Abstract
The lipid phosphatidylinositol 4,5-bisphosphate (PIP2) is critical for a number of physiological functions, and its presence in membrane microdomains (rafts) appears to be important for several of these spatially localized events. However, lipids like PIP2 that contain polyunsaturated hydrocarbon chains are usually excluded from rafts, which are enriched in phospholipids (such as sphingomyelin) containing saturated or monounsaturated chains. Here we tested a mechanism by which multivalent PIP2 molecules could be transferred into rafts through electrostatic interactions with polybasic cytoplasmic proteins, such as GAP-43, which bind to rafts via their acylated N-termini. We analyzed the interactions between lipid membranes containing raft microdomains and a peptide (GAP-43P) containing the linked N-terminus and the basic effector domain of GAP-43. In the absence or presence of nonacylated GAP-43P, PIP2 was found primarily in detergent-soluble membranes thought to correspond to nonraft microdomains. However, when GAP-43P was acylated by palmitoyl coenzyme A, both the peptide and PIP2 were greatly enriched in detergent-resistant membranes that correspond to rafts; acylation of GAP-43P changed the free energy of transfer of PIP2 from detergent-soluble membranes to detergent-resistant membranes by −1.3 kcal/mol. Confocal microscopy of intact giant unilamellar vesicles verified that in the absence of GAP-43P PIP2 was in nonraft microdomains, whereas acylated GAP-43P laterally sequestered PIP2 into rafts. These data indicate that sequestration of PIP2 to raft microdomains could involve interactions with acylated basic proteins such as GAP-43.
INTRODUCTION
Membrane microdomains (“rafts”) are thought to play critical roles in a variety of biological functions such as signal transduction (1–6), cytoskeletal organization (7–9), lipid sorting (10,11), and protein trafficking/recycling (12–14). The composition of membrane rafts is often characterized by analysis of detergent-resistant membranes (DRMs) that are obtained by detergent extraction at 4°C (15–20). DRMs have been found to be enriched in cholesterol and sphingolipids such as sphingomyelin (SM) that primarily have saturated hydrocarbon chains, whereas detergent-soluble membranes (DSMs) are enriched in membrane phospholipids, such as phosphatidylcholine (PC), that contain unsaturated hydrocarbon chains (15,21,22). In addition, several classes of proteins are enriched in DRMs, including acylated cytoplasmic proteins (4,19,23).
A key lipid component of rafts is phosphatidylinositol 4,5-bisphosphate (PIP2), which is critical to a number of important cell functions such as production of second messengers (24–26), enzyme activation (27), regulation of potassium and transient receptor potential (TRP) channels (28–31), membrane trafficking (27,32,33), and regulation of the actin cytoskeleton (7–9,34). The presence of PIP2 in rafts appears to be critical in a number of these spatially localized membrane functions (7–9,26,32,35), although it is important to note that not all PIP2 is localized in rafts (36). Considering only its polyunsaturated hydrocarbon chain composition, one might expect PIP2 to be preferentially localized in nonraft bilayers, as rafts are composed primarily of cholesterol and phospholipids containing saturated and monounsaturated hydrocarbon chains. That is, as noted by Shaw et al. (37), based on partitioning experiments with lipids of different hydrocarbon chain compositions (38,39) it seems likely that the four double bonds typically found in the sn-2 hydrocarbon chain of PIP2 would make it energetically unfavorable for PIP2 to reside in cholesterol-enriched raft domains as compared to nonraft domains.
PIP2 has been shown (7,27,37,40–43) to strongly bind to several cytoplasmic proteins, such as myristoylated alanine-rich protein kinase C substrate (MARCKS), cortical associated protein of 23 kDa (CAP-23), neural axonal myristoylated protein of 22 kDa (NAP-22), and growth associated protein of 43 kDa (GAP-43). These proteins all contain clusters of basic amino acid residues, often called “basic effector domains” (7,27,40–43). In bilayers containing a single lipid domain, it has been shown that peptides containing such basic effector domains laterally sequester PIP2 in the plane of the bilayer, even in the presence of larger concentrations of monovalent negatively charged phospholipids such as phosphatidylglycerol or phosphatidylserine (40–43). GAP-43 is of special interest since it is an abundant raft-associated protein in developing and regenerating neurons and is involved in the development of axons and nerve growth cones (44,45), in nerve regeneration after injury (46,47), and in the regulation of neurotransmitter release (48). Moreover, it has been shown that GAP-43 associates with rafts due to dual palmitoylation of its N-terminus (48,49).
In this article we experimentally test the hypothesis that upon acylation GAP-43 transfers PIP2 from nonraft into raft microdomains. For these experiments we use detergent extraction procedures to determine quantitatively the localization of PIP2 in DRMs and DSMs extracted from bilayers containing microdomains. We also use confocal microscopy to determine the location of fluorescently labeled PIP2 in raft and nonraft microdomains in intact vesicles of the same lipid compositions. We measure the interactions of these bilayers with a peptide (GAP-43P) that contains both the N-terminus and basic effector domains of GAP-43. The N-terminus of GAP-43P contains two cysteine residues that can be acylated with palmitoyl coenzyme A. Before and after palmitoylation of GAP-43P, we quantitatively determine the distribution in DRMs and DSMs of both PIP2 and peptide, and calculate the free energy of transfer of PIP2 between these domains in the presence and absence of peptide.
MATERIALS AND METHODS
Materials
1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DOPG), sphingomyelin (SM), cholesterol (Chol), n-palmitoyl-sphingosine-1-succinyl (methoxy (polyethylene glycol))-2000 (PEG-Cer), phosphatidylinositol 4,5-biphosphate (PIP2), and palmitoyl coenzyme A (CoA) were purchased from Avanti Polar Lipids (Alabaster, AL). Phosphatidylinositol 4,5-biphosphate (myo-inositol-2-3H(N)) (tritiated PIP2) was obtained from American Radiolabeled Chemicals (St. Louis, MO). For confocal microscopy experiments, red fluorescently labeled PIP2 (BODIPY TMR-PIP2-C16) and the green lipid fluorescent marker 3,3′-dilinoleoyloxacarbocyanine perchlorate (DiO) were purchased from Echelon Research Laboratories (Salt Lake City, UT) and Molecular Probes (Eugene, OR), respectively. All lipids were used without further purification.
Phosphorus standard solution and silica gel coated thin layer chromatography plates were purchased from Sigma-Aldrich (St. Louis, MO). The 32-amino-residue peptide Ac-MLCCMRRTKQAHKAATKIQASFRGHITRKKLK-NH2 was synthesized and purified by Bachem California (Torrance, CA). This peptide, which we refer to as GAP-43P, is a fusion of two functionally important regions of GAP-43. Residues 1–10 of the peptide correspond to the N-terminus (membrane binding site) of GAP-43, which contains two cysteine residues known to be acylated in vivo (48,49). The next 22 residues of the peptide correspond to the protein's basic effector domain (residues 31–52 in human GAP-43), containing the PIP2 binding activity. The 2-D Quant kit for peptide assay was purchased from Amersham Biosciences (Piscataway, NJ).
