Abstract
Aim
The Aim of this study was to measure circulating levels of glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), and glucagon in patients who had undergone adjustable gastric banding (BND) or Roux-en-Y gastric bypass (RYGB) in order to understand differences in glucose and insulin regulation after these procedures.
Methods
This was a cross-sectional study of three groups of women matched for age and body mass index: 1) overweight controls (OW, n = 13); 2) BND (n = 10); and 3) RYGB (n = 13). Venous blood was drawn in the fasted state and throughout a 3-h period after a liquid meal.
Results
Fasting glucose was similar between surgery groups, however, fasting insulin concentrations were greater in BND (10.0 μU/ml) compared with RYGB (6.2 μU/ml; P<0.05). Glucose at 60 minutes was significantly lower in RYGB (70 mg/dl; range 38–82) compared with BND (83 mg/dl; range 63–98). GLP-1 levels at 30 minutes were over three-fold higher in RYGB (96 pmol/l) compared with BND and OW (28 pmol/l) controls. GLP-1 and insulin concentrations correlated at 30 minutes only in RYGB (r=0.66; P=0.013). GIP levels at 30 minutes were lower in RYGB (20 pmol/l) compared with BND (31 pmol/l) and OW (33 pmol/l) controls. Peak glucagon levels were similar between groups.
Conclusions/interpretation
Exaggerated postprandial GLP-1 and blunted GIP secretion after RYGB may contribute to greater weight loss and improved glucose homeostasis in comparison to BND.
Keywords: gastric bypass, gastric banding, GLP-1, GIP, glucagon, incretins, bariatric surgery, hyperinsulinemia, glucose homeostasis, obesity
Introduction
The most common surgical weight loss procedures performed in the United States are Roux-en-Y gastric bypass (RYGB) and adjustable gastric banding (BND). In general, RYGB produces a greater reduction in body weight compared with BND. A meta-analysis of bariatric surgery outcomes reveals that diabetes is resolved in 84% of patients after RYGB and 48% after BND1. These different outcomes, and the observation that insulin therapy may often be discontinued shortly after surgery, prior to significant weight loss, beg the question whether improved insulin sensitivity may be a function of the type of bariatric procedure in addition to the degree of weight loss2.
We have previously reported that while fasting insulin concentrations were significantly lower in subjects who had undergone RYGB compared with BMI-matched subjects after BND, there was an exaggerated meal-stimulated insulin response in the former group3. In addition, fasting insulin concentrations and HOMA-IR levels were nearly identical between RYGB subjects and lean controls, yet insulin levels at 30 minutes post-meal were approximately 5.5 fold greater in the RYGB group3, 4. These results have prompted an examination of possible causes of enhanced insulin release after RYGB. Gastrointestinal peptides such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) act as incretins that stimulate insulin release in response to orally administered nutrients5, 6. The aim of the study was to examine the effects of BND and RYGB on fasting and postprandial levels of GLP-1, GIP and glucagon.
Subjects and methods
Study Subjects
Three groups of adult women were studied: Group One – overweight controls (OW) who were BMI and age-matched to subjects in Groups Two and Three (n = 13); Group Two -individuals post-BND (n = 10); and Group Three - individuals post-RYGB (n = 13). BND and RYGB groups were also matched for duration of the post-operative period. Surgical procedures for BND and RYGB have been previously described 3. Subjects were weight stable and were excluded if they had diabetes or used weight loss products within the prior 6 months. After an overnight fast subjects consumed a liquid test meal (Optifast, Novartis, Minneapolis, MN; 474 ml, 320 kcal, 50% carbohydrate, 35% protein, 15% fat). Venous blood was drawn pre-meal and 30, 60, 90, 120 and 180 minutes after meal consumption. All subjects signed an approved informed consent form.
