Abstract
Interferon regulatory factor-1 (IRF-1) is a candidate transcription factor for the regulation of the Toll-like receptor-4 (TLR-4) gene. Using a small interfering RNA-based (siRNA) process to silence IRF-1 gene expression in the leukemic monocytic cell line THP-1, we investigated whether such a modulation would alter TLR-4 expression and activation status in these cells. The siIRF-1 cells expressed elevated levels of TLR-4 mRNA and protein compared to controls by 90% and 77%, respectively. ICAM.1 protein expression and apoptosis levels were increased by 8.35- and 4.25-fold, respectively. The siIRF-1 cells overexpressed Bax mRNA compared to controls. Proteomic analysis revealed upmodulation of the Annexin-II protein in siIRF-1 THP-1 cells. Myelodysplastic syndrome (MDS) patients with an absence of full-length IRF-1 mRNA also overexpressed Annexin-II. It is plausible that this overexpression may lead to the activation of TLR-4 contributing to the increased apoptosis characterizing MDS.
Keywords: IRF-1, TLR-4, Annexin II, RNAi, myelodysplastic syndrome
Introduction
The interferon regulatory factor (IRF) family comprises 10 structurally related transcription factors, each mediating interferon (IFN) signaling and capable of regulating certain cellular processes including cell proliferation, differentiation, and cell death [1]. They all share homology in their amino terminal region that encompasses the DNA binding domain through which IRF family members recognize and bind to similar DNA sequences in the promoter of a variety of genes [2]. IRF-1 is a transcription factor involved in cell growth control, induction of apoptosis, and cell transformation by oncogenes [3–5]. The deletion of one or both alleles of the IRF-1 gene has been observed in acute myeloid leukemia, myelodysplasia, esophageal carcinomas, and gastric adenocarcinomas suggesting that this transcription factor acts as a tumor suppressor gene [6–10]. IRF-1's activities encompass maturation of cells mediating immune responses and regulation of the early events governing myeloid cells' terminal differentiation [11]. IRF-1 usually acts through the formation of induction complexes with other transcription factors. More specifically, IRF-1, PU.1, and IFN consensus sequence binding protein (ICSBP), another IRF family member solely expressed in hematopoietic cells, compose a complex in the bone marrow which recognizes and binds to IRF-PU.1 sites in the promoters of several genes, one of which is the Toll-like receptor-4 (TLR-4) gene [12].
TLR-4 is a member of a conserved family of type I transmembrane receptors, which are characterized by an intracellular signaling domain homolog to the interleukin-1 receptor. These receptors recognize microbial components, particularly bacterial lipopolysaccharide (LPS) [13,14]. Self molecules, including danger signals (heat shock proteins) or products of macromolecular degradation (fibronectin fragments), have been shown to act as TLR-4 ligands [15,16]. On ligation, TLR signaling triggers the expression of proinflammatory cytokines, chemokines, costimulatory, and adhesion molecules (CD54/ICAM.1), priming the adaptive immune system and initiating the inflammatory responses [17–19].
Recently, the involvement of IRFs in innate and adaptive immune responses has gathered substantial interest [20]. The aim of this study was to evaluate the association between the IRF-1 transcription factor and TLR-4 expression. We used RNA interference to silence IRF-1 gene expression in the THP-1 cell line to investigate whether this modulation would affect TLR-4 expression and activation in these cells. Small interfering RNA-based (siRNA) strategy was preferred as knock-out mice studies have found it too complex to define the effects of IRF-1 loss in bone marrow. Moreover, it has been proven that RNA interference is a functional pathway of gene silencing, having a biologic impact on myeloid leukemia cell lines [21]. Additionally, two-dimensional (2D) gel electrophoresis was used for the identification of obliterated or newly expressed proteins after IRF-1 loss.
Materials and Methods
Specific Reagents
PE-conjugated anti-TLR-4 (clone HTA125) was from eBioscience (San Diego, CA). Immunoglobulin G (isotype control) monoclonal antibody, fluorescein isothiocyanate-conjugated Annexin-V and PE-conjugated anti-ICAM.1 were obtained from Becton Dickinson (San Jose, CA). Lipopolysaccharide (Escherichia coli, serotype O55:B5) was purchased from Sigma (St. Louis, MO) and used at a concentration of 1 µg/ml. Titration experiments were carried out to select the optimal concentration of all the reagents. All values presented in the study are the mean value of three independent experiments.
