells (NKT cells) are an important early source of serum IFN-γ and IL-4 after polyclonal activation of T cells in vivo with anti-CD3 mAb (26). It is possible that activation of the NKT cells during the development of lupus contribute to IFN-γ production and disease activity. Mouse NKT cells express invariant Vα14Jα281 TCRs that recognize phospholipid and glycolipid antigens bound to CD1d, a nonpolymorphic, non–MHC-encoded, MHC I–like antigen-presenting molecule expressed on APCs (27–31). α-Galactosylceramide (αGalCer) is a glycolipid that can bind to the invariant TCR and activate NKT cells in vitro and in vivo (29). In nonautoimmune BALB/c and C57BL/6 mice, however, activation of the NKT cells in vivo by αGalCer often resulted in a Th2-type immune response in which IL-4 activity predominated over that of IFN-γ. This, in turn, resulted in a polarization of conventional CD4 T cells toward Th2-type cytokines, increased serum IgE levels, and decreased serum IgG2a levels (32, 33).
Administration of αGalCer in vivo has been reported to ameliorate spontaneous autoimmune diabetes in NOD mice and experimental autoimmune encephalomyelitis (EAE) induced by myelin basic protein in C57BL/6 mice (34–38). In both cases, autoimmune tissue injury is thought to be mediated by a proinflammatory Th1-type immune response, and αGalCer treatment shifts the immune response toward an anti-inflammatory Th2 type (34, 35, 37).
In the current study, we treated lupus in adult NZB/W mice with αGalCer. In contrast to the results of treating diabetes in NOD mice and EAE in C57BL/6, in vivo treatment of female adult NZB/W mice with αGalCer augmented Th1-type immune responses and worsened lupus as judged by an earlier onset of proteinuria and mortality. We also treated adult NZB/W mice with a 6-month course of an anti-CD1d mAb that can block the in vitro interaction between CD1d molecules expressed on B cells and CD1d-reactive T cells (8). The mAb therapy augmented Th2-type immune responses and ameliorated lupus. In addition, sorted CD1d-reactive CD4+ T cells staining positively with a CD1d-αGalCer tetramer markedly augmented the secretion of IgM anti-dsDNA autoantibodies by splenic B cells, but the tetramer-negative CD4 T cells did not. These results link the abnormal Th1-type response observed after in vivo activation of NZB/W NKT cells to lupus disease activity. In addition, the latter cells have been identified as facilitators of autoantibody secretion by B cells early in disease development and potential targets for the treatment of lupus. Anti-CD1d mAb might be useful for treating lupus in adults.
Methods
Mice.
C57BL/6 female mice were obtained from the Department of Comparative Medicine, Stanford University breeding facility. NZB/W female mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). C57BL/6 Jα281–/– mice were obtained from the laboratory of M. Taniguchi (Chiba University, Chiba, Japan) (39) and were maintained at Stanford University animal facility.
Flow-cytometric analysis and sorting.
Single cell suspensions of thymus, spleen, liver, and bone marrow cells were prepared and stained with mAb’s as described previously (20, 39, 40). Analysis and sorting were performed with a FACSVantage (Becton Dickinson Immunocytometry Systems, Mountain View, California, USA), and data were analyzed using FlowJo software (Tree Star, San Carlos, California, USA) (20, 41). The purity of sorted cells was greater than 98%. The FITC-, phycoerythrin-, APC-, or Texas red–conjugated mAb’s to mouse B220, CD19, IgM, IgD, CD1d, TCRαβ, CD4, CD8, NK1.1, CD69, and CD44 were purchased from PharMingen (San Diego, California, USA). Preparation of and staining with CD1d-αGalCer tetramer was reported previously (42).
In vitro secretion of IgM and IgG.
Sorted splenic T and B cell subsets were incubated in 96-well round-bottom plastic plates in complete RPMI medium with 10% FBS for 1–5 days at 37°C in 5% CO2. At the end of the culture period, supernatants were harvested, and the concentrations of IgM and IgG were measured with ELISA, using affinity-purified goat anti-mouse heavy chain–specific Ab’s as described below.
ELISA assays.
A standard sandwich ELISA was used to measure Ab isotype concentrations as described previously (8, 20). The concentration of IgE is expressed in units per milliliter, using a reference-positive standard of pooled serum from 6- to 7-month-old NZB/W mice. A 1:10 dilution of the standard serum was arbitrarily assigned a value of 100 U/ml. Anti-dsDNA titers are expressed in units per milliliter, using a reference-positive standard of pooled serum from 6- to 7-month-old NZB/W mice. A 1:100 dilution of this standard serum was arbitrarily assigned a value of 100 U/ml.
In vitro stimulation of sorted tetramer-positive CD4+ T cells.
Sorted tetramer-positive CD4+ T cells from the spleens of NZB/W or C57BL/6 mice were placed in plastic plates (5 × 103 cells/well) and stimulated with PMA and ionomycin for 48 hours as described previously (41). Supernatants were harvested at the end of the culture period, and the concentrations of IL-4 and IFN-γ were measured by ELISA.
In vivo treatment of NZB/W mice with αGalCer and anti-CD1d mAb.
Mice were injected with αGalCer (Kirin Pharmaceutical Research Institute, Gunma, Japan) or PBS/vehicle at a dose of 4 μg/mouse per injection as described previously (33, 34). Mice were injected with anti-CD1d mAb (rat IgG2b) from the hybridoma 1B1 (43) and the rat IgG2b isotype control (rat anti-human HLA Bw6) from hybridoma HB-152 (American Type Culture Collection, Manassas, Virginia, USA) as described previously (20).
Proteinuria of NZB/W mice was measured on a scale of 1–4+ using a colorimetric assay for albumin (Albustix; Miles Inc., Elkhart, Indiana, USA). Mice were considered to have proteinuria if at least two consecutive urine samples were greater than 2+, according to the scale (100 mg/dl) (8, 20). Serum levels of IgM, IgG, and IgE, and IgG anti-dsDNA Ab’s were measured with the ELISA, as described above.
Statistical analysis.
Differences in proteinuria onset and survival time of groups were analyzed using log-rank test. Differences in percentage of T cell subsets, cytokine, and IgG concentrations in serum and culture supernatants were analyzed with a two-tailed Student’s t test.
Results
In vivo αGalCer treatment induces a Th1-type immune response in adult NZB/W mice.
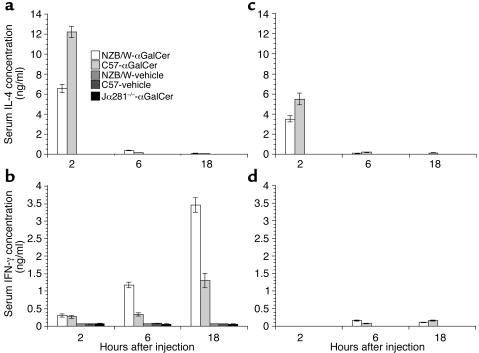
C57BL/6 and BALB/c mice given single injections of the invariant NKT cell TCR ligand, αGalCer, have been reported to develop a Th2-type shift of serum Ig isotypes with high levels of serum IgE (33). Secretion of IgE is augmented by IL-4 from Th2 cells. Immunizing those mice simultaneously with protein antigen and αGalCer directs the T cell response to the antigen to a Th2 pattern (33), although this has not been found in every experiment (44). In the current study, we explored the impact of the administration of αGalCer in lupus-prone NZB/W mice. We first compared the serum levels of IL-4 and IFN-γ at 2, 6, and 18 hours after a single injection of αGalCer or PBS/vehicle in 8- to 12-week-old C57BL/6 and NZB/W mice. As shown in Figure 1, serum levels of IL-4 in both strains of mice given αGalCer increased and peaked at 2 hours after injection, and the IL-4 levels of NZB/W mice were twofold lower than C57BL/6 mice (P < 0.001). The serum levels of IFN-γ peaked at 18 hours after injection, and the IFN-γ levels of NZB/W mice were threefold higher than C57BL/6 mice (P < 0.001). Control NZB/W and C57BL/6 mice injected with the PBS/vehicle did not have detectable amounts of serum IL-4 or IFN-γ. C57BL/6 Jα281–/– mice that are deficient in NKT cells did not have elevated serum levels of IL-4 or IFN-γ after αGalCer injection (Figure 1).
