Abstract
Mucus propelling cilia are excitable by many stimulants, and have been shown to increase their beating frequency up to threefold, by physiological extracellular stimulants, such as adenosine-triphosphate, acetylcholine, and others. This is thought to represent the evolutionary adaptation of mucociliary systems to the need of rapid and efficient cleansing the airways of foreign particles. However, the mucus transport velocity depends not only on the beat frequency of the cilia, but on their beat pattern as well, especially in the case of mucus bearing cilia that beat in a complex, three-dimensional fashion. In this study, we directly measured the force applied by live ciliary tissues with an atomic force microscope, and found that it increases linearly with the beating frequency. This implies that the arc swept by the cilia during their effective stroke remains unchanged during frequency increase, thus leading to a linear dependence of transport velocity on the beat frequency. Combining the atomic force microscope measurements with optical measurements, we have indications that the recovery stroke is performed on a less inclined plane, leading to an effective shortening of the overall path traveled by the cilia tip during this nontransporting phase of their beat pattern. This effect is observed to be independent of the type of stimulant (temperature or chemical), chemical (adenosine-triphosphate or acetylcholine), or concentration (1 μM–100 μM), indicating that this behavior may result from internal details of the cilium mechanical structure.
INTRODUCTION
The mucociliary epithelium is a synchronized and highly effective waste-disposal system. It uses mucus as a vehicle, driven by beating cilia, to transport unwanted particles, trapped in the mucus, away from the organs. The ability of cilia, tiny hairlike protrusions (diameter 0.25 μm, length 5–7 μm and packing density 100–200 per cell), to propel steel beads of 1 mm in diameter at a speed of 0.5 mm/s is amazing (1). This titanic task is achieved due to the high degree of coordination between the individual cilia, and due to the ability of cilia to respond, quickly and for prolonged periods of time, to various stimuli, by increasing drastically the ciliary beat frequency (CBF). Owing to the physiological importance of mucociliary function in respiratory, digestive, and reproductive systems, and the rapidly growing list of diseases caused by dysfunction of the cilia, from chronic respiratory disease to abnormal organ development in the embryo (2), coordinated movement of cilia in time and space (metachronism) and signal transduction pathway(s) which lead to CBF enhancement have been intensively investigated. It was shown that extracellular adenosine-triphosphate (ATP) or acetylcholine (ACH) induces robust CBF enhancements from 10 Hz up to 30 Hz (3–11). Moreover, it was found that, when CBF was rapidly increased by extracellular ATP or ACH, the degree of coordination between beating cilia was not affected (12). These results suggest that transport rate will increase with ciliary stimulation. Nevertheless, the degree of correlation between ciliary frequency and the forces applied by cilia remains to be established. Moreover, since the forces applied by cilia depend not only on the beat frequency, but also on the beat pattern, it remains to be resolved whether the beat pattern depends on the CBF, and to what extent. These fundamental questions remain open, partly due to technical difficulties to measure directly the force produced from intact explants and to estimate the form of ciliary beat versus increase of CBF. Several studies have been made of the dependence of the pattern of ciliary beat upon ciliary beat frequency (13–15). These studies have relied on optical methods, attempting to describe three-dimensional movement from two-dimensional images of moving, dense cilia, thinner than the diffraction limit. The conflicting conclusions about the beat pattern at basal frequencies are an outcome of the inherent complexity of these systems. For example, a recent study (16) concluded that the effective stroke and the recovery stroke are in the same planes, contrary to the common concept (13,17–19).
To address this issue, in this study we examined the activity of cultured mucociliary cells from frog esophagus using simultaneous atomic force microscope (AFM) and photoelectric measurements. We applied our recently developed experimental setup (20) to examine the dependence between the amplitudes of both AFM and photoelectric signals on CBF, stimulated with two chemical stimulants (ATP and ACH) and temperature. Based on these measurements we draw conclusions regarding the dependence of the ciliary beat form on the beat frequency.
MATERIALS AND METHODS
Tissue culture
Experiments were performed on monolayer tissue culture grown from frog esophagus of locally supplied frogs (Rana ridibunda) according to the procedure described elsewhere (10). In addition, the esophagus was bathed in ∼2 ml sterile medium with Mg-ATP 10−5 M, for 1 h. The ATP facilitated firm attachment of the explants in the next step of culturing, by stimulating the cilia to beat faster and thus improve removal of mucus. One tissue piece was then placed at the center of a sterile number 1 glass coverslip (Marienfeld, Lauda-Koenigshofen, Germany) placed in a plastic petri dish (35 mm; Nunc, Rochester, NY) and overlaid with 0.7 ml of culture medium. Tissue cultures 4- to 14-days-old were used for measurements. However, every effort was made to use the cultures at the youngest age possible, to minimize interference of loose tissue with AFM measurements, which increased in quantity as the cultures aged. The coverslip with the culture was placed in a customized sample holder for liquid AFM measurements. The ciliated surfaces used for measurement were free of mucus, and the AFM tip was in direct contact with the cilia, similar to our previous report (20).