Hormone Measurements
Blood was collected in EDTA tubes, centrifuged at 4° C and stored at −80° C until assayed. GIP, GLP-1 and glucagon concentrations were measured after extraction of plasma with 70% ethanol. For the GIP radioimmunoassay we used the C-terminally directed antiserum R 65, which cross-reacts fully with human GIP but not with the so-called GIP 8000, whose chemical nature and relationship to GIP secretion is uncertain7. The antiserum reacts equally with intact GIP and GIP 3–42, the primary metabolite. Human GIP was used for standards and tracer. GLP-1 concentrations were measured against standards of synthetic GLP-1(7–36 amide) using antiserum code no. 89390, which is specific for the amidated C-terminus of GLP-1 and therefore mainly reacts with GLP-1 of intestinal origin8. The assay reacts equally with intact GLP-1 and GLP-1(9–36 amide), the primary metabolite. Because of the rapid and intravascular conversion of both GIP and GLP-1 to their primary metabolites, it is essential to determine both the intact hormone and the metabolite for estimation of the rate of secretion of these hormones. Glucagon concentrations were measured using antiserum code no. 4305 directed against the C-terminus of the glucagon molecule and therefore mainly measures glucagon of pancreatic origin9. Sensitivity for the three assays was below 1 pmol/l, intra-assay coefficient of variation below 6% at 20–30 pmol/l, and recovery of standard, added to plasma before extraction, about 100% when corrected for losses inherent in the plasma extraction procedure. Plasma insulin was measured with the Immulite Analyzer with the lower limit of detection of 2 μU/ml. Serum glucose was measured by the hexokinase method.
Statistical Analysis
Significant differences between groups were determined by one-way ANOVA followed by Fisher’s Protected Least Difference Test. A P-value of <0.05 was considered statistically significant. Insulin resistance was calculated using the homeostasis model assessment (HOMA-IR; fasting insulin (μU/ml) × fasting glucose (mmol/l)/22.5)10. Mean values ± SEM are reported.
Results
Clinical characteristics of each study group are presented in Table 1. There were no significant differences in age, body weight or BMI between groups. The mean post-operative period was 23 ± 2 months (range, 15–36) for the BND, and 24.6 ± 2 months (range, 16–34) for the RYGB group. The RYGB group lost a significantly greater percentage of total body weight than the BND group.
Table 1.
Clinical characteristics of subjects
| OW Controls n=13 | BAND n=10 | BYPASS n=13 | |
|---|---|---|---|
| Age | 41.0 ± 4.1 [20 – 60] | 42.6 ± 4.6 [23 – 64] | 49.5 ± 2.4 [30 – 58] |
| Wt (kg) | 96.5 ± 5.9 [70 – 135] | 99.2 ± 6.1 [79 – 147] | 84.5 ± 4.3 [60 – 119] |
| BMI (kg/m2) | 36.1 ± 2.2 [26 – 51] | 36.1 ± 1.7 [28 – 43] | 31.3 ± 1.3 [26 – 40] |
| Wt loss (% total wt) | na | 24.6 ± 2.3** [15 – 36] | 35.6 ± 2.4 [23 – 51] |
| Fasting glucose (mg/dl) | 97.0 ± 2.0* | 91.9 ± 2.8 | 90.7 ± 2.0 |
| Fasting insulin (μIU/ml) | 13.8 ± 1.2***† | 10.0 ± 1.0* | 6.2 ± 0.7 |
| HOMA-IR | 3.3 ± 0.3***† | 2.3 ± 0.3* | 1.4 ± 0.2 |
Data are presented as mean ± SEM. Range is represented in brackets.
P<0.05;
P<0.01;
P<0.001 vs. bypass.
,P<0.05 vs. band.
OW controls had the highest levels of fasting glucose (Table 1). Postprandial glucose excursions differed between groups, such that at 60, 90 and 120 minutes, levels were significantly lower in RYGB compared with both the OW and BND groups (Fig. 1A). Fasting insulin concentrations and HOMA-IR were lowest in the RYGB group (Table 1). Peak insulin concentrations were greatest in RYGB (99 ± 20 μU/ml) but not statistically different from BND group (69 ± 12 μU/ml, P=0.17) and levels decreased rapidly and remained significantly lower at 90 – 180 minutes compared with both the OW and BND groups (Fig. 1B).
Fig. 1.