Figure 1.
Kinetics of serum levels of IL-4 and IFN-γ after single injection of αGalCer. C57BL/6, NZB/W, and Jα281–/– C57BL/6 mice at age 8–12 weeks or 4 weeks were given a single injection of αGalCer or PBS/vehicle control, and serum levels of IL-4 and IFN-γ were measured at 2, 6, and 18 hours after injection. (a and b) Data from 8- to 12-week-old mice. (c and d) Data from 4-week-old mice. Bars show means of ten mice, and brackets show standard errors.
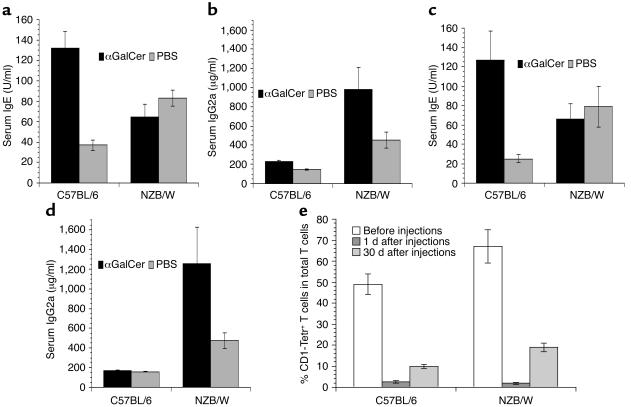
The difference in serum cytokine patterns in adult NZB/W and C57BL/6 mice after αGalCer injection was reflected by changes in serum levels of IgE, an isotype upregulated by IL-4, and IgG2a, an isotype upregulated by IFN-γ (45). As shown in Figure 2, a and b, 9 days after αGalCer injection, C57BL/6 mice had a threefold increase in IgE (P < 0.001) and no significant change (P > 0.1) in serum IgG2a as compared with C57BL/6 mice given PBS/vehicle control. By contrast, NZB/W mice had no significant changes in IgE levels, but there was a twofold increase in IgG2a as compared with NZB/W mice given PBS/vehicle control.
Figure 2.
Treatment with αGalCer induced Th1- or Th2-type Ig isotype pattern in adult NZB/W or C57BL/6 mice. C57BL/6 and NZB/W mice (8–12 weeks old) were given single or multiple injections of αGalCer or PBS/vehicle control. (a and b) Serum levels of IgE and IgG2a in C57BL/6 and NZB/W mice 9 days after having been given a single injection of αGalCer or PBS control. Bars show means of ten mice, and brackets show standard errors. (c and d) Serum levels of IgE and IgG2a in C57BL/6 and NZB/W mice 30 days after completion of injections of αGalCer or PBS control twice a week for 2 weeks. Bars show means of ten mice, and brackets show standard errors. (e) Changes of the percentage of CD1d-αGalCer tetramer-positive T cells in livers of C57BL/6 and NZB/W mice before injection and 1 day and 30 days after last injection (twice a week for 2 weeks). Tetr+, tetramer positive. There are five mice at each time point. Bars show mean percentage of five mice, and brackets show standard errors.
A similar study was performed in 4-week-old instead of 8- to 12-week-old NZB/W and C57BL/6 mice to determine whether the αGalCer-induced Th1-type shift in NZB/W mice precedes the development of IgM anti-dsDNA Ab’s in the serum usually starting at an age of 8–12 weeks (46). As shown in Figure 1, c and d, after a single injection of αGalCer, serum IL-4 levels peaked at 2 hours in both strains of young mice, and mean peak IL-4 levels in NZB/W mice were significantly lower than that in C57BL/6 mice (P < 0.01). The peak IL-4 levels in young NZB/W and C57BL/6 mice were about twofold lower than in adult mice (Figure 1; P < 0.01). IL-4 was not detected in control mice given vehicle/PBS. Serum IFN-γ levels peaked at 6–18 hours after injection in both strains of young mice, and the mean peak IFN-γ level in NZB/W mice was not significantly different from that in C57BL/6 mice (Figure 1d). The mean peak IFN-γ levels in young NZB/W and C57BL/6 mice was about 20-fold lower than in older mice after αGalCer injection (P < 0.001), however, and only slightly above background (Figure 1). Serum levels of IgE and IgG2a were measured before and 9 days after injection. The serum IgE levels were not detectable before or after αGalCer injection in either strain of young mice (data not shown). The serum IgG2a levels were less than 0.2 μg/ml in both strains of mice before αGalCer injection, which is about 1,000-fold lower than in older mice (Figure 2), and αGalCer treatment did not induce a significant change (data not shown). These results indicate that the Th2-type immune responses in C57BL/6 and the Th1-type immune responses in NZB/W mice after αGalCer treatment are detectable only in the older mice. The Th1-type shift in NZB/W mice develops at about the same age as the development of serum IgM anti-dsDNA Ab’s.
In additional experiments, 8- to 12-week-old NZB/W and C57BL/6 mice were injected with αGalCer twice a week for 2 weeks. Four weeks after the last injection, the serum levels of IgE and IgG2a were measured. The results were similar to that after a single αGalCer injection. Whereas IgE was markedly increased in C57BL/6 mice, IgG2a was markedly increased in NZB/W mice (Figure 2, c and d). These results indicate that single as well as multiple injections of αGalCer induces a Th2-type serum Ig shift in adult C57BL/6 mice and a Th1-type shift in adult NZB/W mice. The serum levels of IgE and IgG2a in adult NZB/W mice given PBS/vehicle control were twofold higher than in C57BL/6 mice given PBS/vehicle control (Figure 2, P < 0.01). This is an expected result of spontaneous polyclonal B cell activation in NZB/W mice (46, 47).
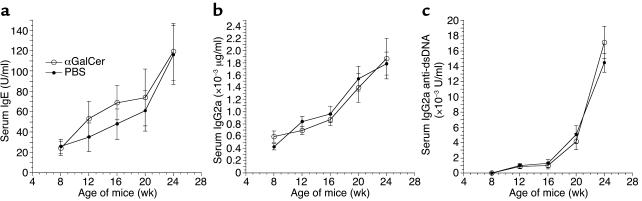
In further experiments, 4-week-old NZB/W and C57BL/6 mice were also injected with αGalCer or PBS/vehicle control twice a week for 2 weeks, and a kinetic measurement of serum levels of IgE, IgG2a, and IgG2a anti-dsDNA Ab’s was carried out monthly for 5 months, starting at the age of 8 weeks (2 weeks after completion of αGalCer injection). As shown in Figure 3, treatment of young NZB/W mice did not significantly (P > 0.1) change the serum levels of IgE, IgG2a, and IgG2a anti-dsDNA Ab’s as compared with PBS/vehicle control at different time points over the 5 month period. In both groups, the IgG2a anti-dsDNA Ab’s, which are the hallmark of lupus disease activity and development (1–4), increased progressively, indicating that the kinetics of disease development were neither accelerated nor slowed by the injection of αGalCer in these young mice. The kinetics of the onset of proteinuria during an 8-month observation period was not significantly different (P > 0.1) in both groups (data not shown). At 9 months of age, five out of ten mice in each group had developed severe (a score greater than 3+) proteinuria, indicating a similar tempo of development of lupus glomerulonephritis in the two groups.