Chemicals and solutions
During the experiments, the cultures were washed and bathed in Ringer solution containing (in mM) 120 NaCl, 2.3 KCl, 1.8 CaCl2, 1.8 MgCl2, 0.85 Na2HPO4, and 0.85 NaH2PO4, pH 7.2. Stimulations were carried out with Mg-ATP (Sigma, St. Louis, MO), or acetylcholine chloride (ACH) as extracellular stimulants, at various concentrations.
The measurement system
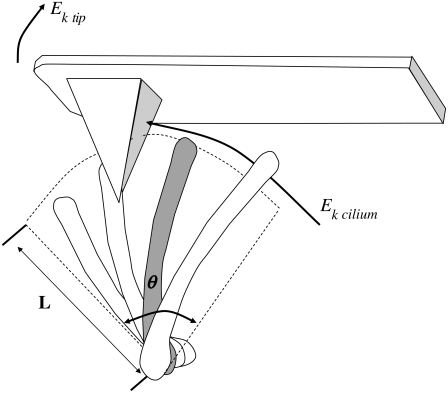
We recently described a measurement system (20) that enables the simultaneous measurement of forces applied by beating cilia and optical measurement of their activity. The forces applied by beating cilia are sensed by the cantilever of an atomic force microscope (AFM), which is dipped into the active field of a ciliated tissue. A schematic drawing is presented in Fig. 1, depicting four cilia along a line perpendicular to the page, performing their effective stroke with a phase shift between them. The gray colored cilium has its imaginary effective stroke arc indicated with dashed lines. It is visible, such that at some point along its path, it collides with the pyramidal AFM tip, thereby conveying to it a part of its kinetic energy (Ek cilium), which causes the AFM tip to recoil upwards, with an equal kinetic energy Ek tip. The well-established optical subsystem (21) continuously collects light scattered by the cilia, thus enabling us to monitor their frequency in the same area where the AFM probe contacts them, before contact and during the measurement, to ensure that no mechanical stimulation or perturbation is induced by the contact of the AFM probe with the cilia. The AFM cantilever is aligned with the (optically observed) direction of the effective stroke; this way, cilia performing their effective stroke hit the AFM tip, transferring the maximal momentum.
FIGURE 1.
Cilia tips hitting the AFM tip. Four cilia, along an imaginary line perpendicular to the page, are depicted performing their effective stroke from right to left. The arc described by the tip of the gray colored cilium is indicated with dashed lines. It spans an angle θ, and since the length of a cilium is L, the length of the arc is Lθ. The cilium has a kinetic energy proportional to the square of its velocity, part of which is transferred to the AFM cantilever via the pyramidal tip. The tip recoils as a result, and the recoil amplitude is measured by the AFM system.
The optical setup is comprised of two opposing optical microscopes, focused in the plane of the ciliary culture, when one of them serves as a condenser, thus focusing light on the tissue and the opposed one collects the light scattered while passing through the active ciliary tissue. An optic fiber positioned in the intermediate image plane of the second microscope collects light from a circular area with a diameter of ∼5 μm in the plane of the sample, adjacent to the AFM cantilever, and delivers it to a photomultiplier tube for conversion to an electric signal. The ability to simultaneously acquire both signals, from the same position on the sample not only allows the validation of the AFM measurements by the well-established electro-optical method, but enables us new insights into the change of the ciliary beat pattern during change of beat frequency.
Stimulation of the CBF
Each stimulation experiment consisted of measuring the optic and AFM signals while the AFM probe and culture were completely stationary (no XY scanning), starting before the approach of the AFM probe to the cilia, and continuing during and after stimulation. Cultures were stimulated only once.
For the measurements of stimulation by increase of temperature, the glass substrate was heated with a laser (Mira Optima, with a Verdi-V5 pump laser, both by Coherent, Santa Clara, CA) coupled through a multimode optical fiber. After temperature stimulation, cells were stimulated with ATP, to verify their normal response and ensure that no temperature-induced damage had occurred.
Treatment of data for derivation of force-CBF relationships
The beating pattern of cilia is pseudo-periodical and exhibits naturally variable frequencies and signal amplitudes, in both AFM and optic methods of measurement. Under these circumstances, the very definition of “frequency” may be problematic; however, Fourier analysis of these signals is well established (21) and common. We employed here the following structured approach to data analysis:
Consecutive segments of the signal were analyzed, each 1–4 s long, with the purpose of calculating the CBF and amplitude representative of that segment.
Segments of the data were selected, in which the frequency was well defined and stable (see further details below). In these sections, the signal was close to periodical, and therefore the standard deviation value was used as the representative value for the signal amplitude.
After identifying the first maximum in the fast Fourier transform (FFT) power spectrum of a one second sampling duration, the program accepts or rejects the segment based on the ratio between the major peak and the next maxima. A clear, prominent peak indicates a period where the frequency is stable and well defined. The program then attempts to widen the segment of data, by adding more sampling points, for as long as the acceptance criterion is met. This way, the longest segments of signal are being selected, for which the frequency is stable and well defined, over the whole stimulation event.