Preparation of sucrose-loaded large unilamellar vesicles
Large unilamellar vesicles (LUVs) were prepared by standard procedures as follows. The appropriate lipids were codissolved in chloroform:methanol (2:1 v/v). Our standard lipid mixtures were DOPC/DOPG/SM/Chol (30:10:30:30, molar ratio) or DOPC/DOPG/SM/Chol/PIP2 (30:10:30:30:1), both with 10-wt% PEG-Cer added to prevent aggregation of the LUVs. After the solvent was removed by rotary evaporation, the dry lipid film was hydrated with 48 mM sucrose in 50 mM Tris buffer, pH 8.0, with five freeze-thaw cycles. The resulting dispersions, at concentrations of 15–20 mg/ml, were extruded 11 times through two stacked polycarbonate filters with pore size of 100 nm to form LUVs. The sucrose solution on the outside of the LUVs was removed by first diluting five times with an isoosmotic buffer (25 mM KCl in 50 mM Tris buffer, pH 8.0), centrifuging at 115,000 × g at 10°C for 1 h, and then resuspending the pellets with an appropriate amount of 25 mM KCl, 50 mM Tris, pH 8.0, buffer (50). The phospholipid content of the LUVs was determined by phosphate assay (51).
GAP-43 peptide binding
The binding to LUVs of either unacylated or acylated GAP-43P was performed with an initial lipid/peptide molar ratio of 50:1 using a centrifugation procedure (50). For the case of unacylated peptide, GAP-43P was mixed with sucrose-loaded LUVs in 25 mM KCl, 50 mM Tris buffer, pH 8.0, and incubated at room temperature for 1 h. In separate experiments, GAP-43P was acylated (palmitoylated) using similar procedures to those described by Quesnel and Silvius (52) by mixing palmitoyl CoA and GAP-43P at a 5:1 molar ratio and incubating with the LUV suspension at 37°C for 1 h. For experiments in either the presence of absence of palmitoyl CoA, bound peptide was separated from unbound peptide by ultracentrifugation for 1 h at 115,000 × g at 10°C. The supernatant (containing unbound peptide) and the pellet (containing LUVs and bound peptide) were collected and the pellet was resuspended in 25 mM KCl, 50 mM Tris buffer, pH 8.0. For both the supernatant and pellet the phospholipid content was determined by phosphate analysis and GAP-43P concentration was analyzed with the 2-D Quant assay. For each assay appropriate standard curves were run for GAP-43P in buffer or in buffer with palmitoyl CoA, with the reaction time for the last step of the assay being the same for each standard curve and sample analysis.
After the incubation of GAP-43P with palmitoyl CoA and LUVs, mass spectrometry was used to verify that the GAP-43P had indeed been palmitoylated. All samples were run on an Applied Biosystems (Foster City, CA) Voyager DE-Pro that was calibrated in linear mode immediately before acquiring the sample spectra. The accelerating voltage was 20,000 volts, the delay time was 100 ns, and the scan range was 500–4000 Da. The matrix used was a saturated solution of 4-hydroxy-alpha-cyano-cinnamic acid purchased from Sigma-Aldrich (MALDI-MS grade). Samples were mixed with the matrix in a ratio of 1:3 and a 1.2 μL aliquot of the solution was spotted on the plate and allowed to dry. A nitrogen laser was light fired onto the spot. All spectra were averages of a minimum of three spectra summed to provide better mass accuracy. In the absence of palmitoyl CoA, spectra of GAP-43P in solution with or without added LUVs displayed a sharp peak at a mass of 3783 Da, corresponding to the expected molecular weight of the peptide. In the presence of palmitoyl CoA an additional large sharp peak was observed at a mass of 4263 Da, corresponding to a doubly palmitoylated GAP-43P. Other smaller peaks were recorded at 4041, 4551, and 4788 Da, corresponding to the peptide containing one, three, or four palmitates. Doubly palmitoylated peptide would be expected since this peptide has two cysteines near the N-terminus and Quesnel and Silvius (52) showed that cysteine residues are palmitoylated by this procedure. Peptides with one palmitate indicate peptides with incomplete acylation of their cysteines, whereas the peptides with three or four palmitates could possibly correspond to additional acylation of lysine residues, as lysines near or within a cluster of basic residues could have a lowered pKa and might also be acylated (Dr. John Silvius, personal communication, 2006).
Detergent extraction and chemical analysis of DRMs and DSMs
Detergent extraction experiments were conducted as follows: LUVs with or without bound GAP-43P were treated with 1% Triton X-100 for 30 min at 4°C and then centrifuged at 15,000 × g for 1 h at 4°C. The supernatant was removed and collected, and the pellets were resuspended with an equal volume of buffer and then vortexed. For both the supernatant (containing detergent-soluble membranes or DSMs) and the resuspended pellets (containing detergent-resistant membranes or DRMs) the phospholipid contents were determined by phosphate assay (51) and the peptide concentrations were analyzed using the 2-D Quant assay. We (53) have previously used both wide-angle and low-angle x-ray diffraction analysis to demonstrate that DRMs prepared in this way are both liquid and more ordered than the DSMs from this lipid system. For each 2-D Quant assay, appropriate standard curves were run for GAP-43P in buffer, in buffer with palmitoyl CoA, in buffer with 1% Triton X-100, or in buffer with palmitoyl CoA and 1% Triton X-100.
To estimate the distribution of DOPC, DOPG, and SM in DSMs and DRMs, thin layer chromatography (TLC) was employed with the solvent chloroform/methanol/ammonium hydroxide 65:25:4 (v:v:v). The DSMs and DRMs were lyophilized, dissolved in the TLC solvent, and loaded onto the TLC plates so that each lane contained the same amount of total phospholipid. Iodine vapor was used to detect the lipid and peptide spots, with control lanes containing only DOPC, DOPG, SM, or GAP-43P identifying the relative positions of these components. The relative intensities of the peaks in TIFF images of the plates were determined by measuring peak areas with NIH Image Version 1.61.
To determine the distribution of PIP2 in the DRMs and DSMs, 0.1% tritiated PIP2 was added to the appropriate lipid mixtures. After GAP-43P binding and detergent extraction, we employed a Packard (Meriden, CT) Tri-Carb 2100TR liquid scintillation analyzer to determine the radioactivity of the tritiated PIP2 in the DRMs and DSMs. Usually 10–15 μL of each sample was added into 3 mL of the high flash point cocktail Safety-Solve scintillation solution (Research Products International, Mount Prospect, IL.) and measured with the scintillation counter.