Figure 3.
Treatment with αGalCer did not induce Th1- or Th2-type Ig isotype pattern in young NZB/W mice. Four-week-old NZB/W mice were injected with αGalCer or control PBS/vehicle twice a week for 2 weeks. Serial measurements of serum IgE, IgG2a, and IgG2a anti-dsDNA Ab’s were shown (a–c), starting from the age of 8 weeks (2 weeks after completion of injection). There were ten mice in each group.
The αGalCer treatment of young C57BL/6 mice did not significantly change the serum levels of IgE or IgG2a as compared with the control mice given PBS/vehicle (data not shown). The IgG2a levels in these mice remained in the 200–300 μg/ml range during the entire observation period and were about tenfold lower than that of the NZB/W mice at 24 weeks of age. The IgG2a anti-dsDNA Ab’s were undetectable at all the points in the C57BL/6 mice. These results indicate that single as well as multiple injections of αGalCer did not augment either Th1-type or Th2-type immune responses in young NZB/W or young C57BL/6 mice, although the same treatment augmented Th1-type immune responses in adult NZB/W mice and Th2-type immune response in adult C57BL/6 mice. Taken together, the impact of αGalCer treatment on immune response was age dependent.
In vivo αGalCer treatment results in the depletion of the CD1d-αGalCer tetramer-positive (henceforth called tetramer positive) NKT cells in the spleen and liver of C57BL/6 and BALB/c mice (42). Thus, the different changes induced by αGalCer treatment of adult 8- to 12-week-old C57BL/6 and NZB/W mice might be explained by different patterns of NKT cell depletion rather than the results of NKT cell activation. Accordingly, we observed the changes of CD1d-reactive tetramer-positive T cells in the spleen and liver of C57BL/6 and NZB/W mice before and after multiple αGalCer injections. As shown in Figure 2e, before injection about 50% of T cells in C57BL/6 liver were tetramer positive and about 65% of T cells in NZB/W liver were tetramer positive. One day after the last injection, less than 3% of T cells in the liver of both strains were tetramer positive, and 30 days after the last injection there was a partial recovery of tetramer-positive T cells in the liver. At the latter point, 12% of the T cells in C57BL/6 liver and 20% of the T cells in NZB/W liver were tetramer positive. The change in the absolute numbers of tetramer-positive T cells in the livers of the two strains of mice had patterns similar to the percentage of change (data not shown). Therefore, the Th1-type shift after αGalCer injection in adult NZB/W mice is not due to a profound or prolonged depletion of NKT cells as compared with C57BL/6 mice.
Treatment with αGalCer worsens lupus in adult NZB/W mice.
Treatment with αGalCer induced Th2-type immune responses in NOD and adult C57BL/6 mice and ameliorated autoimmune diabetes and EAE (34, 35, 37). Since αGalCer administration induced a Th1-type immune response in NZB/W mice at age 8–12 weeks, we expected αGalCer given to prenephritic NZB/W mice would also induce Th1-type immune responses and accelerate lupus development. Accordingly, groups of 15 female NZB/W mice at age 20 weeks were injected with αGalCer or PBS/vehicle control twice a week for 2 weeks. All the mice were monitored weekly for proteinuria and survival. Serum samples were collected monthly, starting at age 26 weeks, an expected time for the onset of proteinuria (15, 46).
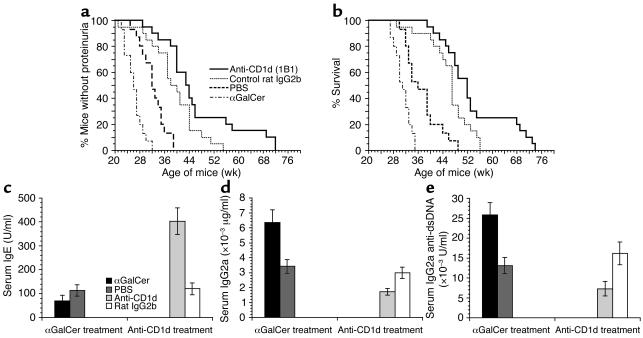
As shown in Figure 4, a and b, all the mice in the PBS/vehicle control group developed proteinuria by 39 weeks of age. In contrast, all the mice in the αGalCer-treated group developed proteinuria by 32 weeks of age. The αGalCer treatment accelerated the onset of proteinuria by about 7 weeks (P = 0.002). Similarly, when the survival of the groups was compared, αGalCer treatment shortened the survival by about 14 weeks as compared with PBS/vehicle control (49 versus 35 weeks for all mice to die; P = 0.0007).
Figure 4.
Exacerbation or amelioration of lupus by αGalCer or anti-CD1d mAb treatment in adult NZB/W mice. NZB/W mice were treated with αGalCer, PBS/vehicle, anti-CD1d mAb, and rat IgG2b isotype control. (a and b) Groups of 20-week-old NZB/W mice were given four injections of αGalCer or PBS/vehicle control at a dose of 4 μg/mouse over a period of 2 weeks. There were 15 mice in each group. In addition, groups of 8- to 12-week-old NZB/W mice were given 15 injections of anti-CD1d mAb or control rat IgG2b at a dose of 500 μg/mouse over a period of 6 months (five injections for the first month, two injections for each month thereafter). There were 20 mice in each group. All mice were monitored weekly for proteinuria (a) and survival (b). (c–e) The serum concentrations of IgE, IgG2a, and IgG2a anti-dsDNA Ab’s of the above-treated mice at the age of 26 weeks. Bars show means of 15 or 20 mice, and brackets show standard errors.
Serum samples from the control and experimental groups at 26 weeks of age were measured for levels of IgE, IgG2a, and IgG2a anti-dsDNA Ab’s. Later time points were not compared due to the rapid development of lupus and death in the αGalCer-treated group. As shown in Figure 4, c–e, αGalCer-treated mice had a significant decrease in serum IgE (P < 0.01), but about a twofold increase in serum IgG2a and IgG2a anti-dsDNA Ab’s (P < 0.001) as compared with PBS/vehicle control. These results demonstrated that αGalCer treatment not only augments the Th1-type immune responses but also worsens lupus in NZB/W mice. It is possible that αGalCer may worsen lupus by stimulating B cells that express surface CD1d to secrete Ab’s by binding to the CD1d. αGalCer did not, however, significantly change the in vitro secretion of IgM autoantibodies by sorted B220+CD1dhigh B cells from the spleen of 8- to 12-week-old NZB/W mice as compared with vehicle control (culture supernatant IgM concentration, αGalCer: 1,647 ± 76 versus vehicle 1,492 ± 96 μg/ml). This indicated that αGalCer did not directly activate the CD1dhigh B cells.
Anti-CD1d mAb treatment induces a Th2-type shift and ameliorates lupus in adult NZB/W mice.