RESULTS
The amplitude of the AFM signal is proportional to the force applied on it by the cilia. A thorough calibration of the AFM system may be performed, allowing readings of this amplitude in force units, as we have previously demonstrated (20). For the purpose of this study the absolute force units are not required (therefore the calibration steps were skipped); however, it is important to realize that the amplitude of the AFM signal is proportional to the force applied on it by the beating cilia. The various measurements, on various samples, were performed with uncalibrated AFM probes, and no attempt was made to establish the precise distance of the AFM tip from the base of the cilia. Therefore, in various measurements, the AFM tip may measure the force applied by a different number of cilia.
The force applied by a cilium increases linearly with CBF increase
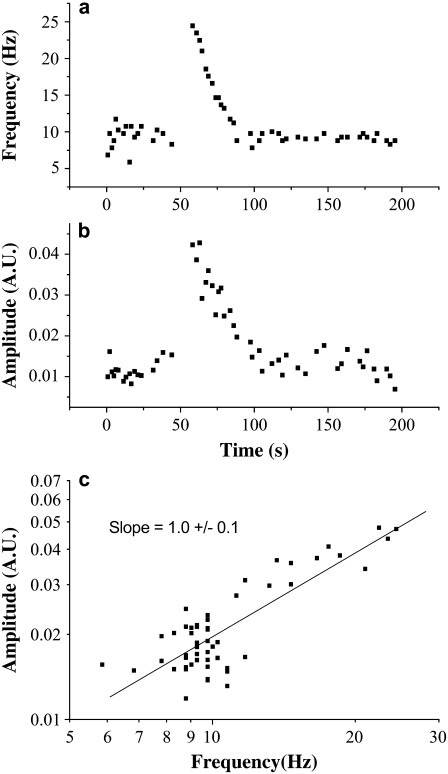
All AFM measurements of stimulations events were monitored simultaneously with the photoelectric measurement subsystem. The frequency measured by the electro-optical system was identical to that measured by the AFM. Fig. 2 presents a typical stimulation experiment result, after analyzing the measured AFM signal as described in Materials and Methods. The AFM signal frequency (Fig. 2 a) is at its basal level (∼10 Hz) during the first ∼50 s, then it increases dramatically to ∼25 Hz as a result of stimulation with extracellular ATP, followed by a gradual decrease back to the basal level. The AFM signal amplitude, proportional to the force sensed by the AFM probe (Fig. 2 b), is following the same course: it increases with increasing frequency and decreases with decreasing frequency. A plot of amplitude versus frequency yields an apparent linear dependence (not shown). However, a more rigorous way to extract the power of a polynomial dependence is to present the variables on a log-log plot (if y = axα + b, then log(y-b) = log(a) + α log(x), thus the slope of the log-log plot is α). In Fig. 2 c, we are plotting the force versus the frequency on a log-log scale, and show that the dependence is precisely linear since the slope of the log-log plot is 1.
FIGURE 2.
Stimulation of ciliary beat frequency with extracellular ATP (10 μM). (a) Frequency as extracted from AFM signal, according to the protocol described in Materials and Methods. Frequency is at its basal level (∼10 Hz) during the first 50 s, then abruptly increases to ∼25 Hz in response to extracellular ATP and gradually decreases back to its basal level. (b) The amplitude of the AFM signal follows the pattern of the frequency, increasing abruptly then decreasing gradually, in a similar way. (c) Amplitude versus frequency on a log-log scale has a slope of 1; the amplitude of the AFM signal has a linear dependence on the frequency.
To test whether the linear relationship depends on stimulant concentration, we performed experiments with ATP concentrations of 1, 10, 100 μM, on different cultures, from different frogs. The results were practically identical to the experiment described in Fig. 2 and are detailed in Table 1. The linear relationship between force and frequency is concentration-independent, over the whole duration of the experiment, i.e., through acceleration and deceleration of the beat frequency.
TABLE 1.
Force-CBF relationship for stimulation of the beat frequency with various stimulants
| Stimulant | Number of samples | Number of frogs | Slope | Standard deviation |
|---|---|---|---|---|
| ATP, 1 μM | 5 | 4 | 1.1 | 0.1 |
| ATP, 10 μM | 3 | 2 | 1.0 | 0.2 |
| ATP, 100 μM | 3 | 3 | 1.0 | 0.2 |
| ACH, 10 μM | 8 | 3 | 1.1 | 0.1 |
| Temperature | 3 | 3 | 1.0 | 0.2 |
The force-CBF relationship is independent of the stimulant
Acetylcholine is another well-known stimulant of CBF (3,10), although it stimulates the beat frequency using different receptors (muscarinic receptors for ACH as opposed to purinergic receptors for ATP) (11). It is interesting, thus, to compare the force-frequency relationship for stimulation by ACH, with that measured for stimulation by ATP. We measured the response to 10 μM ACH, a concentration for which the CBF exhibits a strong change after stimulation. The experiments were performed in a similar way with the experiments with ATP.
The results are summarized in Table 1, where we report the slopes of the amplitude versus frequency plots on a log-log scale (plots not shown). The linear force-frequency relationship is maintained with ACH as a stimulant, despite the fact that it acts through different receptors. For ATP as stimulant we obtained an average slope of 1.0 ± 0.2 (three samples, two frogs, concentration 10 μM), and with ACH the average slope is 1.1 ± 0.1 (eight samples, three frogs, concentration 10 μM). A t-test comparison of the two sets of measurements, for the two stimulants, shows the sets of data are identical with a confidence >95%.