Calculations of molar partition coefficients and apparent free energies of transfer
The mol-fraction partition coefficient for GAP-43P from DSM to DRM was calculated from
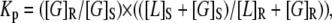 |
(1) |
where [G]R and [G]S represent the molar concentrations of GAP-43P in the DRM and DSM phase, respectively, and [L]R and [L]S are the molar concentrations of total lipid (phospholipid plus cholesterol) in the DRM and DSM phase, respectively. The phospholipid content in DRMs and DSMs was determined by phosphate assay (51) and the cholesterol content was calculated based on our previous measurements of cholesterol/total lipid (0.2 for DSMs and 0.4 for DRMs) for similar lipid systems (54). The mol-fraction partition coefficient for PIP2 from DSM to DRM was calculated from
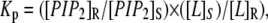 |
(2) |
where [PIP2]R and [PIP2]S represent the molar concentrations of PIP2 in the DRM and DSM phase, respectively. The apparent free energies of transfer from DSMs to DRMs were obtained using
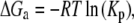 |
(3) |
where R is the molar gas constant and T is temperature in degrees Kelvin. The thermal energy (RT) is 0.55 kcal/mol at 277 °K (4°C). An implicit extra thermodynamic assumption of these calculations is that the presence of 1% Triton X-100 does not alter the distribution (or Kp) of the lipids or peptides between DSMs and DRMs. We therefore refer to the energies as apparent free energies (ΔGa).
Confocal microscopy
Confocal microscopy was used to determine the distribution of BODIPY TMR-labeled PIP2 in intact raft-containing giant unilamellar vesicles (GUVs) in the presence and absence of GAP-43P. GUVs were made following the procedures of Akashi et al. (55) with slight modifications. Small amounts (typically <1 mol %) of BODIPY TMR-PIP2 and DiO were added to DOPC/DOPG/SM/Chol/PEG-ceramide in chloroform/methanol. These chloroform/methanol solutions were deposited on a roughened Teflon surface and allowed to dry under vacuum; 100 mM sucrose was then added at 37°C and incubated for 2–3 h. The resulting lipid cloud that detached from the Teflon plate contained GUVs. This lipid cloud was carefully collected in plastic tubes and prepared for microscopy by diluting with 100 mM glucose (external solution), placing on a microscope slide, and then covering with a glass coverslip. Since the inside of the vesicles contained sucrose and the outside contained the lower density glucose, the GUVs sank to the microscope slide.
The GUVs were observed with a 63× NA 1.4 Plan Apochromat oil objective on a LSM 510 Meta Zeiss (Jena, Germany) confocal microscope. Configurations for double channel excitation and the choice of emission of the fluorochromes were made to prevent cross talk and the two colors were scanned using multitrack line switching. The green DiO lipid labels were observed with the use of a 488-nm filter, whereas the red-labeled BODIPY TMR-PIP2 was visualized with a 543-nm filter. Quantification of lipid probe colocalization was performed with the Zeiss LSM AIM 3.2 enhanced colocalization software that permitted the determination of the number of green and red pixels in each region of the bilayer. To determine if the proportion of DiO and BODIPY TMR-PIP2 pixels were significantly different in the observed bilayer microdomains, we employed a sample test for equality of proportions without continuity correction using the χ-square test (α = 0.05%) (56,57).
RESULTS
Peptide binding
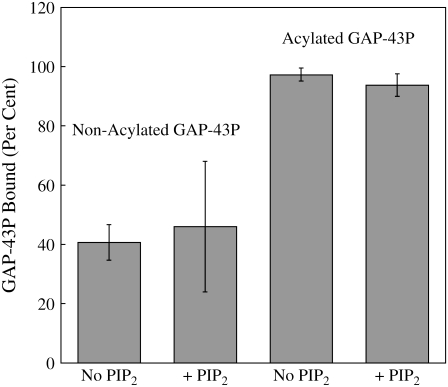
Fig. 1 shows the percentage of nonacylated and acylated GAP-43P bound to DOPC/DOPG/SM/Chol LUVs in the presence or absence of PIP2. The addition of 1% PIP2 to these bilayers caused no significant change in GAP-43P binding. However, acylation of GAP-43P produced a large increase in binding. For example, in the absence of PIP2 only 43% GAP-43P bound to DOPC/DOPG/SM/Chol bilayers, whereas 97% of acylated GAP-43P bound to bilayers of that composition.
FIGURE 1.
Percentage of acylated or nonacylated GAP-43P bound to LUVs containing 30:10:30:30 DOPC/DOPG/SM/Chol or 30:10:30:30:1 DOPC/DOPG/SM/Chol/PIP2. Each bar indicates mean ± SD for three to five experiments.
Distribution of phospholipids, GAP-43P, and PIP2 between DRMs and DSMs
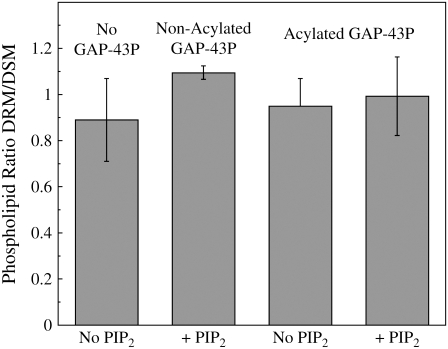
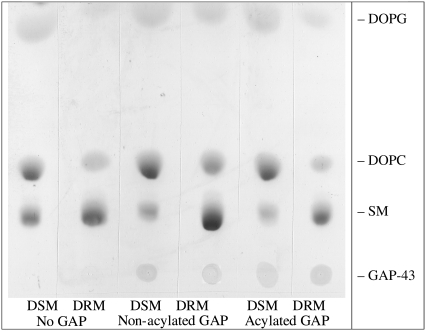
After detergent extraction, phosphate analysis showed that for all the tested systems the total phospholipids were more or less equally distributed between the DSMs and DRMs (Fig. 2). Thin layer chromatography (Fig. 3) was used to determine the distributions of DOPC, DOPG, and SM in DSMs and DSMs obtained from three DOPC/DOPG/SM/Chol preparations: 1), with no GAP-43P, 2), with nonacylated GAP-43P, and 3), with acylated GAP-43P. For each system SM was enriched in the DRMs, whereas DOPC and DOPG were enriched in the DSMs (Fig. 3 and Table 1). In addition, the presence of GAP-43P could be observed at the origin of the relevant TLC lanes. Nonacylated GAP-43P was preferentially observed in DSMs, whereas acylated GAP-43P was enriched in DRMs (Fig. 3; Table 1).
FIGURE 2.