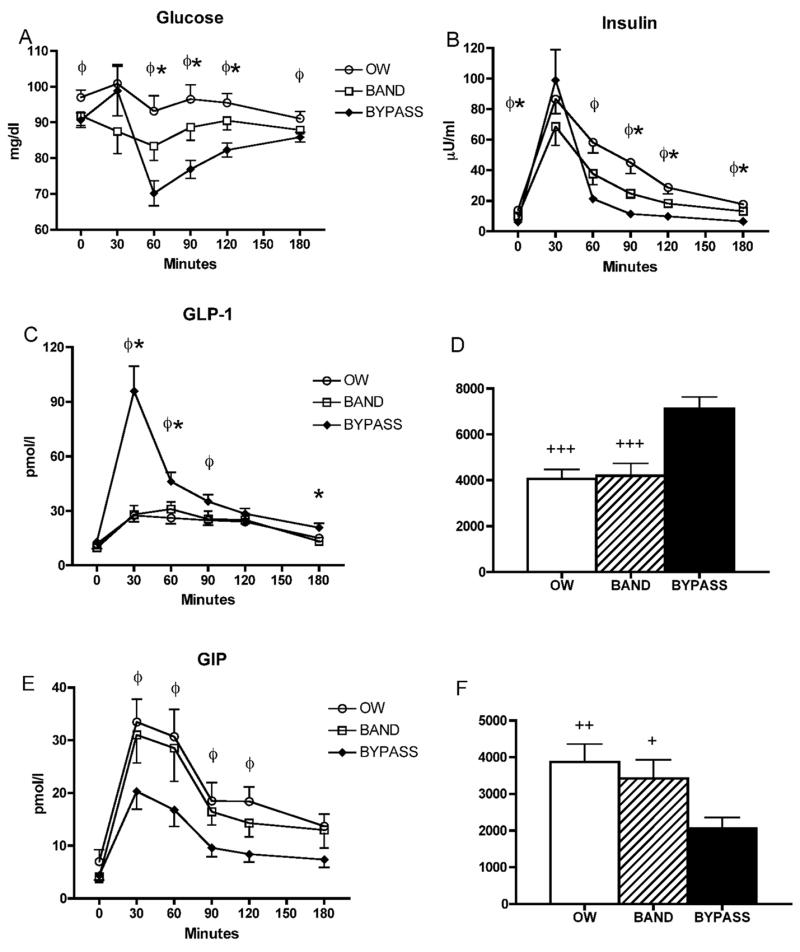
Circulating concentrations of glucose (A), insulin (B), GLP-1 (C), and GIP (E) in response to a test meal.φ,P<0.05 for OW vs RYGB; *,P<0.05 for BND vs RYGB. AUC from fasting to 180 minutes post-meal of GLP-1 (D) and GIP (F). +,P<0.05; ++,P<0.01; +++,P<0.001 vs. bypass.
Fasting levels of GLP-1 were similar between groups, but peak levels were three-fold higher in the RYGB compared with OW and BND groups at 30 minutes post-meal (Fig. 1C). AUC for the 180-minute period was significantly greater in RYGB compared with both other groups (Fig. 1D). GLP-1 concentrations at 30 minutes correlated significantly with insulin concentrations at 30 minutes only in the RYGB group (r=0.663; P=0.013). Fasting levels of GIP tended to be lowest in BND (4.1 ± 1.1) and RYGB (4.4 ± 0.9) compared with OW controls (7.0 ± 2.2 pmol/l), but these differences did not reach statistical significance. All subjects exhibited a postprandial rise in GIP levels, however, peak levels at 30 minutes were blunted by over 35% in RYGB compared with OW and BND groups (Fig. 1E); AUC was also significantly lower in the RYGB group (Fig. 1F). There was no significant correlation between peak GIP and peak insulin levels in any group. Fasting glucagon concentrations were similar between groups as was AUC over the 180 minute period, however, at 180 minutes glucagon was significantly lower (P<0.05) in RYGB (7.9 ± 1.0) compared with OW (13.1 ± 2.1) and BND (13.0 ± 1.8 pmol/l) groups (Fig. 2).
Fig. 2.
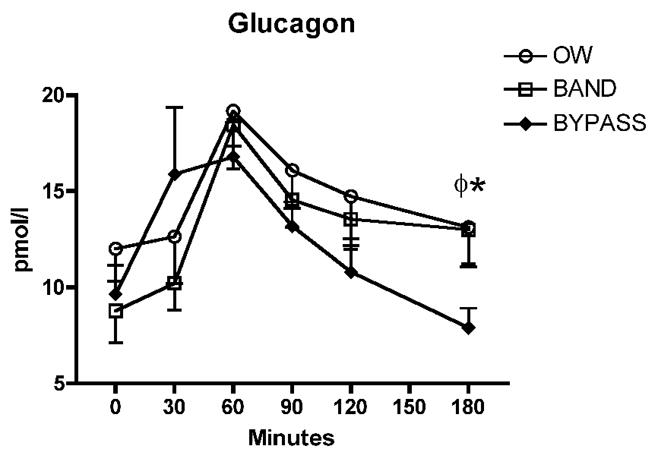
Circulating concentrations of glucagon in response to a test meal.φ,P<0.05 for OW vs RYGB; *P<0.05 for BND vs RYGB.