Temperature stimulation of CBF
To test the force-frequency relationship during stimulation by a nonchemical stimulant, we stimulated the CBF by increasing briefly the temperature of the culture and then allowed it to return to room temperature. The frequency increased with temperature, as expected, and thus enabled us to monitor the force and frequency simultaneously, as they change.
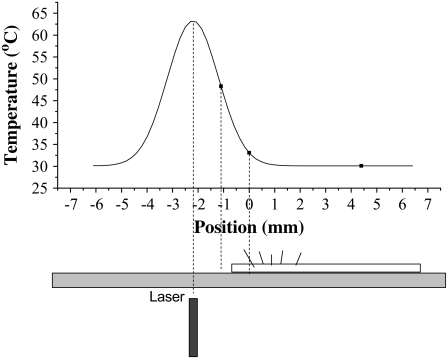
We used a powerful laser coupled to an optical fiber, to heat the sample. We chose this method of heating, to avoid electrical wires contacting the AFM liquid cell, which would have induced mechanical vibrations. The setup is described schematically in Fig. 3. The end of the optic fiber was placed under the glass substrate on which the ciliary tissue resided, 2.2 mm away (horizontally) from the position of the AFM probe.
FIGURE 3.
Experimental setup for temperature stimulation of the ciliary beat frequency. An optic fiber connected to a powerful laser is positioned underneath the coverslip on which the ciliated tissue culture resides. The graph shows the temperature profile in the liquid chamber. The AFM probe measures cilia that are at a temperature of ∼33°C. The temperature of the medium in contact with the ciliated tissue does not exceed 40°C. Maximal temperature in the liquid chamber is above the optic fiber, and does not exceed 65°C. However, no tissue exists at this location.
We took extensive measures to ensure that the heating of the chamber did not damage the ciliary tissue. Tissue damage may be followed by cell death and release of ATP to the growth medium, thus causing stimulation of the CBF indirectly by ATP and not directly by temperature. Before beginning experiments, we measured the temperature profile in the liquid chamber with a copper-constantan thermocouple at three points, after shining the laser for several minutes. The temperatures at this time are representative of thermal equilibrium, therefore the temperature profile in the whole cell can be assumed to be a Gaussian centered above the optic fiber (see Fig. 3). The distance of the fiber from the bottom of the liquid chamber was adjusted so that the temperature did not exceed 35°C at the point where the AFM cantilever was positioned. The temperature directly above the fiber did not exceed 65°C. Then, the bare glass substrate was replaced with an identical glass substrate with ciliated tissue culture, for measurement. Importantly, it was verified that no tissue was placed directly above the optic fiber (see Fig. 3).
The AFM probe measured in a region where the temperature raised from room temperature to ∼33°C, when heating. Temperature was not monitored during the experiment, to avoid mechanical vibrations induced by a thermocouple connected to the AFM liquid chamber. The scope of this study is to characterize the force-frequency relationship, and not the frequency-temperature relationship, thus, once it has been established that attained temperatures are not damaging, we treated the temperature as a stimulant of CBF. The measurements were started with the heating laser off, verification of the AFM reported frequency with the electro-optical system reported frequency, then the laser radiation was shone on the sample for ∼60 s and shut off again. AFM signal was continuously collected before, during, and after heating, for a total of ∼10 min. The optical subsystem signal was also continuously collected during this time. After completion of the measurement, it was verified that the ciliary tissue responded to ATP stimulation in the usual fashion, to ensure yet again that no damage was inflicted by heating.
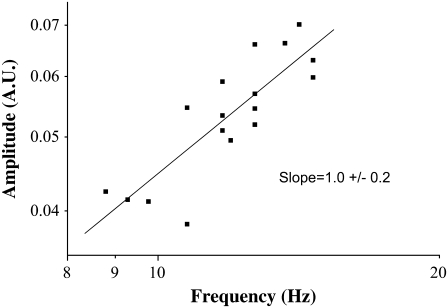
In Fig. 4 we plot on a log-log scale the amplitude versus the frequency from one experiment and show that in this case the dependence is linear as well (the slope is 1). The average slope of three experiments from three different frogs is 1.0 ± 0.2. For temperature stimulation of CBF, the relationship force-frequency is also linear.
FIGURE 4.
Dependence of AFM signal amplitude on the frequency, upon stimulation of CBF by temperature. Plot is on a log-log scale and the slope of the linear fit is 1; the amplitude of the AFM signal has a linear dependence on the frequency.
The optic signal amplitude decreases with CBF increase
In this study, we used the optical subsystem to validate the AFM measurements, especially to ensure that the mechanical contact of the probe did not disturb the cilia. However, important information is found in the optical signal amplitude as well. Our system in fact provides simultaneously and colocally four ciliary parameters: frequency measured by AFM; frequency measured by the optical system; amplitude of the AFM signal; and amplitude of the optical signal. The frequency measurements by AFM and the electro-optical method are identical.