The phospholipid ratio in DRMs/DSMs extracted from LUVs containing 30:10:30:30 DOPC/DOPG/SM/Chol or 30:10:30:30:1 DOPC/DOPG/SM/Chol/PIP2 in the absence GAP-43P, in the presence of nonacylated GAP-43P, or in the presence of acylated GAP-43P. Each bar indicates mean ± SD for three to five experiments.
FIGURE 3.
Thin layer chromatogram of DRMs and DSMs from 30:10:30:30 DOPC/DOPG/SM/Chol in the absence GAP-43P, in the presence of nonacylated GAP-43P, or in the presence of acylated GAP-43P.
TABLE 1.
Density ratios (DRM/DSM) of spots in TLC
| Molecule | No GAP-43P | Nonacylated GAP-43P | Acylated GAP-43P |
|---|---|---|---|
| DOPC | 0.34 ± 0.06 | 0.52 ± 0.14 | 0.28 ± 0.12 |
| DOPG | 0.35 ± 0.07 | 0.49 ± 0.19 | 0.29 ± 0.04 |
| SM | 1.90 ± 0.52 | 2.87 ± 1.15 | 2.10 ± 0.04 |
| GAP-43P | – | 0.78 ± 0.44 | 1.40 ± 0.58 |
2D Quant analysis of the distribution of GAP-43P in DSMs and DRMs (Fig. 4) showed quantitatively that the acylation of GAP-43P had a marked effect on the distribution of the peptide in DSMs and DRMs. In the presence or absence of PIP2 the ratio of GAP-43P in DRMs to DSMs increased upon GAP-43P acylation (Fig. 4). For example, in the absence of PIP2 the DRM/DSM ratio of GAP-43P increased from <1 to >5.
FIGURE 4.
Distribution of nonacylated or acylated GAP-43P in DRMs and DSMs extracted from 30:10:30:30 DOPC/DOPG/SM/Chol or 30:10:30:30:1 DOPC/DOPG/SM/Chol/PIP2 LUVs. Each bar indicates mean ± SD for three to five experiments.
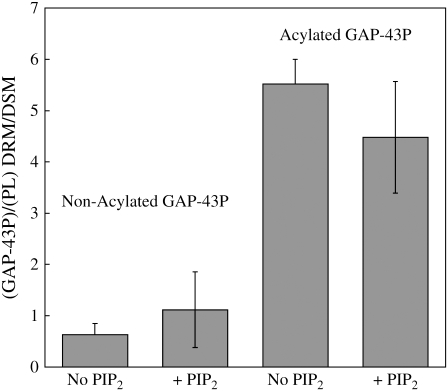
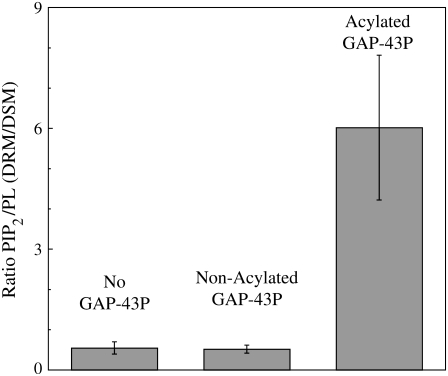
Experiments with tritiated PIP2 (Fig. 5) showed that, either in the absence or in the presence of nonacylated GAP-43P, PIP2 was enriched in DSMs compared to DRMs. That is, when normalized to the total phospholipid (PL) in each component, the PIP2/PL ratio was ∼0.5 for DRMs compared to DSMs. However, in the presence of acylated GAP-43P the PIP2 was highly enriched in DRMs compared to DSMs, so that the PIP2/PL ratio was ∼6.5 for DRMs compared to DSMs (Fig. 5).
FIGURE 5.
Distribution of radio-labeled PIP2 between DRMs and DSMs in the absence GAP-43P, in the presence of nonacylated GAP-43P, or in the presence of acylated GAP-43P. Each bar indicates mean ± SD for three to five experiments.
Apparent free energies of transfer of GAP-43P or PIP2 from DSMs to DRMs
With the assumption that the Triton X-100 does not change the composition of bilayer DRMs, the peptide and PIP2 distributions can be put on a thermodynamic basis by calculations of the molar partition coefficients (Kp) and apparent free energies of transfer (ΔGa) from DSMs to DRMs. Table 2 presents the molar partition coefficients (Kp) and the apparent free energies of transfer (ΔGa) for GAP-43P and PIP2 from DSMs to DRMs. In either the presence or absence of nonacylated peptide, ΔGa for PIP2 was about +0.5 kcal/mol, indicating that it was energetically favorable for this lipid to be in DSMs. For nonacylated GAP-43P, ΔGa was also positive, +0.33 kcal/mol in the absence of PIP2 and +0.21 kcal/mol in the presence of PIP2, indicating that it was also energetically favorable for the nonacylated peptide to be associated with DSMs. However, when GAP-43P was acylated, the free energies of transfer of either peptide or PIP2 were negative, indicating that under these conditions it was energetically favorable for these molecules to be in DRMs. For example, the binding of palmitoylated GAP-43P to DOPC/DOPG/SM/Chol/PIP2 bilayers changed the free energy of transfer of PIP2 from DSMs to DRMs from +0.45 kcal/mol to −0.87 kcal/mol.
TABLE 2.
Partition coefficients (Kp) and apparent free energies of transfer (ΔGa) of PIP2 and GAP-43P from DSMs to DRMs
| Molecule | PIP2 present | GAP-43P present | Kp | ΔGa (kcal/mol) |
|---|---|---|---|---|
| PIP2 | Yes | No | 0.44 ± 0.06 | +0.45 ± 0.08 |
| PIP2 | Yes | Nonacylated | 0.41 ± 0.08 | +0.49 ± 0.12 |
| GAP-43P | No | Nonacylated | 0.56 ± 0.16 | +0.33 ± 0.16 |
| GAP-43P | Yes | Nonacylated | 0.68 ± 0.62 | +0.21 ± 0.47 |
| PIP2 | Yes | Acylated | 5.0 ± 1.4 | −0.87 ± 0.16 |
| GAP-43P | No | Acylated | 4.4 ± 0.4 | −0.81 ± 0.05 |
| GAP-43P | Yes | Acylated | 3.6 ± 0.8 | −0.70 ± 0.11 |
Values represent either mean ± SD for three to five experiments. For comparison, thermal energy is 0.55 kcal/mol.