Small Interfering RNA-Based Process
The siRNA strategy [22] was employed to silence the endogenous IRF-1 in THP-1 cells. IRF-1 and scrambled siRNA were generated using Donze's procedure and an in vitro transcription system (T7 RiboMAX Express RNAi; Promega, Madison, WI). Briefly, the siRNA sequences for IRF-1 and primers were chosen using a web-based tool (siRNA Target Designer; Promega). The primers are: IRF-1 si-SS sense 5′-AAGTAATTTCCCTTCCTCATCTATAGTGAGTCGTATTAGGATCC-3′ and IRF-1 si-AS antisense 5′-AAGATGAGGAAGGGAAATTACTATAGTGAGTCGTATTAGGATCC-3′. Target sequences for IRF-1 gene are underlined, and the remaining 3′ regions correspond to T7 promoter sequences. T7 si primer, 5′-GGATCCTAATACGACTCACTATAG-3′, was synthesized and scrambled primers (sense and antisense) were also created as siRNA control. The double-stranded 21-nucleotide RNA were generated according to the manufacturer's recommendations. The concentration of siRNA was optimized at 1.8 µg in each transfection.
THP-1 Cell Cultures and Transfection
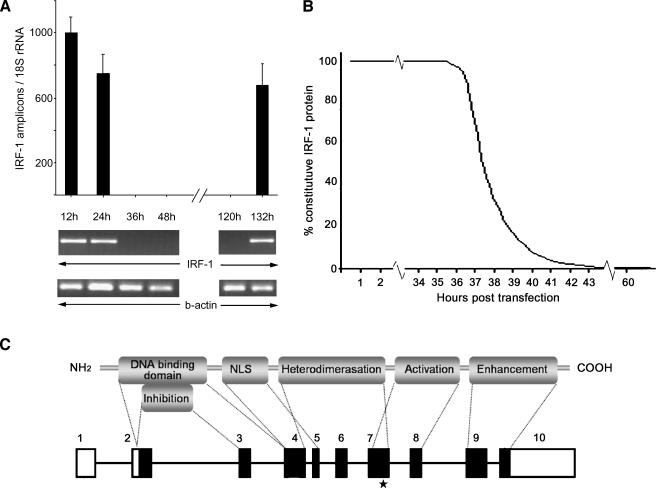
THP-1 cells were cultured in RPMI-1640 (Gibco, Carlsbad, CA) supplemented with 10% FBS, 5 x 10-5 M 2-mercaptoethanol, 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells were regularly passaged to maintain exponential growth. Twenty-four hours before transfection, cells were diluted in fresh medium without antibiotics and transferred to 24-well plates. Transient transfection of siRNA was performed using a reagent (GeneEraser; Stratagene, La Jolla, CA) according to the manufacturer's recommendations. The efficiency of the transfection was monitored every 12 hours. At 36 hours, IRF-1 expression was totally inhibited. Taking this into account and given that IRF-1 protein has a half-life of 0.5 hours, we estimated the decreased rate to be (1/2)2v with v representing each hour after IRF-1 transcriptional inactivation (Figure 1B). Using this model, we set harvesting time at 60 hours posttransfection. Specific silencing was confirmed by at least three independent experiments. The cells were also treated with transfection reagent alone or with the nonsilencing scrambled siRNA (scrIRF-1 siRNA) to be used as controls.
Figure 1.