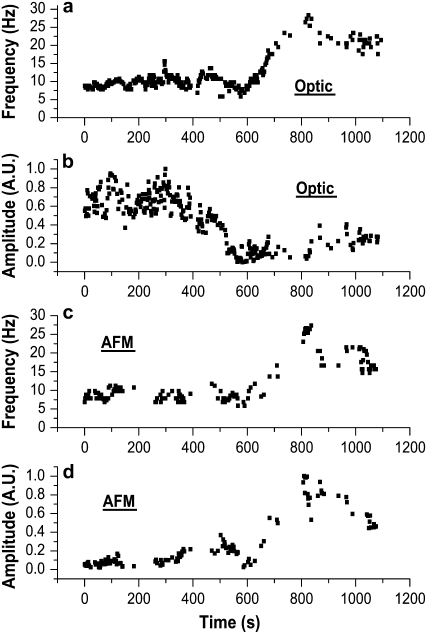
In our measurements, we find that, while the AFM signal amplitude rises linearly with the beat frequency, the amplitude of the optical signal decreases with increasing frequency, simultaneously and colocally. A typical result is shown in Fig. 5. All data presented have been acquired on the same patch of cilia during an ATP stimulation experiment. ATP was slowly added to the liquid chamber between 450 and 480 s. Frequency is increasing at ∼550 s (∼9 min) from the start of the experiment. The frequencies of the optical signal and AFM signal (Fig. 5, a and c, respectively) are identical, confirming proper operation of the AFM system and the fact that we are measuring the same cilia by both systems. The amplitude of the AFM signal increases (linearly) with increase of the frequency (Fig. 5 d) while simultaneously, the amplitude of the optic signal decreases (Fig. 5 b). The apparent decay of the optic signal before the rise in frequency occurs within the signal noise.
FIGURE 5.
Optical and AFM simultaneous and colocal measurement during a stimulation experiment with extracellular ATP (10 μM). (a) Frequency of the optical signal. (b) Amplitude of the optical signal (normalized). (c) Frequency of the AFM signal. (d) Amplitude of the AFM signal (normalized). The frequency (measured both optically and with the AFM) increases after stimulation with ATP and then gradually decreases to the prestimulation, basal value. While the amplitude of the AFM signal increases linearly with the frequency (d), the amplitude of the optical signal decreases with increasing frequency (b).
Previous measurements of the amplitude of the optic signal while changing the CBF by stimulation were performed (3) showing a decrease of the optic amplitude with the increase of the CBF. Our results show that this effect is not disconnected from the linear increase of the AFM signal amplitude, but in fact provides additional information regarding the change of the ciliary beat patterns. We discuss in depth these findings in what follows.
DISCUSSION
The measurement of the force applied by cilia as a function of beat frequency is reported here for the first time. It is important to mention that the absolute values of force have not been measured in each experiment. Not only is this not required to assess whether the force depends linearly on the beat frequency, but in fact it strengthens the reliability of the results: the linear dependence is not a function of the initial force sensed by the AFM cantilever at the beginning of the experiment (initial approach and dipping of the tip into the field of cilia). The log-log plots in Fig. 2 c and Fig. 4 represent on a logarithmic scale the relationship y = axα + b, where y is the force sensed by the AFM tip and x is the beating frequency. In this equation, a is the proportionality coefficient, that has dimensions of N/Hz, and describes how much force per Hz of beating frequency is sensed by the cantilever. We have shown previously that this number is in its essence a geometrical factor describing the number of cilia that hit the AFM tip, the angles and position along the individual cilia where they meet the pyramidal tip, and the geometry of the tip (in addition, it includes the proportionality between the bending of the AFM cantilever and the actual voltage difference that is reported by the AFM electronics). It can be extracted from an experiment with a calibrated cantilever, by varying the depth to which the AFM tip penetrates the field of cilia (20). This a-value changes very sharply as a function of the initial dipping depth (approximately one order of magnitude for 2 μm change in penetration depth), and in this study it varies between the various experiments; however, it is constant along a single experiment. The results reported here are therefore an average over several experiments in which the geometry was different, the cantilever force coefficient was different and the initial alignment of the AFM was different (the monitoring laser hitting the back of the cantilever is manually adjusted for each mounted cantilever, thus the voltage recorded by the acquisition electronics varies from one cantilever to another). The fact that the force-frequency dependence is linear under these conditions lends much credibility to these results, and shows that it is a general effect, not just a coincidence of a specific system setup. This information provides a unique opportunity to analyze the form of the ciliary beat upon change of frequency in a mucociliary system. The obtained results have a number of implications on the form of beat of these cilia, discussed below.