Confocal microscopy
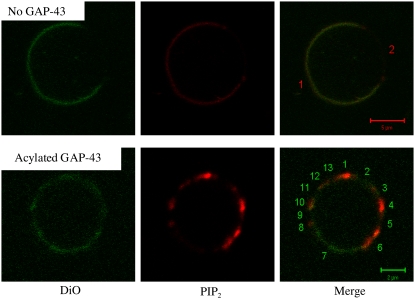
Fig. 6 shows confocal images of equatorial slices of GUVs containing the green fluorescent lipid DiO and the red fluorescently labeled PIP2. The top row of Fig. 6 shows a typical GUV in the absence of GAP-43P. The red BODIPY TMR-PIP2 was colocalized with the green lipid marker DiO in a large microdomain (labeled 1) in the left-hand side of the GUV. As shown in Table 3, for this particular vesicle >99% of both the red and green pixels were localized in this microdomain 1, with <1% of the pixels in the nonlabeled microdomain (domain 2). This colocalization analysis was also performed on six other GUVs in the absence of GAP-43, with each showing >99% colocalization of red and green pixels. Because the unsaturated DiO preferentially localizes to nonraft microdomains (58,59), these images indicate that, in the absence of peptide, BODIPY TMR-PIP2 was enriched in nonraft (green-containing) membranes. This observation was consistent with the data showing that, in the absence of GAP-43P, PIP2 was found preferentially in DSMs (Fig. 5).
FIGURE 6.
Confocal images of 30:10:30:30 DOPC/DOPG/SM/Chol GUVs containing fluorescently labeled DiO and PIP2. The top row shows a vesicle in the absence of GAP-43P with filters showing the green DiO (left), the red BODIPY TMR-PIP2 (middle), and a dual-colored merged image (right). The bottom row shows a vesicle in the presence of acylated GAP-43P with filters showing the green DiO (left), the red PIP2 (middle), and a merged image (right). For each vesicle, microdomains are numbered in the right image.
TABLE 3.
Pixel distributions in regions of GUVs
| Without GAP-43P
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pixel color | Region 1 | Region 2 | |||||||||||
| Green | 1574 | 10 | |||||||||||
| Red
|
1737
|
3
|
|||||||||||
| With acylated GAP-43P
| |||||||||||||
| Pixel color | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Green | 204 | 126 | 112 | 107 | 48 | 239 | 553 | 54 | 32 | 135 | 43 | 90 | 19 |
| Red | 532 | 6 | 113 | 545 | 86 | 844 | 8 | 63 | 3 | 242 | 1 | 98 | 13 |
See Fig. 6.
The addition of acylated GAP-43P changed the distribution of the lipids in the GUVs (Fig. 6, bottom row). In the presence of acylated GAP-43P, much smaller microdomains containing green and/or red probes were observed (Fig. 6, bottom row). To quantitate the overlap of green and red probes we divided the equatorial section in Fig. 6 into 13 small regions and measured the green (DiO) and red (TMR-PIP2) pixels in each region. As can be seen in Table 3, regions 2, 7, 9, and 11 contained mostly green pixels, regions 1, 4, and 6 contained mostly red pixels, whereas regions 3, 5, 8, 10, 12, and 13 contained roughly equal amounts of green and red pixels. Thus, the presence of acylated GAP-43P removed much of the TMR-PIP2 from the green nonraft bilayers. A sample test for equality of proportions was used testing the null hypothesis that the red and green markers had the same distribution among the regions. The p-values were between 0.4 and 1.0 for regions 3, 8, 12, and 13, but were <2.2 × 10−16 for regions 1, 2, 4, 6, and 7, with intermediate p-values for the other regions. Similar results and p-values were obtained with five other vesicles from similar preparations. Thus, compared to the control vesicles (Fig. 6, top), the introduction of acylated GAP-43P significantly changed the distribution of TMR-PIP2 relative to the nonraft marker DiO.
DISCUSSION
Several water-soluble cytoplasmic proteins, including the protein kinase C substrates MARCKS, CAP-23, and GAP-43, contain clusters of positively charged amino acids that bind the membrane lipid PIP2 (27,40,42). In our studies we have examined the interactions with lipid bilayers of a 32-amino-acid peptide (GAP-43P) corresponding to the N-terminus domain linked to the basic effector domain of GAP-43. Quantitative analyses of the distribution of GAP-43P and PIP2 were performed with detergent extraction experiments, which have been extensively used to study the composition of microdomains in both cell membranes and lipid bilayers (15–17,23,26,59–63). Previous x-ray diffraction experiments (53) have analyzed the structure of the DRMs and DSMs from the bilayer system used in this study. However, detergents partition into and break apart bilayers, potentially modifying lipid-lipid interactions (36,60,64,65). Therefore, to verify and supplement the detergent extraction results, we also used confocal microscopic observations of intact vesicles of the same lipid compositions (59).
Previous studies have shown that a peptide corresponding to the effector domain of GAP-43 (without the N-terminus) binds to LUVs containing negatively charged lipids (40). This binding of the effector domain was shown to be due to nonspecific electrostatic interactions (40). Our experiments with GAP-43P, containing both the N-terminus and the effector domain, showed that acylation significantly increased peptide binding to LUVs containing negatively charged lipids to the extent that virtually all of the peptide was bound (Fig. 1).
The acylation of GAP-43P markedly increased the concentration of both the peptide (Fig. 4) and PIP2 (Fig. 5) in DRMs compared to DSMs. That is, the acylation of GAP-43P changed the apparent free energy of transfer of PIP2 from DSMs to DRMs (Table 2) from ΔGa = +0.45 kcal/mol to ΔGa = −0.87 kcal/mol. This energy difference of 1.32 kcal/mol is significantly larger than thermal energy (0.55 kcal/mol), suggesting that electrostatic interactions between PIP2 and acylated positively charged proteins such as GAP-43 could have a significant impact on the sequestering of PIP2 into membrane rafts.
The binding of either acylated or nonacylated GAP-43P had little effect on the amount of DSMs or DRMs extracted with detergent (Fig. 2). The observation that the peptide did not markedly change the distribution of monovalent DOPG between DRMs and DSMs (Fig. 3; Table 1) is consistent with the observations of Golebiewska et al. (43) that showed that peptides with clusters of basic residues do not sequester monovalent acidic phospholipids such as phosphatidylglycerol.
In the absence of GAP-43P, PIP2 was preferentially extracted in DSMs (Fig. 5) and BODIPY TMR-PIP2 was preferentially localized in large nonraft microdomains in intact vesicles (Fig. 6; Table 3). Thus, in the absence of GAP-43P the detergent extraction and confocal experiments were in complete agreement indicating that PIP2 prefers nonraft to raft microdomains. In the case of native PIP2 this is expected given that one of its hydrocarbon chains is polyunsaturated. The location of both native PIP2 and BODIPY TMR-PIP2 in nonraft domains is also consistent with the results of Gambhir et al. (41) who observed that both lipids are excluded from cholesterol-enriched domains in monolayers. Moreover, the amount of phospholipid was similar in DRMs and DSMs (Fig. 2) and the relative sizes of the nonraft and raft domains were approximately equal as viewed in intact vesicles (typical vesicle is shown in Fig. 6). Therefore, in the absence of peptide the detergent extraction appears to provide a valid representation of the bilayer microdomain composition.