Discussion
We have shown that RYGB is associated with unique changes in postprandial plasma concentrations of incretin hormones in comparison with BND subjects and overweight controls matched for post-operative BMI. RYGB subjects exhibited an exaggerated GLP-1 response and a suppression of GIP secretion after administration of a test meal. Our previous studies showed significantly greater insulin levels 30 minutes after identical test meals in RYGB compared with both BND and BMI-matched controls3,4. While peak insulin levels were also greatest in RYGB in this study, the differences did not reach statistical significance. More detailed studies are necessary to better characterize glucose metabolism and insulin sensitivity in these subjects, however, the lower HOMA-IR in RYGB subjects is suggestive of greater insulin sensitivity compared with BND. If this is the case, one may have predicted less postprandial insulin secretion in RYGB. In contrast, there was at least equal, if not greater, postprandial insulin secretion in the RYGB group. Given that GLP-1 augments insulin secretion it is possible that enhanced secretion of this incretin, rather than insulin resistance, is the stimulus for much of the insulin response in RYGB subjects. In support of this notion is the finding that in this group only, peak GLP-1 levels correlated with insulin concentrations. The initial rise in glucose concentration in RYGB compared with BND may have also contributed to increased insulin secretion.
It has been suggested that in the setting of improved insulin sensitivity post-RYGB, enhancement of GLP-1 secretion may on rare occasions result in hyperinsulinemic hypoglycemia11–14. None of our RYGB participants experienced clinical dumping syndrome, but their glucose levels were significantly lower than both other groups at 60, 90 and 120 minutes, with the lowest values for two different subjects being 38 and 48 mg/dl. Although GLP-1 may also inhibit glucagon secretion, we observed an early robust increase in glucagon levels in the RYGB group, indicating that lower levels of glucose in the RYGB group were not due to impaired glucagon secretion. At 180 minutes, however, lower glucagon levels in the RYGB group (perhaps as a result of elevated GLP-1) may have contributed to lower glucose levels. GIP is another gut hormone that stimulates glucose-dependent insulin secretion, however, we show here that postprandial concentrations are significantly decreased in the RYGB group. The blunted GIP response may help abet further decreases in glucose concentrations. Other glucoregulatory factors, such as cortisol, growth hormone and autonomic nervous system function were not examined in this study.
What accounts for the differences in incretin secretion in our study groups? The most obvious answer is the different paths of nutrient flow. After bypass, nutrients pass directly from the gastric pouch to the distal small intestine. Thus, delivery of concentrated nutrients to L cells in the distal small intestine where GLP-1 is primarily produced may enhance GLP-1 secretion, as was described after jejunoileal bypass15 and total gastrectomy16. It has also been shown that postprandial levels of peptide YY (PYY), which is also produced by L cells, are enhanced after RYGB compared BND and lean subjects, and BMI-matched controls3, 17. Since GIP is synthesized primarily in the proximal small intestine, diversion of nutrients from this segment would be expected to reduce postprandial GIP levels as we have shown. It is unlikely that the difference in weight loss contributed to the different patterns of incretin secretion given that the degree of weight loss did not correlate with either GLP-1 or GIP secretion.
Other studies have also examined the effect of bariatric surgery on gastrointestinal peptide hormones18 and have shown that GLP-1 secretion is enhanced after RYGB17, 19 although to a much lesser degree (less than three-fold over baseline) than the nearly ten-fold increase we observed. After biliopancreatic diversion, a malabsorptive bariatric procedure that bypasses the foregut and part of the hindgut, fasting GLP-1 levels were shown to increase compared to pre-surgical values, however, circulating levels after an OGTT were nearly flat-line20. Postprandial GLP-1 and enteroglucagon levels also increase after jejunoileal bypass, whereas results of this procedure on GIP concentrations are inconsistent18. We are not aware of other studies that examine the effect of RYGB on GIP in non-diabetic subjects. Animal models such as ileal transposition in rodents also show that expedited delivery of nutrients to a segment of foregut increases levels of hormones synthesized in the hindgut, such as GLP-1, enteroglucagon and PYY21, 22.