Small interfering RNA for the transcriptional inhibition of IRF-1. (A) The IRF-1 transcriptional expression as assessed by real-time PCR. At 36 hours posttransfection, no IRF-1 mRNA was detected. IRF-1 expression was restored 136 hours posttransfection. The transcriptional expression of β-actin (PCR) and 18S rRNA (real-time PCR) were not disturbed by the process. The THP-1 cells treated with transfection reagent alone or non-silencing scrambled siRNA revealed no change in IRF-1 expression. (B) A mathematical approach of the degradation rate of the constitutive IRF-1 protein levels posttransfection. At 36 hours after the addition of the siRNA, the transcription of IRF-1 was inhibited. The already formed IRF-1 protein levels (constitutive) would drop by 50% every 30 minutes (half-life). We estimated that, at 42 hours posttransfection, the IRF-1 protein levels would be less than 0.1% of the constitutive. To further minimize the margin of error, we set the LPS induction at 52 hours and the harvesting time at 60 hours posttransfection. (C) Schematic representation of the IRF-1 protein's functional domains and the mRNA. The known functional domains of the IRF-1 protein are depicted and correlated with the genomic arrangement of the full-length transcript. The black boxes represent the translated areas. The asterisk indicates the complementary site of the siRNA, which is outside the conserved region shared by the IRF family members.
Two-Dimensional Gel Electrophoresis
Protein was extracted from 2 x 106 THP-1 cells using a lysis buffer (0.5% w/v CHAPS, 8 M urea, 0.2% w/v DTT, and 0.5% v/v immobilized pH gradient buffer pH 3–10). Immobilized pH gradient strips [18 cm, pH 3–10] were rehydrated for 20 hours with the sample solution (total protein amount of 150 µg assessed by Bradford analysis). Proteins were focused using a platform (Ettan IPGphor, San Francisco, CA) for 1 hour at 1000 V and for 4 hours at 8000 V. Strips were then equilibrated (50 mM Tris-HCl pH 6.8, 6 M urea, 30% glycerol, 2% SDS, and 1% DTT) and run overnight onto SDS-PAGE (acrylamide/bisacrylamide ratio of 4:0.232) at 90 V. Finally, the protein zones were identified with silver staining.
Protein Identification By Mass Spectrometry
The spots of interest were excised from gels and digested overnight with sequence grade trypsin (Promega). The eluate was analyzed on an ion trap mass spectrometer with a nanospray source (LCQ Deca; ThermoFinnigan, Baltimore, MD). The interpretation of both the mass spectrometry (MS) and tandem mass spectrometry (MS/MS) data was carried out by search in protein databases (SwissProt) using the Turbo-SEQUEST search engine.
mRNA Analysis
Total RNA was collected as previously described [23]. Real-time polymerase chain reaction (PCR) was used to confirm the integrity and perform the normalization of all cDNA samples through the amplification of the 18S ribosomal RNA (rRNA), which served as the housekeeping gene. The primers' sequences used are described in Table 1.
Table 1.
Characteristics of Primer Sets.
| Target Gene | Accession Number | Amplicon Position | Amplicon Size | Amplification Efficiency |
| 18S rRNA | NG_002801 | 1151–1453 | 302 | 1.98 |
| IRF-1 | NM_002198 | 391µ605 | 214 | 1.83 |
| TLR-4 | NM_003266 | 3035–3370 | 355 | 1.91 |
| Bax | NM_138761 | 1804–2053 | 249 | 1.86 |
| Bcl-2 | NM_000633 | 1078–1313 | 236 | 1.88 |
Quantitative Real-Time PCR
mRNA levels were quantified by real-time PCR. We designed oligonucleotide primers for each mRNA, in accordance with the published sequences (Table 1). All values were measured as the number of amplicons normalized against 18S rRNA, a housekeeping gene, as previously described [23,24].
Flow Cytometry
The expression of various proteins was investigated by flow cytometry analysis, either constitutively or following appropriate stimulation, as previously described [24].
Immunohistochemistry
We investigated the expression of Annexin-II protein by immunohistochemistry, using a monoclonal antibody specific to the Annexin-II (Becton Dickinson). We used paraffin-embedded bone marrow biopsy tissues from 10 myelodysplastic syndrome (MDS) patients characterized by absence (n = 5) or presence (n = 5) of full-length IRF-1 mRNA, three acute promyelocytic leukemia patients with documented Annexin-II overexpression (positive controls) and three normal individuals (negative controls) [23,25]. Annexin-II expression was measured as the percentage of immunoreactive cells per 1000 nucleated cells in 10 fields per preparation.
Statistical Analysis
Mann-Whitney U tests were used for data comparison.