In the presence of acylated GAP-43P the detergent extraction and confocal experiments were in agreement in that they both indicated that the acylated peptide modified the distribution of PIP2 in the plane of the bilayer. Several of the observed 13 equatorial regions (Fig. 6, bottom) contained mostly green pixels, as expected for nonraft bilayers not containing red BODIPY TMR-PIP2, whereas other regions contained mostly red pixels corresponding to BODIPY TMR-PIP2 in raft bilayers (Table 3). Other regions contained a mixture of green and red pixels, corresponding to the presence of BODIPY TMR-PIP2 in nonraft bilayers. Thus, the confocal experiments confirm that acylated GAP-43P targets at least some of the PIP2 away from nonraft bilayers into rafts. However, in comparing the detergent extraction and confocal experiments there are three factors to consider. First, during detergent extraction GAP-43P had access to both sides of the bilayer, whereas in the confocal experiments the added peptide presumably had access to only the outer lipid monolayer of the intact GUV, explaining why GUVs exposed to GAP-43P contained regions with both green and red pixels (Table 3). Second, because the fluorescently labeled PIP2 contains the BODIPY moiety in its hydrocarbon chain region, its partitioning properties could be somewhat different than unlabeled PIP2. Third, although the detergent extraction method indicated that the DRMs and DSMs contained similar amounts of lipid (Fig. 2), it provided no information on the lateral dimensions of the microdomains. The confocal images indicated that the raft and nonraft microdomains were of smaller lateral dimensions in the presence of acylated GAP-43P. Shaw et al. (37) previously observed that myristoylated NAP-22 broke up raft domains in a similar ternary lipid system.
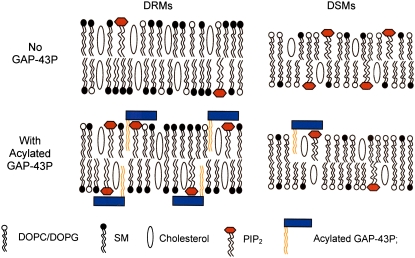
Our results are summarized in diagrammatic form in Fig. 7. In the absence of peptide, DRMs and DSMs had different compositions; DRMs were enriched in SM (with its mostly saturated hydrocarbon chains) and cholesterol, whereas DSMs were enriched in the unsaturated lipids DOPC, DOPG, and PIP2. We found that palmitoylated GAP-43P bound primarily to DRMs, as do a variety of acylated membrane proteins (4,19,23). The effector domain of GAP-43P bound PIP2 and therefore sequestered this lipid to DRMs (Fig. 7 B).
FIGURE 7.
Schematic diagrams showing the distribution of PIP2 in DRMs and DSMs in the absence (top row) and presence (bottom row) of acylated GAP-43P. In the absence of GAP-43P (top) the PIP2 is primarily located in DSMs along with the other unsaturated lipids DOPC and DOPG, whereas the DRMs are enriched in SM and cholesterol. When acylated GAP-43P is added (bottom), the peptide binds and preferentially sequesters the PIP2 into the raft microdomains (DRMs). Note that during detergent extraction procedures the GAP-43P gains access to both sides of the bilayer.
In a recent study Golebiewska et al. (43) present an intriguing model whereby proteins with clusters of basic residues, such as MARCKS, can nucleate raft lipids. In this model proteins with interfacial phenylalanines that penetrate into the interfacial region increase local lateral surface pressure and decrease the local thickness of the lipid monolayer, thereby attracting lipids with small polar headgroups, such as cholesterol. Our experiments indicate that preformed raft microdomains can sequester acylated proteins such as GAP-43.
For several cell lines, Laux et al. (7) showed that GAP-43 is extracted in DRMs and in intact cells colocalizes with PIP2 in plasma membrane microdomains. They also showed that the binding of PIP2 and its effects on cell actin dynamics depend on the effector domain of GAP-43. Our results point to the importance of GAP-43 diacylation in colocalizing the protein and PIP2 in the raft microdomains. That is, in terms of sequestering PIP2 to rafts, these experiments demonstrate the importance of both the effector domain and the acylated N-terminus of GAP-43. As a corollary, we propose that some of the cellular PIP2 not localized to raft microdomains in cells (36) could represent PIP2 that is not bound to acylated proteins such as GAP-43.
Once in a raft, the PIP2 would be in close physical proximity to raft-localized actin binding proteins and signaling proteins. Since it seems likely that a given molecule of PIP2 could not bind to GAP-43 and to another protein at the same time, the PIP2 would have to be released from GAP-43 to participate in classical PIP2 signaling pathways. This would mean that the availability of PIP2 for signaling would be a function of the off-rate of PIP2 from GAP-43 as well as the diffusion rate of free PIP2 out of the raft microdomain. As a result PIP2 would be transiently available at high concentrations only to signaling molecules that are themselves highly concentrated within the raft. Therefore it seems possible that PIP2 released from GAP-43 would be in a position to interact with concentrated signaling proteins before diffusing out of the raft. As PIP2 diffuses, it could rebind to another GAP-43 molecule, bind to phospholipase C (PLC), or partition into the surrounding nonraft microdomain. An important implication of these findings is that both the concentration of PIP2 in raft domains, and the dynamics of its release for signaling, could be regulated by other signals that modify the affinity of the GAP-43 effector domain for PIP2. The effector domain of GAP-43 binds apocalmodulin with higher affinity than calcium-calmodulin (66,67) and also contains a site for PKC phosphorylation (45). Therefore the binding of apocalmodulin would compete with PIP2 binding to the effector domain and PKC phosphorylation would reduce binding of either calmodulin or PIP2. Although the specific consequences for signal transduction remain to be elucidated, our findings suggest that in raft domains GAP-43-mediated concentration of PIP2 would allow the availability of PIP2 for signaling that would be modulated dynamically by calcium and PKC. Once the PIP2 is hydrolyzed, the resulting diacyl glycerol should rapidly diffuse out of the raft into the surrounding bilayer.
In summary, we have demonstrated a mechanism by which PIP2 can be sequestered into lipid rafts. The mechanism involves electrostatic binding of PIP2 to proteins, such as GAP-43, that have effector domains containing clusters of positively charged amino acids. We argue that, when GAP-43 is acylated by cellular enzymes, the diacylated GAP-43 and its bound PIP2, preferentially partition into raft microdomains.