Bariatric surgery, particularly RYGB, confers long-term maintenance of a reduced body weight and improvement in glucose homeostasis. GLP-1 analogues and methods to decrease the inactivation of GLP-1 by dipeptidyl peptidase IV (DPP IV) are now in clinical use and are being extensively studied for the treatment of type 2 diabetes23. Manipulation of incretin hormone concentrations may also affect body weight. For example, the use in humans of the GLP-1 receptor agonist, exenatide, is associated with weight loss23, and mice lacking the GIP receptor are resistant to diet-induced obesity24. Our results suggest that enhancement of postprandial GLP-1 concentrations after RYGB and suppression of GIP secretion may contribute to increased weight loss and improved glucoregulation.
Acknowledgments
We would like to acknowledge the participants of this study and the excellent technical assistance of Lene Albaek, Lone Bagger Thielsen and Robert Sundeen. This work was supported by National Institutes of Health grants RO1-DK072011 and RR00645 (to General Clinical Research Center).
Abbreviations
- BND
adjustable gastric banding
- GIP
gastric inhibitory polypeptide
- GLP
glucagon-like peptide
- OW
overweight
- RYGB
Roux-en-Y gastric bypass
Footnotes
Disclosures:
Dr. Bessler has received lecture fees from Ethicon and Inamed
Dr. Inabnet has received consulting fees from the Surgical Review Corporation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–15. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 3.Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity. 2006;14:1553–61. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 4.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–65. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology. 2001;142:521–7. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- 6.Meier JJ, Nauck MA. GIP as a potential therapeutic agent? Horm Metab Res. 2004;36:859–66. doi: 10.1055/s-2004-826176. [DOI] [PubMed] [Google Scholar]
- 7.Krarup T, Madsbad S, Moody AJ, et al. Diminished immunoreactive gastric inhibitory polypeptide response to a meal in newly diagnosed type I (insulin-dependent) diabetics. J Clin Endocrinol Metab. 1983;56:1306–12. doi: 10.1210/jcem-56-6-1306. [DOI] [PubMed] [Google Scholar]
- 8.Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–9. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 9.Holst JJ. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33–69) of glicentin. Biochem J. 1982;207:381–8. doi: 10.1042/bj2070381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–54. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 12.Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes Care. 2006;29:1554–9. doi: 10.2337/dc06-0392. [DOI] [PubMed] [Google Scholar]
- 13.Patti ME, McMahon G, Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48:2236–40. doi: 10.1007/s00125-005-1933-x. [DOI] [PubMed] [Google Scholar]
- 14.Cummings DE. Gastric bypass and nesidioblastosis--too much of a good thing for islets? N Engl J Med. 2005;353:300–2. doi: 10.1056/NEJMe058170. [DOI] [PubMed] [Google Scholar]
- 15.Holst JJ, Sorensen TI, Andersen AN, et al. Plasma enteroglucagon after jejunoileal bypass with 3:1 or 1:3 jejunoileal ratio. Scand J Gastroenterol. 1979;14:205–7. doi: 10.3109/00365527909179871. [DOI] [PubMed] [Google Scholar]
- 16.Miholic J, Orskov C, Holst JJ, Kotzerke J, Meyer HJ. Emptying of the gastric substitute, glucagon-like peptide-1 (GLP-1), and reactive hypoglycemia after total gastrectomy. Dig Dis Sci. 1991;36:1361–70. doi: 10.1007/BF01296800. [DOI] [PubMed] [Google Scholar]
- 17.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aylwin S. Gastrointestinal surgery and gut hormones. Curr Opin Endocrinol Diabetes. 2005;12:89–98. [Google Scholar]
- 19.Morinigo R, Moize V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–40. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 20.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–31. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 21.Koopmans HS, Ferri GL, Sarson DL, Polak JM, Bloom SR. The effects of ileal transposition and jejunoileal bypass on food intake and GI hormone levels in rats. Physiol Behav. 1984;33:601–9. doi: 10.1016/0031-9384(84)90378-0. [DOI] [PubMed] [Google Scholar]
- 22.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–53. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 23.Todd JF, Bloom SR. Incretins and other peptides in the treatment of diabetes. Diabet Med. 2007;24:223–32. doi: 10.1111/j.1464-5491.2006.02071.x. [DOI] [PubMed] [Google Scholar]
- 24.Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–42. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]