Results
siIRF-1 THP-1 Cell Cultures
The IRF-1 mRNA expression was inhibited 36 hours posttransfection, as assessed by PCR or real-time PCR (Figure 1A) in the cells treated with the silencing siRNA (siIRF-1 THP-1 cells). The cells treated with transfection reagent alone and those transfected with no silencing scrambled siRNA (scrIRF-1 THP-1 cells) showed no change in IRF-1 mRNA expression, supporting the efficiency of the siRNA strategy. The expression of β-actin (PCR) and 18S rRNA (real-time PCR) among the three groups remained unchanged (data not shown), confirming the high specificity of the siRNA strategy (Figure 1).
TLR-4 Is Upregulated in THP-1 siIRF-1 Cells
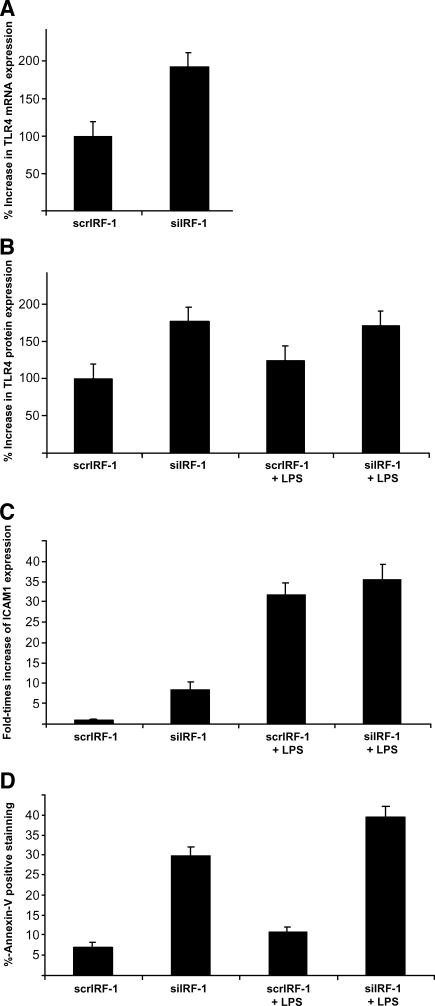
TLR-4 expression was examined by quantitative real-time PCR and FACS analysis. We observed significantly higher levels of TLR-4 in siIRF-1 THP-1 cells in comparison with the scrIRF-1 THP-1 cells. The siIRF-1 THP-1 cells presented a 90 ± 10% and 77 ± 8% increase in the TLR-4 mRNA and protein levels, respectively (P < .01) (Figure 2, A and B). To investigate the potential implication of IRF-1 in the LPS-driven TLR-4 levels, LPS was added at 52 hours posttransfection (8 hours of induction) in all cell groups. The scrIRF-1 THP-1 cells treated with LPS showed 25 ± 3% increase in TLR-4 protein levels in comparison with the untreated scrIRF-1 THP-1 cells. The LPS-stimulated siIRF-1 THP-1 cells showed no change in the TLR-4 protein levels compared to the untreated siIRF-1 THP-1 cells. Overall, the LPS-treated siIRF-1 THP-1 cells presented a 46 ± 8% increase in TLR-4 protein levels in contrast to the LPS-triggered scrIRF-1 THP-1 cells (Figure 2B). These results suggest that IRF-1 is implicated in the expression of TLR-4 because its silencing fully activated TLR-4 on siIRF-1 THP-1 cells to the extent that their levels remained unchanged despite mild LPS treatment.
Figure 2.