Since activation of the NKT cells augmented Th1-type immune responses and worsened lupus in adult NZB/W mice, we tested whether treatment with a blocking anti-CD1d mAb would induce a Th2-type shift and ameliorate lupus. We reported recently that a short-term treatment (1 month) of NZB/W mice with anti-CD1d mAb delayed the onset of lupus for 4–8 weeks, but the pattern of immune response was not investigated (20). In the current study, groups of 20 female NZB/W mice (8 weeks old) were injected with anti-CD1d mAb or rat IgG2b isotype control (500 μg/mouse) daily for the first 3 days, then twice a month for 6 months. As in the αGalCer treatment experiments, all the mice were monitored weekly for proteinuria and survival. Serum samples were collected monthly, starting at 26 weeks of age. As shown in Figure 4, a and b, all the mice in the PBS/vehicle control group developed proteinuria by 39 weeks of age, and all the mice in the control rat IgG2b-treated group developed proteinuria by 53 weeks of age. In contrast, all the mice in the anti-CD1d mAb–treated group developed proteinuria by 72 weeks of age. As compared with PBS/vehicle control or rat IgG2b control, anti-CD1d mAb treatment delayed the onset of proteinuria 33 weeks (P < 0.0001) or 19 weeks (P = 0.03), respectively (Figure 4, a and b).
Similarly, when the survival of the groups was compared, anti-CD1d treatment prolonged the survival 23 weeks as compared with PBS/vehicle control (72 versus 49 weeks for all mice to die, P < 0.0001) or by 16 weeks as compared with rat IgG2b isotype controls (72 versus 56 weeks for all mice to die, P = 0.006). These results indicate that long-term (6 month) anti-CD1d treatment can also achieve a more prolonged amelioration of lupus as compared with the short-term (1 month) treatment used in our previous report (20). IgG2b isotype treatment significantly delayed the onset of proteinuria and prolonged survival as compared with PBS/vehicle control (P < 0.01). This may be due to the local anti-inflammatory activity mediated by inhibitory Fcγ receptor IIB when large amounts of IgG2b are infused (48, 49). Rat IgG2b treatment did not change serum levels of pathogenic IgG2a anti-dsDNA as compared with the PBS/vehicle control (Figure 4e).
Serum samples from the anti-CD1d mAb and isotype control–treated groups at 26 weeks of age were also measured for levels of IgE, IgG2a, and IgG2a anti-dsDNA Ab’s. As shown in Figure 4, c–e, the anti–CD1d-treated group had a fourfold increase in IgE (P < 0.0001) and a twofold decrease in serum IgG2a and anti-dsDNA IgG2a Ab’s (P < 0.01) as compared with the rat IgG2b isotype control–treated group. As reported previously, in vivo anti-CD1d mAb treatment did not significantly change the percentage of total B cells among spleen cells or CD1dhigh B cells among total B cells and did not reduce the serum levels of IgM and IgM anti-dsDNA Ab’s (20, 50). These results indicate that anti-CD1d mAb treatment induces a Th2-type shift of serum Ig’s and ameliorates lupus in NZB/W mice. The marked improvement of lupus by anti-CD1d mAb treatment as compared with PBS controls is likely due to both the specific reduction of pathogenic IgG2a anti-dsDNA Ab’s and the nonspecific Fcγ receptor IIB–mediated inhibition of local inflammation activity after IgG infusion (48, 49).
The majority of CD1d-reactive tetramer-positive T cells in the NZB/W spleen are NK1.1–.
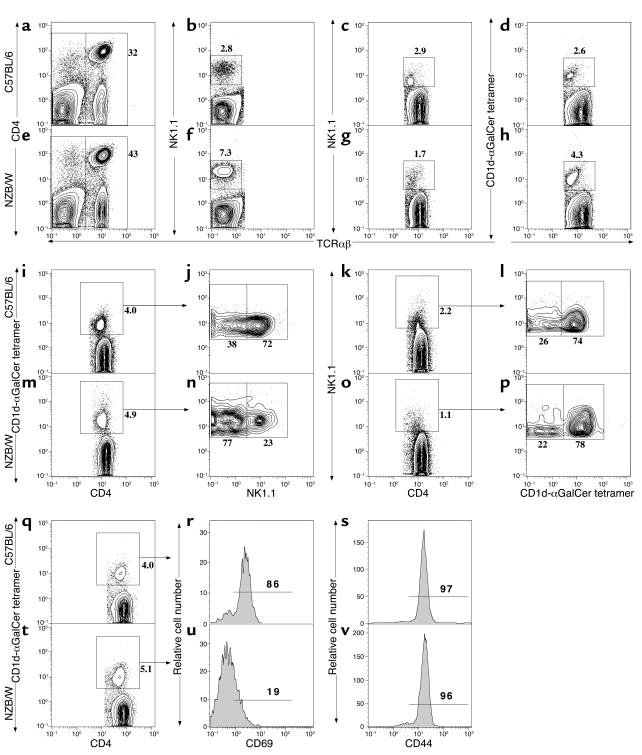
We compared the percentage and absolute numbers of NK1.1+ T cells and T cells expressing the CD1d-reactive invariant Vα14Jα281TCRs, as judged by staining with a CD1d-αGalCer tetramer reagent in 8- to 12-week-old C57BL/6 and NZB/W mice. About 32% of C57BL/6 spleen cells (Figure 5a) and 43% of NZB/W spleen cells (Figure 5e) were TCRαβ+. The mean (plus or minus SE) percentage in six C57BL/6 and six NZB/W mice was 30% ± 3% and 38% ± 5%, respectively. To determine whether NK1.1 expression on conventional NK cells was similar in NZB/W and C57BL/6 mice, gated TCRαβ– spleen cells from both strains were analyzed for the staining of NK1.1. Figure 5, b and f, show that 2.8% of the TCRαβ– cells in the C57BL/6 spleen were NK1.1+ cells (mean 3% ± 1.5%), and 7.3% of the TCRαβ– cells in NZB/W spleen were NK1.1+ (mean 9% ± 2%). The intensity of NK1.1 staining was similar. Gated TCRαβ+ T cells were also analyzed for staining of NK1.1 (Figure 5, c and g) and for staining with the CD1d-αGalCer tetramer reagent (Figure 5, d and h). The percentage of NK1.1+ T cells among TCRαβ+ T cells was reduced in the NZB/W spleen (1.7%) (mean 1.6% ± 0.4%) as compared with the C57BL/6 spleen (2.9%) (mean 3.4% ± 0.3%). On the other hand, the percentage of tetramer-positive T cells was increased in NZB/W spleen (4.3%) (mean 4.8% ± 0.9%) as compared with the C57BL/6 spleen (2.6%) (mean 3.5% ± 0.5%). The gated T cells from Jα281–/– C57BL/6 spleens had less than 0.1% tetramer-positive T cells (data not shown). The absolute number of NK1.1+ T cells in C57BL/6 spleen (mean 899 ± 39 × 103) was about twofold higher than that in NZB/W spleen (mean 480 ± 67 × 103). The difference was significant (P < 0.01).
Figure 5.