The length of the effective stroke arc is constant
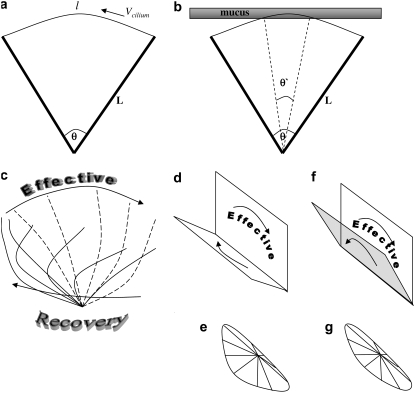
The AFM signal represents only the effective stroke, in both unstimulated and stimulated beats, since the dip of the AFM tip into the field of cilia is limited (20), and the recovery stroke is relatively close to the cell surface during the cycle of the beat (22). The precisely linear dependence of the AFM signal amplitude on the beating frequency has an important implication on the path drawn by the tip of the cilium. This is schematically described in Fig. 6 a, where a cilium of length L, is depicted performing its effective stroke from right to left and drawing an arc of angle θ (and a length l = Lθ), with a tangential velocity Vcilium. The kinetic energy of the cilium is proportional to 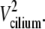 During the collision of the cilium with the AFM probe, some of this energy is transferred to the AFM probe (inelastic collision), so that Ek tip (the kinetic energy of the AFM probe just after the collision) is proportional to
During the collision of the cilium with the AFM probe, some of this energy is transferred to the AFM probe (inelastic collision), so that Ek tip (the kinetic energy of the AFM probe just after the collision) is proportional to 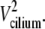 This energy is converted into spring potential energy, by the vertical recoiling of the AFM cantilever to a displacement Z—the amplitude of the signal measured by the system—such that this spring potential energy, proportional to Z2, is a measure of the kinetic energy of the cilium. This is summarized as
This energy is converted into spring potential energy, by the vertical recoiling of the AFM cantilever to a displacement Z—the amplitude of the signal measured by the system—such that this spring potential energy, proportional to Z2, is a measure of the kinetic energy of the cilium. This is summarized as
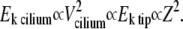 |
FIGURE 6.
Schematic representation of the form of ciliary beat. (a) A cilium performing its effective stroke in the direction indicated by the arrow. L is the length of the cilium, θ is the angle swept by the cilium during the effective stroke, l = θ L is the length of this arc, and Vcilium is the tangential velocity of the cilium's tip. (b) The cilium's tip is embedded into the mucus layer for an arc length corresponding to θ′ only, and not during the whole arc of the effective stroke. The movement of the mucus is only Lθ′, even though the cilium performs a trip of Lθ > Lθ′. However, the tangential velocity and thus the velocity of mucus transport is determined by θ, since Vcilium = Lθf. (c) A two-dimensional projection of the three-dimensional beat form of the cilium. The effective stroke is performed on a plane parallel to the page, while the recovery stroke is on a plane almost perpendicular to the page, leaning in the direction of the reader. (d) The plane of the effective stroke is perpendicular to the cell surface; the plane of the recovery stroke is inclined toward the cell surface. (e) A projection on the cell surface of the path drawn by the cilium's tip when viewed from above. (f) Upon frequency stimulation, the plane on which the recovery stroke is performed is less inclined and more erect. (g) A projection on the cell surface of the path drawn by the cilium's tip when viewed from above, after frequency increase. The area on which shadow is cast by the cilium on the cell surface decreases and the length of the total path traveled by the cilium tip is shortened.
Thus, the measured AFM amplitude, Z, is proportional to the tangential velocity of the cilium's tip Vcilium: Z ∝ Vcilium (the same conclusion can be drawn also from momentum transfer considerations).
Since our measurements show that Z is linear in the beat frequency, f, it follows that
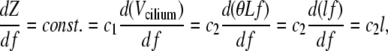 |
where L is the length of the cilium, θ is the angle swept by the cilium during the effective stroke, and l is the length of this arc (see Fig. 6 a). Therefore, the length of the arc drawn by the tip of the cilium remains constant during change of beat frequency. This leads to an increase of the tangential velocity exactly proportional to the increase in beat frequency. The constants c1 and c2 are some of the constituents of the a-value discussed above.
It is important to note that an alternative scenario could have taken place: as we sketch in Fig. 6 b, the angle swept during the effective stroke could have been decreased to a minimal value of θ′ (between the two dashed lines) while still displacing the mucus layer by the same distance during each cycle, since during the rest of the stroke the cilium tip is not embedded in the mucus layer. However, our findings indicate that this is not the case; instead, the arc length remains constant while the frequency changes. A hypothetical scenario like the one described in Fig. 6 b would have saved energy consumption; however, this would have decreased the tangential velocity of the cilium's tip, leading to a reduced transport velocity (since a reduced length of arc swept at a given frequency means a lower tangential velocity). Our results indicate that the cilia follow a scenario that leads to the maximal increase of transport velocity, by keeping the length of the effective stroke constant.
Switching occurs at constant positions
The fact that this behavior does not depend on the type (chemical or temperature), kind (ATP or ACH), or concentration of the stimulant, indicates that the switching from effective to recovery stroke and from recovery stroke to effective stroke (regardless of the existence of a pause phase) occurs at constant positions, and possibly originates in mechanochemical details of the cilium structure. The switching positions seem to be completely independent of the mechanism leading to CBF increase.
Both ATP and ACH activate phospholipase C, in turn mobilizing Ca2+ from intracellular Ca2+ stores (10,23). However, extracellular ATP acts through the Purinergic receptors to cause continuous influx of Ca2+ from outside the ciliary cell (8), while ACH does not (10). Instead, ACH acts through muscarinic receptors on the ciliary cell membrane to directly increase intracellular cAMP levels (11), which enables maintaining the CBF higher than prestimulation level, independent of (resumed prestimulation) Ca2+ level. Thus, seemingly, two different ways are utilized to maintain increased CBF activity, by each stimulant.