siIRF-1 THP-1 cells present elevated levels of TLR-4 and ICAM.1 protein levels as well as increased Annexin-V+ staining. (A) The siIRF-1 THP-1 cells presented a 90 ± 10% increase of the TLR-4 mRNA levels when compared to the scrIRF-1 THP-1 cells (P < .01). (B) The siIRF-1 THP-1 cells presented a 77 ± 8% increase of the TLR-4 protein levels when compared to the scrIRF-1 THP-1 cells (P < .01). The scrIRF-1 THP-1 cells treated with LPS presented elevated TLR-4 protein levels by 25 ± 3% in comparison with the untreated cells. The siIRF-1 THP-1 cells challenged with LPS showed no differences in the TLR-4 protein levels when compared to the untreated siIRF-1 THP-1 cells. Finally, the LPS-treated siIRF-1 THP-1 cells presented a 46 ± 8% increase in the TLR-4 protein levels in contrast to the LPS-triggered scrIRF-1 THP-1 cells. (C) The siIRF-1 THP-1 cells presented an 8.35-fold increase in their ICAM.1 protein levels, in contrast to the scrIRF-1 THP-1 cells (P < .01). The scrIRF-1 THP-1 cells treated with LPS presented a 31.7-fold increase in ICAM.1 protein levels in comparison with the untreated cells (P < .01). The siIRF-1 THP-1 cells challenged with LPS revealed a 27.3-fold increase in ICAM.1 levels when compared to the untreated siIRF-1 THP-1 cells (P < .01). Finally, the LPS-treated siIRF-1 THP-1 cells presented a 3.98-fold increase in ICAM.1 levels in contrast to the LPS-triggered scrIRF-1 THP-1 cells (P < .01). (D) The constitutive levels of apoptosis (Annexin-V+) in the scrIRF-1 THP-1 cells were calculated at 7 ± 2% of the total population. The siIRF-1 THP-1 cells presented a significant increase in apoptosis (4.25-fold) with the Annexin-V+ positive cells reaching 29 ± 3% of the total population, indicating that IRF-1 interference resulted in a 23% increase in cellular apoptotic levels (P < .01). The scrIRF-1 THP-1 cells treated with LPS presented a 0.55-fold increase in Annexin-V+ staining (10.85 ± 2%) in comparison with the untreated cells. The siIRF-1 THP-1 cells challenged with LPS (39.5 ± 4%), revealed a 1.4-fold increase in the apoptotic levels when compared to the untreated siIRF-1 THP-1 cells (P < .05). Finally, the LPS-treated siIRF-1 THP-1 cells presented a 3.6-fold increase in Annexin-V+ staining in contrast to the LPS-triggered scrIRF-1 THP-1 cells (P < .01).
ICAM.1 (CD54) Is Upregulated in THP-1 siIRF-1 Cells
The adhesion molecule ICAM.1 is a surrogate marker for TLR-mediated activation. The siIRF-1 THP-1 cells presented an 8.35-fold increase (P < .01) in their ICAM.1 protein levels, in contrast to the scrIRF-1 THP-1 cells (Figure 2C). Following LPS stimulation, the scrIRF-1 THP-1 cells presented a 31.7-fold increase in ICAM.1 protein levels in comparison with the untreated cells (P < .01). The siIRF-1 THP-1 cells challenged with LPS, revealed a 27.3-fold increase in ICAM.1 levels when compared to the untreated siIRF-1 THP-1 cells (P < .01). Overall, the LPS-stimulated siIRF-1 THP-1 cells presented a 3.98-fold increase in ICAM.1 levels in contrast to the LPS-stimulated scrIRF-1 THP-1 cells (P < .01) (Figure 2C). The increase of ICAM.1 expression in the siIRF-1 THP-1 cells advocates that IRF-1 inhibition is suffice to activate TLRs.
Annexin-V+ Staining and Proapoptotic Bax mRNA Are Upregulated in THP-1 siIRF-1 Cells
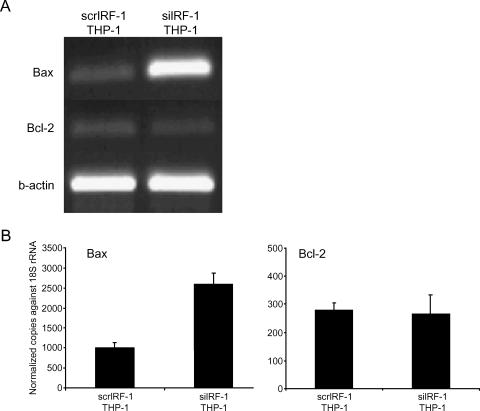
Annexin-V positive staining was employed to estimate early and intermediate apoptosis. The constitutive levels of apoptosis (Annexin-V+) in the scrIRF-1 THP-1 cells were calculated at 7 ± 2% of the total population. The siIRF-1 THP-1 cells presented a significant increase in apoptosis (4.25-fold) (P < .01) with the Annexin-V+ positive cells reaching 29 ± 3% of the total population indicating that IRF-1 interference resulted in a 23% increase in cellular apoptotic levels (P < .01) (Figure 2D). To further investigate apoptosis in the scrIRF-1 THP-1 cells and siIRF-1 THP-1 cells, we examined the mRNA expression levels of Bax (proapoptotic) and Bcl-2 (antiapoptotic). The expression levels of Bax were elevated 1.6 times in the siIRF-1 THP-1 cells when compared to the scrIRF-1 THP-1 cells (P < .05). The expression levels of Bcl-2 did not present any differences between the two groups (Figure 3).