Multicolor flow-cytometric analyses of spleen cells. Spleen cells from 8- to 12-week-old C57BL/6 and NZB/W mice were stained with anti-TCRαβ, CD4, NK1.1, and CD1d-αGalCer tetramer. (a and e) Staining of CD4 versus TCRαβ for both strains. (b and f) NK1.1 versus TCRαβ on gated TCRαβ– cells. (c and g) NK1.1 versus TCRαβ on gated TCRαβ+ cells. (d and h) Tetramer versus TCRαβ on gated TCRαβ+ cells. (i and m) gated TCRαβ+CD4+ T cells were analyzed for CD4 versus tetramer. (j and n) Tetramer versus NK1.1 on gated TCRαβ+CD4+tetramer-positive cells. (k and o) NK1.1 versus CD4 on the gated TCRαβ+CD4+ cells. (l and p) NK1.1 versus tetramer on the gated TCRαβ+CD4+NK1.1+ cells. The percentage of each subset was shown beside the gating box. Data were representative of six mice in each strain. (q and t) Gated TCRαβ+CD4+ T cells were analyzed for CD4 versus tetramer. (r and s) Expression of CD69 and CD44, respectively, on the gated C57BL/6 cells and (u and v) expression of CD69 and CD44 on the gated NZB/W cells. The background staining with control CD1d-vehicle-tetramer for the gated T cells was less than 0.1%.
The absolute number of tetramer-positive T cells in the spleen of C57BL/6 and NZB/W mice was similar (923 ± 72 × 103 and 989 ± 88 × 103, respectively; P > 0.1). Approximately 80% of the tetramer-positive T cells and 60% of the NK1.1+ T cells in the spleen of both strains were CD4+ T cells (data not shown). As shown in Figure 5, i–p, while 72% of gated CD4+ tetramer-positive T cells in the spleen of C57BL/6 and NZB/W were NK1.1+ (mean 71% ± 4%), only 23% of those from NZB/W spleen were NK1.1+ (mean 20% ± 5%). Conversely, 74% of CD4+NK1.1+ T cells in the C57BL/6 spleen were tetramer positive (mean 75% ± 4%), and 78% of CD4+NK1.1+ T cells from NZB/W spleen were tetramer positive (mean 78% ± 6%). These results indicate that there is no reduction of the invariant CD1d-reactive T cells in the spleen of 8- to 12-week-old NZB/W mice as compared with C57BL/6 mice, and the majority of the CD1d-reactive T cells in NZB/W spleen are NK1.1–.
Since tetramer-positive NK1.1–CD4+ T cells in the NZB/W spleen may represent immature NKT cells similar to those found in the thymus of nonautoimmune mice (51–53) or mature NKT cells that have downregulated the NK1.1 surface receptors after spontaneous activation (54), we attempted to distinguish these two possibilities by examining additional surface receptors and the cytokine secretion patterns of the tetramer-positive CD4+ T cells in C57BL/6 and NZB/W mice. Figure 5, t–v, shows that the tetramer-positive CD4+ T cells in adult NZB/W mice express low levels of CD69 and a discrete high level of CD44. This pattern is atypical of mature NKT cells that express high levels of both markers (51–53). The typical pattern was observed in the tetramer-positive CD4+ T cells from the adult C57BL/6 spleen (Figure 5, q–s). The NZB/W pattern, however, is not typical of immature NK1.1– tetramer-positive T cells, since the latter cells express low levels of CD69, but are heterogeneous for CD44 expression (53).
In further experiments, 5 × 103 sorted tetramer-positive CD4+ and tetramer-positive NK1.1–CD4+ T cells from the spleen of NZB/W mice were stimulated in vitro with PMA and ionomycin, and the cytokine pattern in culture supernatants was analyzed for the concentrations of IL-4 and IFN-γ after 48 hours. A similar analysis was performed on C57BL/6 tetramer-positive CD4+ T cells. At this cell number, spontaneous secretion of cytokines was not detectable using NZB/W or C57BL/6 mice. Whereas the mean (plus or minus SE) concentrations of IFN-γ and IL-4 from quadruplicate cultures were 23 ± 2 and 630 ± 53 pg/ml, respectively, using all NZB/W tetramer-positive CD4+ T cells, the mean concentrations using NZB/W tetramer-positive NK1.1–CD4+ T cells were 48 ± 7 and 1,786 ± 78 pg/ml, respectively. On a per cell basis, the concentrations of both IFN-γ (mean 21 ± 2) and IL-4 (323 ± 15) were lower when C57BL/6 tetramer-positive CD4+ T cells were used instead of the NZB/W tetramer-positive NK1.1–CD4+ T cells. The more vigorous secretion of IFN-γ by the NZB/W tetramer-positive NK1.1– T cells is more characteristic of mature rather than immature NKT cells (51, 53). The very high level of IL-4 is atypical of mature cells (51–53), however.
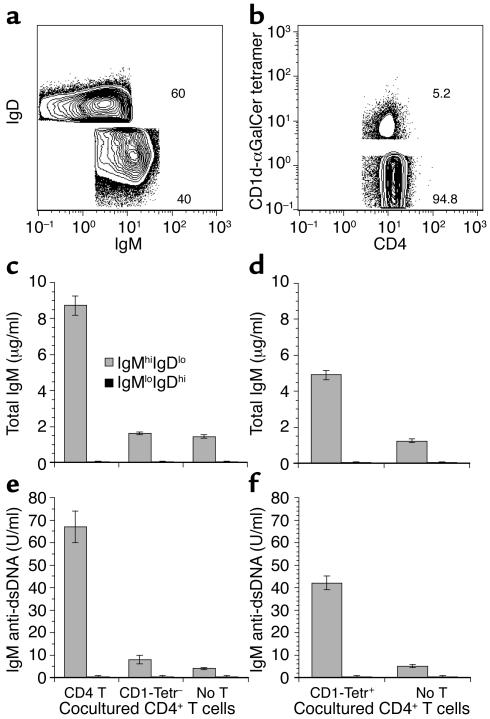
CD1d-reactive tetramer-positive CD4+ T cells are required for the augmentation of in vitro secretion of IgM anti-dsDNA Ab’s by CD4+ T cells in NZB/W mice. We have reported that CD1dhigh B cells are the predominant source of spontaneous secretion of IgM anti-dsDNA Ab’s by splenic B cells in NZB/W mice, and CD4+ T cells augment the Ab secretion (20). In the current study, we determined whether the CD1d-reactive CD4+ T (CD1d-αGalCer tetramer-positive) cells were required for the augmentation. As shown in Figure 6, splenic B cells (CD19+ gated) from 8- to 12-week-old NZB/W mice were sorted into IgMhighIgDlow and IgMlowIgDhigh B cells (Figure 6a). Previous studies have shown that the former B cell subset is CD1dhigh and the latter is CD1d1ow (55, 56). The CD4+ T cells from the same spleens were sorted into tetramer-positive and tetramer-negative cells (Figure 6b). Since tetramer staining may activate the NKT cells by cross-linking the invariant Vα14Jα281TCRs, we first tested if depletion of tetramer-positive T cells removed the capacity of CD4+ T cells to augment in vitro secretion of autoantibodies. Sorted IgMhighIgDlow or IgMlowIgDhigh B cells (200 × 103) were cultured alone or cocultured with 500 × 103 total CD4+ T cells or tetramer-negative CD4+ T cells in vitro for 5 days. The supernatants were assayed for the concentrations of IgM and IgM anti-dsDNA Ab’s.
Figure 6.