There is a very limited amount of data describing the form of beat of mucociliary systems, nevertheless it is instructive to compare our findings with other members of the cilia family, even though they perform different functions.
In a comparative study of native and mutant Chlamydomonas, the mutants beat with a slower frequency, but with an arc of identical length (24). A study of the waveform of the metachronal wave at various CBFs, in Mytilus edulis' short (lateral) cilia and other organisms' cilia (25), led to the conclusion that the angular velocity of the effective stroke is higher in cilia that beat faster, since the waveform remains unchanged. A study of the metachronal wave in the same cilia as used in this study (12) also demonstrated that the metachronal wavelength does not change with CBF increase. In a photoelectronic measurement of the beats of respiratory tract cilia (6), both recovery and effective strokes were faster with increase of CBF. These studies support the conclusion from our AFM measurements of faster tangential velocity during effective stroke with CBF increase and independence of arc length on CBF.
Other imaging results in the literature are less straightforward for comparisons with our results. In optic measurement of cilia from Paramecium (14) the angular velocity of the effective stroke first sharply increased with CBF increase, followed by plateau with further CBF increase. In a study of rabbit tracheal cilia (26), the effective stroke shortened its duration linearly with CBF increase within the range 4–12 Hz but did not change at higher frequencies.
The recovery stroke is performed on a more perpendicular plane
The amplitude of the optic signal decreases with increasing beat frequency, in contrast to the increase of the amplitude in the AFM signal. This is so because the origin of the two signals is different, and they report on different parameters of the ciliary beat. The AFM signal originates in the effective stroke, as discussed above. The optical signal, however, provides information on the recovery stroke. The cilia project a shadow on the cell's surface when light is shone upon them from above the cell. The shadow is larger when the cilia perform the recovery stroke in an inclined plane, while during the effective stroke, performed in an upright plane, they scatter less light (27) (see Fig. 6 c). Thus, the decrease in amplitude of the optical signal should be interpreted as the recovery stroke being performed in a more perpendicular plane to the cell's surface (see Fig. 6, d and f, representing the planes of the recovery stroke during basal and stimulated frequency, respectively). A shorter recovery stroke arc length at the same inclination to the cell surface would also lead to a decrease in the amplitude of the optical signal, but this option is not acceptable, since the arc of the effective stroke does not change. This conclusion about the change of the asymmetry of the beat after ciliary stimulation was formerly suggested by the results of measuring the metachronal activity of the same type of cilia (12).
In addition, since the length of the effective stroke arc is constant, the recovery stroke is also performed faster. However, since the cilium is more erect during recovery stroke at high frequencies, the total path traveled by the cilium during a whole beat cycle shortens. To illustrate this, we schematically draw a top-down projection of the path described by the cilium tip in Fig. 6, e and g, during basal and stimulated frequency, respectively. Thus, when increasing the beat frequency, the cilium maintains a constant effective stroke arc, resulting in a linear increase in the tangential velocity and consequently a linear increase in mucus transport velocity, while during recovery stroke it becomes more erect, thus shortening the distance to the end of the cycle and the beginning of the next recovery stroke. The shortening of the total path should save on energy consumption, without sacrificing maximal transport velocity increase.
CONCLUSIONS
The simultaneous electro-optical and AFM measurements performed in this study reveal new details about the beat form of mucus propelling cilia, when they accelerate their beating frequency.
The length of the arc swept by the cilia during effective stroke remains constant during stimulation of CBF. This leads to a tangential velocity of the cilium tip precisely linear with the beating frequency. Consequently, the mucus transport velocity is expected to increase linearly with the beating frequency.
This feature does not depend on the type of stimulant (chemical or temperature), on the species of chemical stimulant (ATP or ACH), or on the concentration of the chemical stimulant (ATP: 1 μM, 10 μM, 100 μM). This seems to indicate that the switching occurs at constant positions of the cilium, presumably related to its inner structure, positions that are not affected by the mechanism leading to CBF increase.
While the effective stroke geometry remains unchanged, the recovery stroke is being performed in an increasingly perpendicular (to the cell surface) plane with increasing beat frequency, as indicated by the optical measurement. This fact leads to a shortening of the total path swept by the tip of the cilium during a beat cycle.
Editor: Gaudenz Danuser.