Acknowledgments
We thank Dr. George Dubay, Department of Chemistry, Duke University, for performing the mass spectroscopy analysis and Dr. Tim Oliver of the Department of Cell Biology, Duke University Medical Center, for assistance with the confocal experiments.
This work was supported by National Institutes of Health grant GM27278 and by grants from Philip Morris Inc. USA and Philip Morris International.
Editor: Lukas K. Tamm.
References
- 1.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39. [DOI] [PubMed] [Google Scholar]
- 2.Field, K. A., D. Holowka, and B. Baird. 1997. Compartmentalized activation of the high affinity immunoglubulin E receptor within membrane domains. J. Biol. Chem. 272:4276–4280. [DOI] [PubMed] [Google Scholar]
- 3.Baird, B., E. D. Sheets, and D. Holowka. 1999. How does the plasma membrane participate in cellular signaling by receptors for immunoglobulin E? Biophys. Chem. 82:109–119. [DOI] [PubMed] [Google Scholar]
- 4.Moffett, S., D. A. Brown, and M. E. Linder. 2000. Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem. 275:2191–2198. [DOI] [PubMed] [Google Scholar]
- 5.Pierchala, B. A., J. Milbrandt, and E. M. Johnson, Jr. 2006. Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation. J. Neurosci. 26:2777–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young, R. M., X. Zheng, D. Holowka, and B. Baird. 2005. Reconstitution of regulated phosphorylation of FcepsilonRI by a lipid raft-excluded protein-tyrosine phosphatase. J. Biol. Chem. 280:1230–1235. [DOI] [PubMed] [Google Scholar]
- 7.Laux, T., K. Fukami, M. Thelen, T. Golub, D. Frey, and P. Caroni. 2000. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Biol. Chem. 149:1455–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caroni, P. 2001. New EMBO members' review: actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts. EMBO J. 20:4332–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwik, J., S. Boyle, D. Fooksman, L. Margolis, M. P. Sheetz, and M. Edidin. 2003. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc. Natl. Acad. Sci. USA. 100:13964–13969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons, K., and E. Ikonen. 2000. How cells handle cholesterol. Science. 290:1721–1726. [DOI] [PubMed] [Google Scholar]
- 11.Brown, R. E. 1998. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J. Cell Sci. 111:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- 13.Sharma, D. K., A. Choudhury, R. D. Singh, C. L. Wheatley, D. L. Marks, and R. E. Pagano. 2003. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278:7564–7572. [DOI] [PubMed] [Google Scholar]
- 14.Ikonen, E. 2001. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 13:470–477. [DOI] [PubMed] [Google Scholar]
- 15.Hanada, K., M. Nishijima, Y. Akamatsu, and R. E. Pagano. 1995. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline-phosphatase, a glycosylphosphatidylinositol-anchored protein, in mammalian membranes. J. Biol. Chem. 270:6254–6260. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 36:10944–10953. [DOI] [PubMed] [Google Scholar]
- 17.London, E., and D. A. Brown. 2000. Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta. 1508:182–195. [DOI] [PubMed] [Google Scholar]
- 18.Kay, J. G., R. Z. Murray, J. K. Pagan, and J. L. Stow. 2006. Cytokine secretion via cholesterol-rich lipid raft-associated SNAREs at the phagocytic cup. J. Biol. Chem. 281:11949–11954. [DOI] [PubMed] [Google Scholar]
- 19.Shogomori, H., A. T. Hammond, A. G. Ostermeyer-Fay, D. J. Barr, G. W. Feigenson, E. London, and D. A. Brown. 2005. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J. Biol. Chem. 280:18931–18942. [DOI] [PubMed] [Google Scholar]
- 20.Umlauf, E., M. Mairhofer, and R. Prohaska. 2006. Characterization of the stomatin domain involved in homo-oligomerization and lipid raft association. J. Biol. Chem. 281:23349–23356. [DOI] [PubMed] [Google Scholar]
- 21.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221–17224. [DOI] [PubMed] [Google Scholar]
- 22.Fridriksson, E. K., P. A. Shipkova, E. D. Sheets, D. Holowka, B. Baird, and F. W. McLafferty. 1999. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 38:8056–8063. [DOI] [PubMed] [Google Scholar]
- 23.Melkonian, K. A., A. G. Ostermeyer, J. Z. Chen, M. G. Roth, and D. A. Brown. 1999. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274:3910–3917. [DOI] [PubMed] [Google Scholar]
- 24.Majerus, P. W., T. M. Connolly, H. Deckmyn, T. S. Ross, T. E. Bross, H. Ishii, V. S. Bansal, and D. B. Wilson. 1986. The metabolism of phosphoinositide-derived messenger molecules. Science. 234:1519–1526. [DOI] [PubMed] [Google Scholar]
- 25.Berridge, M. J., and R. F. Irvine. 1989. Inositol phosphates and cell signalling. Nature. 341:197–205. [DOI] [PubMed] [Google Scholar]
- 26.Pike, L. J., and J. M. Miller. 1998. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J. Biol. Chem. 273:22298–22304. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin, S., J. Wang, A. Gambhir, and D. Murray. 2002. PIP(2) and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 31:151–175. [DOI] [PubMed] [Google Scholar]
- 28.Hilgemann, D. W., S. Feng, and C. Nasuhoglu. 2001. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE. 2001:RE19. [DOI] [PubMed] [Google Scholar]
- 29.Lopes, C. M., H. Zhang, T. Rohacs, T. Jin, J. Yang, and D. E. Logothetis. 2002. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron. 34:933–944. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, L., J. K. Lee, S. A. John, N. Uozumi, and I. Kodama. 2004. Mechanosensitivity of GIRK channels is mediated by protein kinase C-dependent channel-phosphatidylinositol 4,5-bisphosphate interaction. J. Biol. Chem. 279:7037–7047. [DOI] [PubMed] [Google Scholar]
- 31.Liu, B., and F. Qin. 2005. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25:1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, T. F. 2001. PI(4,5)P(2) regulation of surface membrane traffic. Curr. Opin. Cell Biol. 13:493–499. [DOI] [PubMed] [Google Scholar]
- 33.Czech, M. P. 2003. Dynamics of phosphoinositides in membrane retrieval and insertion. Annu. Rev. Physiol. 65:791–815. [DOI] [PubMed] [Google Scholar]
- 34.Yin, H. L., and P. A. Janmey. 2003. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 65:761–789. [DOI] [PubMed] [Google Scholar]
- 35.Remillard, C. V., and J. X. Yuan. 2006. Transient receptor potential channels and caveolin-1: good friends in tight spaces. Mol. Pharmacol. 70:1151–1154. [DOI] [PubMed] [Google Scholar]