CD1d-αGalCer tetramer-positive CD4+ T cells augment in vitro secretion of IgM anti-dsDNA Ab’s. (a) Spleen B cells (CD19+ gated) from 8- to 12-week-old NZB/W mice were sorted into IgMhighIgDlow and IgMlowIgDhigh B cell subsets. (b) Spleen CD4+ T cells (TCRαβ+CD4+ gated) were sorted into CD1d-αGalCer tetramer-positive and CD1d-αGalCer tetramer-negative subsets. (c and e) Concentrations of IgM and IgM anti-dsDNA Ab’s in the culture supernatants of sorted CD4+ T cells or tetramer-negative CD4+ T cells (500 × 103 each) cocultured with sorted IgMhighIgDlow B or IgMlowIgDhigh B cells (200 × 103 each) in vitro for 5 days. (d and f) Concentrations of IgM and IgM anti-dsDNA Ab’s in the culture supernatants of sorted CD1d-αGalCer tetramer-positive CD4+ T cells (50 × 103) cocultured with 200 × 103 IgMhighIgDlow or IgMlowIgDhigh B cells for 5 days. Bars show means of quadruple cultures from three replicate experiments, and brackets show standard errors. lo, low; hi, high; Tetr–, tetramer negative; Tetr+, tetramer positive; T, T cells.
In Figure 6, c and e, IgMhighIgDlow B cells alone secreted about 1.5 μg/ml of IgM and 4 U/ml of IgM anti-dsDNA Ab’s, but IgMlowIgDhigh B cells alone did not secrete detectable amounts of IgM or IgM anti-dsDNA Ab’s. The addition of total CD4+ T cells augmented the secretion of IgM by IgMhighIgDlow B cells about fivefold and IgM anti-dsDNA Ab’s about 15-fold (P < 0.001). Total CD4+ T cells did not help the IgMlowIgDhigh B cells to secrete Ab’s (Figure 6, c and e), however. Sorted tetramer-negative CD4+ T cells (accounting for 95% of total CD4+ T cells) lost the capacity to significantly augment Ab secretion by the IgMhighIgDlow B cells (Figure 6, c and e; P > 0.1). In addition, the sorted tetramer-positive CD4+ T cells (50 × 103) augmented the secretion of IgM threefold (P < 0.01) and IgM anti-dsDNA tenfold (P < 0.001) as compared with IgMhighIgDlow B cells alone (Figure 6, d and f). On the other hand, depletion of the NK1.1+CD4+ T cells (about 1.5%) from total CD4+ T cells did not significantly reduce the augmentation of the Ab secretion by total CD4+ T cells (data not shown). These results indicate that the tetramer-positive (but not NK1.1+) CD4+ T cells are required for the augmentation of in vitro spontaneous secretion of IgM and IgM anti-dsDNA autoantibodies. Addition of anti-CD1d mAb, or anti–IL-4 mAb, or anti–IFN-γ mAb, or anti–IL-4 and anti–IFN-γ mAb in the culture of CD4+ T and IgMhighIgDlow B cells, however, failed to significantly block the augmentation by CD4+ T cells (data not shown). This may be due to spontaneous in vivo activation of CD1dhigh B cells and CD1d-reactive T cells in NZB/W mice such that augmentation in vitro is by activation-dependent cytokines (other than IL-4 and IFN-γ) and/or costimulatory molecules, since our previous studies showed that in vitro activation of nonautoimmune BALB/c B cells by CD1d-reactive transgenic T cells was blocked by the same anti-CD1d mAb (8).
We also tried to determine whether sorted tetramer-positive CD4+ T cells could augment the in vitro spontaneous secretion of IgG and IgG anti-dsDNA Ab’s by splenic B cells in older NZB/W mice. Splenic B cells from 8- to 12-week-old NZB/W mice spontaneously secrete little IgG or IgG anti-dsDNA Ab’s in vitro (20), but those from 24- to 28-week-old NZB/W mice with proteinuria secrete large amounts of IgG and IgG anti-dsDNA Ab’s in vitro (20). We used the sorted B220+ cells from 24- to 28-week-old NZB/W as the B cell source for those in vitro cultures. We found that, although both total CD4+ T and tetramer-positive CD4+ T cells significantly augmented the IgM and IgM anti-dsDNA secretion, neither total CD4+ T cells nor tetramer-positive CD4+ T cells augmented the in vitro secretion of IgG or IgG anti-dsDNA Ab’s (data not shown).
Discussion
Hereditary lupus of NZB/W mice is an Ab-mediated systemic autoimmune disease in which the Th1 cytokine IFN-γ has been shown to play an important role in the pathogenesis of tissue injury (57). Anti–IFN-γ mAb treatment has been reported to ameliorate the immune complex glomerulonephritis, the hallmark of the disease (21). In addition, introduction of a transgene encoding the Th2 cytokine IL-4 into lupus-prone (NZW × C57BL/6.Yaa) F1 mice prevented lupus development (58). We have recently reported that adoptive transfer of CD1d-reactive transgenic CD4 T cells with a Th1-like cytokine-secretion pattern induced lupus in BALB/c nu/nu recipients (8). CD1d-reactive T cells have also been suggested to play a role in augmenting IgG2a anti-dsDNA secretion and lupus development in lupus-prone NZB/W mice (20). It is not yet clear, however, whether activation of the CD1d-reactive T cells in NZB/W mice contributed to the IFN-γ secretion that shifted the autoantibody secretion toward the pathogenic IgG2a isotype.
In vivo activation of NKT cells with αGalCer treatment over a 2-week interval induced Th2-type immune responses with high levels of serum IgE in C57BL/6, BALB/c, and NOD mice and ameliorated autoimmune diabetes in NOD mice (33, 35). In the current study, we used a 2-week αGalCer regimen at a similar dose (4 μg/mouse) to treat 8- to 12-week-old NZB/W mice that have already developed IgM anti-dsDNA Ab’s (46). These NZB/W mice treated with αGalCer developed increased levels of IFN-γ and decreased levels of IL-4 in serum as compared with the treated C57BL/6 mice. This Th1-like serum cytokine profile in the treated NZB/W mice was associated with decreased levels of serum IgE and increased levels of serum IgG2a and IgG2a anti-dsDNA autoantibodies. The in vivo αGalCer-induced Th1 immune response was age related, since 4-week-old NZB/W mice injected with αGalCer showed a significant elevation of serum IL-4 but not IFN-γ. Thus, the young NZB/W mice showed a Th2 pattern that 1–2 months later shifted to a Th1 pattern at about the time of anti-dsDNA development. The levels of serum IL-4 in the young mice were, however, below the levels of serum IL-4 in the older mice after αGalCer treatment. This may be related to the lower absolute numbers of NKT cells in the spleen of young NZB/W mice that were about two-thirds of that in the older mice (data not shown). The αGalCer treatment of young NZB/W mice did not have a significant impact on the tempo of the progressive increase in levels of IgG2a anti-dsDNA Ab’s and the development of proteinuria that are the hallmarks of lupus disease activity over a subsequent 8-month observation period.