References
- 1.King, M., A. Gilboa, F. A. Meyer, and A. Silberberg. 1974. On the transport of mucus and its rheologic stimulants in ciliated systems. Am. Rev. Respir. Dis. 110:740–745. [DOI] [PubMed] [Google Scholar]
- 2.Stephan, M. M. 2004. Chasing the cilium. Scientist. 18:14–16. [Google Scholar]
- 3.Ovadyahu, D., D. Eshel, and Z. Priel. 1988. Intensification of ciliary motility by extracellular ATP. Biorheology. 25:489–501. [DOI] [PubMed] [Google Scholar]
- 4.Johnson, N. T., M. Villalon, F. H. Royce, R. Hard, and P. Verdugo. 1991. Autoregulation of beat frequency in respiratory ciliated cells—demonstration by viscous loading. Am. Rev. Respir. Dis. 144:1091–1094. [DOI] [PubMed] [Google Scholar]
- 5.Weiss, T., L. Gheber, V. Shoshan-Barmatz, and Z. Priel. 1992. Possible mechanism of ciliary stimulation by extracellular ATP: involvement of calcium-dependent potassium channels and exogenous Ca2+. J. Membr. Biol. 127:185–193. [DOI] [PubMed] [Google Scholar]
- 6.Lansley, A. B., M. J. Sanderson, and E. R. Dirksen. 1992. Control of the beat cycle of respiratory-tract cilia by Ca2+ and CAMP. Am. J. Physiol. 263:L232–L242. [DOI] [PubMed] [Google Scholar]
- 7.Gheber, L., Z. Priel, C. Aflalo, and V. Shoshanbarmatz. 1995. Extracellular ATP binding-proteins as potential receptors in mucociliary epithelium—characterization using [P-32] 3′-O-(4-benzoyl)benzoyl ATP, a photoaffinity label. J. Membr. Biol. 147:83–93. [DOI] [PubMed] [Google Scholar]
- 8.Korngreen, A., and Z. Priel. 1996. Purinergic stimulation of rabbit ciliated airway epithelia: control by multiple Ca2+ sources. J. Physiol. 497:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uzlaner, N., and Z. Priel. 1999. Interplay between the NO pathway and elevated [Ca2+]i enhances ciliary activity in rabbit trachea. J. Physiol. 516:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zagoory, O., A. Braiman, L. Gheber, and Z. Priel. 2001. Role of calcium and calmodulin in ciliary stimulation induced by acetylcholine. Am. J. Physiol. Cell Physiol. 280:C100–C109. [DOI] [PubMed] [Google Scholar]
- 11.Zagoory, O., A. Braiman, and Z. Priel. 2002. The mechanism of ciliary stimulation by acetylcholine—roles of calcium, PKA, and PKG. J. Gen. Physiol. 119:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gheber, L., and Z. Priel. 1994. Metachronal activity of cultured mucociliary epithelium under normal and stimulated conditions. Cell Motil. Cytoskeleton. 28:333–345. [DOI] [PubMed] [Google Scholar]
- 13.Sleigh, M. A., J. R. Blake, and N. Liron. 1988. The propulsion of mucus by cilia. Am. Rev. Respir. Dis. 137:726–741. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto, K., and Y. Nakaoka. 1994. Reconstitution of metachronal waves in ciliated cortical sheets of paramecium. J. Exp. Biol. 192:73–81. [DOI] [PubMed] [Google Scholar]
- 15.Robertson, A., W. Stannard, C. Passant, C. O'Callaghan, and A. Banerjee. 2004. What effect does isoflurane have upon ciliary beat pattern: an in-vivo study. Clin. Otolaryngol. 29:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chilvers, M. A., and C. O'Callaghan. 2000. Analysis of ciliary beat pattern and beat frequency using high speed imaging: comparison with the photomultiplier and photodiode methods. Thorax. 55:314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanderson, M. J., and M. A. Sleigh. 1981. Ciliary activity of cultured rabbit tracheal epithelium: beat pattern and metachrony. J. Cell Sci. 47:331–347. [DOI] [PubMed] [Google Scholar]
- 18.Marino, M. R., and E. Aiello. 1982. Ciliary beat dynamics of rabbit tracheal cilia in monolayer-culture. Am. Rev. Respir. Dis. 125:159. [Google Scholar]
- 19.Sugino, K., and H. Machemer. 1988. The ciliary cycle during hyperpolarization-induced activity—an analysis of axonemal functional parameters. Cell Motil. Cytoskeleton. 11:275–290. [DOI] [PubMed] [Google Scholar]
- 20.Teff, Z., Z. Priel, and L. A. Gheber. 2007. Forces applied by cilia measured on explants from muco-ciliary tissue. Biophys. J. 92:1813–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eshel, D., Y. Grossman, and Z. Priel. 1985. Spectral characterization of ciliary beating: variations in frequency with time. Am. J. Physiol. 249:C160–C165. [DOI] [PubMed] [Google Scholar]
- 22.Gueron, S., and N. Liron. 1993. Simulations of three-dimensional ciliary beats and cilia interactions. Biophys. J. 65:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gertsberg, I., V. Hellman, M. Fainshtein, S. Weil, S. D. Silberberg, M. Danilenko, and Z. Priel. 2004. Intracellular Ca2+ regulates the phosphorylation and the dephosphorylation of ciliary proteins via the NO pathway. J. Gen. Physiol. 124:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brokaw, C. J., and R. Kamiya. 1987. Bending patterns of Chlamydomonas flagella. 4. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskeleton. 8:68–75. [DOI] [PubMed] [Google Scholar]
- 25.Aiello, E., and M. A. Sleigh. 1972. The metachronal wave of lateral cilia of Mytilus edulis. J. Cell Biol. 54:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sleigh, M. A. 1983. Ciliary function in transport of mucus. Eur. J. Respir. Dis. 64:287–292. [PubMed] [Google Scholar]
- 27.Gheber, L., and Z. Priel. 1997. Extraction of cilium beat parameters by the combined application of photoelectric measurements and computer simulation. Biophys. J. 72:449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]