- 36.van Rheenen, J., E. M. Achame, H. Janssen, J. Calafat, and K. Jalink. 2005. PIP2 signaling in lipid domains: a critical re-evaluation. EMBO J. 24:1664–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw, J. E., R. F. Epand, K. Sinnathamby, Z. Li, R. Bittman, R. M. Epand, and C. M. Yap. 2006. Tracking peptide-membrane interactions: insights from in situ coupled confocal-atomic microscopy imaging of NAP-22 peptide insertion and assembly. J. Struct. Biol. 155:458–469. [DOI] [PubMed] [Google Scholar]
- 38.Niu, S. L., and B. J. Litman. 2002. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 83:3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvius, J. R. 2005. Partitioning of membrane molecules between raft and non-raft domains: insights from model-membrane studies. Biochim. Biophys. Acta. 1746:193–202. [DOI] [PubMed] [Google Scholar]
- 40.Wang, J., A. Gambhir, G. Hangyas-Mihalyne, D. Murray, U. Golebiewska, and S. McLaughlin. 2002. Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. J. Biol. Chem. 277:34401–34412. [DOI] [PubMed] [Google Scholar]
- 41.Gambhir, A., G. Hangyas-Mihalyne, I. Zaitseva, D. S. Cafiso, J. Wang, D. Murray, S. N. Pentyala, S. O. Smith, and S. McLaughlin. 2004. Electrostatic sequestration of PIP(2) on phospholipid membranes by basic/aromatic regions of proteins. Biophys. J. 86:2188–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLaughlin, S., and D. Murray. 2005. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 438:605–611. [DOI] [PubMed] [Google Scholar]
- 43.Golebiewska, U., A. Gambhir, G. Hangyas-Mihalyne, I. Zaitseva, J. Radler, and S. McLaughlin. 2006. Membrane-bound basic peptides sequester multivalent (PIP2), but not monovalent (PS), acidic lipids. Biophys. J. 91:588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skene, J. H. P. 1989. Axonal growth-associated proteins. Annu. Rev. Neurosci. 12:127–156. [DOI] [PubMed] [Google Scholar]
- 45.Benowitz, L. I., and A. Routtenberg. 1997. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 20:84–91. [DOI] [PubMed] [Google Scholar]
- 46.Strittmatter, S. M., T. Vartanian, and M. C. Fishman. 1992. GAP-43 as a plasticity protein in neuronal form and repair. J. Neurobiol. 23:507–520. [DOI] [PubMed] [Google Scholar]
- 47.Bomze, H. M., K. R. Bulsara, B. J. Iskandar, P. Caroni, and J. H. Skene. 2001. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat. Neurosci. 4:38–43. [DOI] [PubMed] [Google Scholar]
- 48.Arni, S., S. A. Keilbaugh, A. G. Ostermeyter, and D. A. Brown. 1998. Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J. Biol. Chem. 273:28478–28485. [DOI] [PubMed] [Google Scholar]
- 49.Skene, J. H. P., and I. Virag. 1989. Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. J. Cell Biol. 108:613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buser, C. A., and S. McLaughlin. 1998. Ultracentrifugation techniques for measuring the binding of peptides and proteins to sucrose-loaded phospholipid vesicles. Methods Mol. Biol. 84:267–281. [DOI] [PubMed] [Google Scholar]
- 51.Chen, P. S., Jr., T. Y. Toribara, and H. Warner. 1956. Microdetermination of phosphorous. Anal. Chem. 28:1756–1758. [Google Scholar]
- 52.Quesnel, S., and J. R. Silvius. 1994. Cysteine-containing peptide sequences exhibit facile uncatalyzed transacylation and acyl-CoA-dependent acylation at the lipid bilayer interface. Biochemistry. 33:13340–13348. [DOI] [PubMed] [Google Scholar]
- 53.Gandhavadi, M., D. Allende, A. Vidal, S. A. Simon, and T. J. McIntosh. 2002. Structure, composition, and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys. J. 82:1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McIntosh, T. J., A. Vidal, and S. A. Simon. 2003. Sorting of lipids and transmembrane peptides between detergent-soluble bilayers and detergent-resistant rafts. Biophys. J. 85:1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akashi, K.-I., H. Miyata, H. Itoh, and K. Kinosita. 1996. Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys. J. 71:3242–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher, L. D., and G. van Belle. 1993. Biostatistics: A Methodology for Health Sciences. John Wiley and Sons, New York.
- 57.Pirie, W. R., and M. A. Hamdan. 1972. Some revised continuity corrections for discrete distributions. Biometrics. 28:693–701. [Google Scholar]
- 58.Kahya, N., D. Scherfeld, K. Bacia, B. Poolman, and P. Schwille. 2003. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem. 278:28109–28115. [DOI] [PubMed] [Google Scholar]
- 59.Vidal, A., and T. J. McIntosh. 2005. Transbilayer peptide sorting between raft and nonraft bilayers: comparisons of detergent extraction and confocal microscopy. Biophys. J. 89:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shogomori, H., and D. A. Brown. 2003. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol. Chem. 384:1259–1263. [DOI] [PubMed] [Google Scholar]
- 61.Wilson, B. S., S. L. Steinberg, K. Liederman, J. R. Pfeiffer, Z. Surviladze, J. Zhang, L. E. Samelson, L. H. Yang, P. G. Kotula, and J. M. Oliver. 2004. Markers for detergent-resistant lipid rafts occupy distinct and dynamic domains in native membranes. Mol. Biol. Cell. 15:2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simons, K., and W. L. Vaz. 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33:269–295. [DOI] [PubMed] [Google Scholar]
- 63.Lagerholm, B. C., G. E. Weinreb, K. Jacobson, and N. L. Thompson. 2005. Detecting microdomains in intact cell membranes. Annu. Rev. Phys. Chem. 56:309–336. [DOI] [PubMed] [Google Scholar]
- 64.Heerklotz, H., H. Szadkowska, T. Anderson, and J. Seelig. 2003. The sensitivity of lipid domains to small perturbations demonstrated by the effect of Triton. J. Mol. Biol. 329:793–799. [DOI] [PubMed] [Google Scholar]
- 65.Lichtenberg, D., F. M. Goni, and H. Heerklotz. 2005. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 30:430–436. [DOI] [PubMed] [Google Scholar]
- 66.Alexander, K. A., B. T. Wakim, G. S. Doyle, K. A. Walsh, and D. R. Storm. 1988. Identification and characterization of the calmodulin-binding domain of neuromodulin, a neurospecific calmodulin-binding protein. J. Biol. Chem. 263:7544–7549. [PubMed] [Google Scholar]
- 67.Liu, Y. C., and D. R. Storm. 1990. Regulation of free calmodulin levels by neuromodulin: neuron growth and regeneration. Trends Pharmacol. Sci. 11:107–111. [DOI] [PubMed] [Google Scholar]