The same treatment, however, accelerated the development of lupus in adult NZB/W mice as judged by earlier onset of proteinuria and mortality than that which occurred in PBS/vehicle–treated mice. These results indicate that in vivo activation of NKT cells in adult NZB/W mice with this αGalCer regimen contributes to IFN-γ secretion and autoantibody isotype switching to IgG2a. These results also link the Th1-type immune responses and lupus disease activity in adult NZB/W mice. It is of interest that coinjection of αGalCer and myelin basic protein augmented Th1-type immune responses and exacerbated EAE in B10.PL mice, but the same treatment augmented Th2-type immune responses and prevented EAE in C57BL/6 mice (37). Thus, some autoimmune-prone strains of mice can differ from other autoimmune or nonautoimmune strains in the pattern of immune responses observed after NKT cell activation in vivo, such that autoimmune disease may be ameliorated or exacerbated. We did not examine different doses, dosing schedules, or durations of αGalCer treatment other than the one reported herein. It is possible that more extended αGalCer treatment regimens (34), alternative synthetic glycolipid regimens (59), or daily (35) rather than twice weekly injections that have been used to ameliorate autoimmune diabetes might result in a different outcome of disease activity in lupus.
Sorted NKT cells from the adult NZB/W and C57BL/6 spleen activated with PMA and Ionomycin secreted similar amounts of IFN-γ in vitro, but NZB/W NKT cells secreted fivefold higher amounts of IL-4 as compared with C57BL/6 mice, indicating a Th2-type cytokine profile. Thus, there is a discrepancy between cytokine profiles generated after αGalCer stimulation in vivo versus in vitro in NZB/W mice. Pure populations of NKT cells in NZB/W mice may be Th2-biased instead of Th1-biased. NK cells, however, present in higher levels in NZB/W as compared with C57BL/6 mice, may contribute to the higher serum levels of IFN-γ in NZB/W mice after αGalCer treatment in vivo. IFN-γ from the activated NKT cells have been found to initiate the activation and IFN-γ secretion of NK cells 90 minutes after αGalCer injection (60), and IFN-γ from NK cells was reported to enhance isotype switching to IgG2a (61, 62). It has also been reported that NKT cells can modulate the cytokine secretion capacity of dendritic cells (38, 63).
In the current study, adult NZB/W mice treated with a 6-month course of anti-CD1d mAb had increased serum levels of IgE and decreased levels of serum IgG2a and IgG2a anti-dsDNA Ab’s as compared with controls. This treatment significantly delayed the onset of proteinuria and mortality. The reduced serum levels of IgG2a Ab after anti-CD1d treatment suggests that suppression of in vivo spontaneous IFN-γ secretion occurs after blocking activation of CD1d-reactive NKT cells. It is not yet clear, however, whether the spontaneous IFN-γ secretion is directly from NKT cells themselves or from other cells such as NK cells.
The majority of the CD1 αGalCer tetramer-positive T cells in the NZB/W spleen were NK1.1–, although the majority of the tetramer-positive cells were NK1.1+ in C57BL/6 mice. The reduction of NK1.1 expression on tetramer-positive T cells may be explained by the downregulation of NK1.1 markers on the spontaneously activated tetramer-positive T cells in vivo. NKT cells have been reported to lose the NK1.1 marker after in vitro activation (54). On the other hand, tetramer-positive NK1.1– T cells in the thymus of nonautoimmune mice have been reported to be the immature precursors of the NK1.1+ tetramer-positive T cells (51–53). To determine whether the majority of NK1.1– tetramer-positive T cells in the spleen of NZB/W were the immature type, we analyzed additional surface markers and their cytokine secretion pattern. Although these cells expressed low levels of CD69, they expressed homogeneous high levels of CD44, a pattern that is atypical for either mature or immature NKT cells (51–53). Analysis of cytokine secretion showed that the NK1.1– tetramer-positive CD4 T cells in the NZB/W spleen made more IFN-γ and IL-4 than mature tetramer-positive NKT cells in the spleen of C57BL/6 mice. The vigorous secretion of IFN-γ indicates that these cells are more likely to be mature NKT cells (51–53) and are likely to have been activated in vivo.
In the current study, we demonstrated that CD1d-reactive tetramer-positive CD4+ T cells, accounting for about 5% of all NZB/W splenic CD4+ T cells, are required for the in vitro augmentation of IgM and IgM anti-dsDNA Ab secretion by splenic CD4+ T cells. One hypothesis that explains the experimental results is that CD1dhigh autoreactive B cells accumulate in the NZB/W spleen marginal zone and are stimulated to secrete IgM and then IgG autoantibodies by CD1d-reactive CD4+ NKT cells in adult mice. NKT cells may not be pathogenic in NZB/W mice, however, in the first few weeks of life, since αGalCer treatment of 4-week-old NZB/W mice did not significantly augment the development of lupus disease parameters. CD1d–/– NZB/W mice that are deficient in NKT cells have been reported recently to have more severe lupus as compared with CD1+/+ NZB/W mice (64). This raises the possibility that there is an ameliorating role for NKT cells in early stages of immune maturation and that NKT cells contribute to and modulate development of disease activity as the mice age.
Acknowledgments
We thank Aditi Mukhopadhyay for technical assistance and Mary Hansen for her assistance in preparation of the manuscript. We are grateful to Yasuhiko Koezuka at the Kirin Pharmaceutical Research Institute for providing us with αGalCer. This research was supported by an Arthritis Foundation Investigator Award (to D. Zeng), NIH grant CA-52511 (to M. Kronenberg), and NIH grant AI-40093 (to S. Strober).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: double-stranded DNA (dsDNA); natural killer T cells (NKT cells); α-galactosylceramide (αGalCer); experimental autoimmune encephalomyelitis (EAE).
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Theofilopoulos AN, Kofler R, Singer PA, Dixon FJ. Molecular genetics of murine lupus models. Adv. Immunol. 1989;46:61–109. doi: 10.1016/s0065-2776(08)60651-3. [DOI] [PubMed] [Google Scholar]
- 3.Hahn BH. Antibodies to DNA. N. Engl. J. Med. 1998;338:1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 4.O’Keefe TL, Datta SK, Imanishi-Kari T. Cationic residues in pathogenic anti-DNA autoantibodies arise by mutations of a germ-line gene that belongs to a large VH gene subfamily. Eur. J. Immunol. 1992;22:619–624. doi: 10.1002/eji.1830220302. [DOI] [PubMed] [Google Scholar]
- 5.Via CS, Shearer GM. T-cell interactions in autoimmunity: insights from a murine model of graft-versus-host disease. Immunol. Today. 1988;9:207–213. doi: 10.1016/0167-5699(88)91215-7. [DOI] [PubMed] [Google Scholar]
- 6.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J. Exp. Med. 1994;180:2341–2346. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendlovic S, et al. Induction of a systemic lupus erythematosus-like disease in mice by a common human anti-DNA idiotype. Proc. Natl. Acad. Sci. U. S. A. 1988;85:2260–2264. doi: 10.1073/pnas.85.7.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng D, et al. Subsets of transgenic T cells that recognize CD1 induce or prevent murine lupus: role of cytokines. J. Exp. Med. 1998;187:525–536. doi: 10.1084/jem.187.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ando DG, Sercarz EE, Hahn BH. Mechanisms of T and B cell collaboration in the in vitro production of anti-DNA antibodies in the NZB/NZW F1 murine SLE model. J. Immunol. 1987;138:3185–3190. [PubMed] [Google Scholar]
- 10.Borchers A, Ansari AA, Hsu T, Kono DH, Gershwin ME. The pathogenesis of autoimmunity in New Zealand mice. Semin. Arthritis Rheum. 2000;29:385–399. doi: 10.1053/sarh.2000.7173. [DOI] [PubMed] [Google Scholar]
- 11.Sobel ES, et al. An intrinsic B cell defect is required for the production of autoantibodies in the lpr model of murine systemic autoimmunity. J. Exp. Med. 1991;173:1441–1449. doi: 10.1084/jem.173.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roths JB, Murphy ED, Eicher EM. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J. Exp. Med. 1984;159:1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wofsy D, Kerger CE, Seaman WE. Monocytosis in the BXSB model for systemic lupus erythematosus. J. Exp. Med. 1984;159:629–634. doi: 10.1084/jem.159.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsao BP, et al. Structural characteristics of the variable regions of immunoglobulin genes encoding a pathogenic autoantibody in murine lupus. J. Clin. Invest. 1990;85:530–540. doi: 10.1172/JCI114469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theofilopoulos, A.N. 1992. Murine models of systemic lupus erythematosus. In Systemic lupus erythematosus. R.G. Lahita, editor. Churchill Livingstone. New York, New York, USA. 121 pp.
- 16.Wofsy D, Seaman WE. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J. Exp. Med. 1985;161:378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J. Exp. Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebling FM, Tsao BP, Singh RR, Sercarz E, Hahn BH. A peptide derived from an autoantibody can stimulate T cells in the (NZB × NZW)F1 mouse model of systemic lupus erythematosus. Arthritis Rheum. 1993;36:355–364. doi: 10.1002/art.1780360311. [DOI] [PubMed] [Google Scholar]
- 19.Singh RR, et al. T cell determinants from autoantibodies to DNA can upregulate autoimmunity in murine systemic lupus erythematosus. J. Exp. Med. 1995;181:2017–2027. doi: 10.1084/jem.181.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng D, Lee MK, Tung J, Brendolan A, Strober S. Cutting edge: a role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J. Immunol. 2000;164:5000–5004. doi: 10.4049/jimmunol.164.10.5000. [DOI] [PubMed] [Google Scholar]
- 21.Jacob CO, van der Meide PH, McDevitt HO. In vivo treatment of (NZB × NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J. Exp. Med. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engleman EG, et al. Treatment of NZB/NZW F1 hybrid mice with Mycobacterium bovis strain BCG or type II interferon preparations accelerates autoimmune disease. Arthritis Rheum. 1981;24:1396–1402. doi: 10.1002/art.1780241110. [DOI] [PubMed] [Google Scholar]
- 23.Datta SK, Patel H, Berry D. Induction of a cationic shift in IgG anti-DNA autoantibodies. Role of T helper cells with classical and novel phenotypes in three murine models of lupus nephritis. J. Exp. Med. 1987;165:1252–1268. doi: 10.1084/jem.165.5.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 25.Stevens TL, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 26.Mendiratta SK, et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 27.Porcelli SA. The CD1 family: a third lineage of antigen-presenting molecules. Adv. Immunol. 1995;59:1–98. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- 28.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 29.Kawano T, et al. CD1d-restricted and TCR-mediated activation of Valpha 14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 30.Joyce S, et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998;279:1541–1544. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- 31.Gumperz JE, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 32.Burdin N, Brossay L, Kronenberg M. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 1999;29:2014–2025. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 33.Singh N, et al. Cutting edge: activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 1999;163:2373–2377. [PubMed] [Google Scholar]
- 34.Hong S, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat. Med. 2001;7:1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 35.Sharif S, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune type 1 diabetes. Nat. Med. 2001;7:1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 36.Wang B, Geng YB, Wang CR. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J. Exp. Med. 2001;194:313–320. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jahng AW, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naumov YN, et al. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13838–13843. doi: 10.1073/pnas.251531798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng D, et al. Heterogeneity of NK1.1+ T cells in the bone marrow: divergence from the thymus. J. Immunol. 1999;163:5338–5345. [PubMed] [Google Scholar]
- 40.Zeng D, et al. Bone marrow NK1.1– and NK1.1+ T cells reciprocally regulate acute graft versus host disease. J. Exp. Med. 1999;189:1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng D, et al. Unique patterns of surface receptors, cytokine secretion, and immune functions distinguish T cells in the bone marrow from those in the periphery: impact on allogeneic bone marrow transplantation. Blood. 2002;99:1449–1457. doi: 10.1182/blood.v99.4.1449. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda JL, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brossay L, et al. Mouse CD1 is mainly expressed on hemopoietic-derived cells. J. Immunol. 1997;159:1216–1224. [PubMed] [Google Scholar]
- 44.Cui J, et al. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Valpha14 natural killer T cells. J. Exp. Med. 1999;190:783–792. doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coffman RL, Lebman DA, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 1993;54:229–270. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 46.Steward MW, Hay FC. Changes in immunoglobulin class and subclass of anti-DNA antibodies with increasing age in N/ZBW F1 hybrid mice. Clin. Exp. Immunol. 1976;26:363–370. [PMC free article] [PubMed] [Google Scholar]
- 47.Reininger L, et al. Intrinsic B cell defects in NZB and NZW mice contribute to systemic lupus erythematosus in (NZB × NZW)F1 mice. J. Exp. Med. 1996;184:853–861. doi: 10.1084/jem.184.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 49.Clynes R, et al. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J. Exp. Med. 1999;189:179–185. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szalay G, et al. Cutting edge: anti-CD1 monoclonal antibody treatment reverses the production patterns of TGF-beta 2 and Th1 cytokines and ameliorates listeriosis in mice. J. Immunol. 1999;162:6955–6958. [PubMed] [Google Scholar]
- 51.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 52.Pellicci DG, et al. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1–CD4+ CD1d-dependent precursor stage. J. Exp. Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gadue P, Stein PL. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J. Immunol. 2002;169:2397–2406. doi: 10.4049/jimmunol.169.5.2397. [DOI] [PubMed] [Google Scholar]
- 54.Chen H, Huang H, Paul WE. NK1.1+CD4+ T cells lose NK1.1 expression upon in vitro activation. J. Immunol. 1997;158:5112–5119. [PubMed] [Google Scholar]
- 55.Amano M, et al. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: beta 2-microglobulin-dependent and independent forms. J. Immunol. 1998;161:1710–1717. [PubMed] [Google Scholar]
- 56.Roark JH, et al. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J. Immunol. 1998;160:3121–3127. [PubMed] [Google Scholar]
- 57.Peng SL, Moslehi J, Craft J. Roles of interferon-gamma and interleukin-4 in murine lupus. J. Clin. Invest. 1997;99:1936–1946. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santiago ML, et al. Interleukin-4 protects against a genetically linked lupus-like autoimmune syndrome. J. Exp. Med. 1997;185:65–70. doi: 10.1084/jem.185.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 60.Carnaud C, et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 61.Amigorena S, Bonnerot C, Fridman WH, Teillaud JL. Recombinant interleukin 2-activated natural killer cells regulate IgG2a production. Eur. J. Immunol. 1990;20:1781–1787. doi: 10.1002/eji.1830200824. [DOI] [PubMed] [Google Scholar]
- 62.Wilder JA, Koh CY, Yuan D. The role of NK cells during in vivo antigen-specific antibody responses. J. Immunol. 1996;156:146–152. [PubMed] [Google Scholar]
- 63.Racke FK, Clare-Salzer M, Wilson SB. Control of myeloid dendritic cell differentiation and function by CD1d-restricted (NK) T cells. Front. Biosci. 2002;7:d978–d985. doi: 10.2741/racke. [DOI] [PubMed] [Google Scholar]
- 64.Hong S, et al. Genetic deletion of CD1 in lupus: evidence of a regulatory role. Arthritis Rheum. 2001;44(Suppl.):283. (Abstr.) [Google Scholar]