Figure 3.
mRNA expression of Bax and Bcl-2. (A) The mRNA expression of Bax, Bcl-2, and β-actin in scrIRF-1 THP-1 cells and siIRF-1 THP-1 cells. (B) The mRNA expression of Bax, Bcl-2, and β-actin in scrIRF-1 THP-1 cells and siIRF-1 THP-1 cells, as it was studied with quantitative real-time PCR. The siIRF-1 THP-1 cells presented a 1.6-fold increase in the Bax mRNA levels when compared to the scrIRF-1 THP-1 cells (P < .05). The Bcl-2 mRNA expression presented no statistically significant difference between the two groups.
The scrIRF-1 THP-1 cells treated with LPS presented a 0.55-fold increase in Annexin-V+ staining (10.85 ± 2%) in comparison with the untreated cells. The siIRF-1 THP-1 cells challenged with LPS (39.5 ± 4%) revealed a 1.4-fold increase in the apoptotic levels when compared to the untreated siIRF-1 THP-1 cells (P < .05). Overall, the LPS-treated siIRF-1 THP-1 cells presented a 3.6-fold increase in Annexin-V+ staining in contrast to the LPS-triggered scrIRF-1 THP-1 cells (P < .01) (Figure 2D).
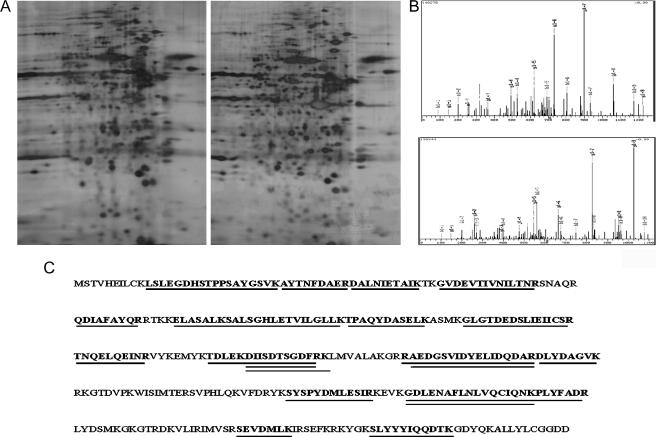
Identification of Differentially Expressed Proteins After siRNA Treatment By 2D Gel Electrophoresis
IRF-1 inhibition caused alterations in the protein expression of THP-1 cells, in regard to 21 peptides (Figure 4). More specifically, Annexin-II, tryptophanyl-tRNA synthetase, ubiquitinactivating enzyme E1, esterase D, and keratine type II cytoskeletal 1 were detected in the siIRF-1 THP-1 cells but not in the scrIRF-1 THP-1 cells. The protein coverage of Annexin-II, as calculated by MS, was 64.2% by amino acid count and 63.2% by mass.
Figure 4.
Identification of differentially expressed proteins after IRF-1 interference. (A) Two-dimensional electrophoresis was employed to investigate any differentiated protein expression profile before (right gel) and after (left gel) IRF-1 transcriptional interference. The mass spectrometry that followed identified the appearance of five protein spots solely in the siIRF-1 gel, namely Annexin II, tryptophanyl-tRNA synthetase, ubiquitin-activating enzyme E1, esterase D, and keratine type II cytoskeletal 1. (B) Two of the peptides as analyzed by MS/MS. (C) The Annexin-II protein sequence. The positions of the peptides that were identified by MS are underlined.
Annexin-II Expression in MDS Bone Marrows
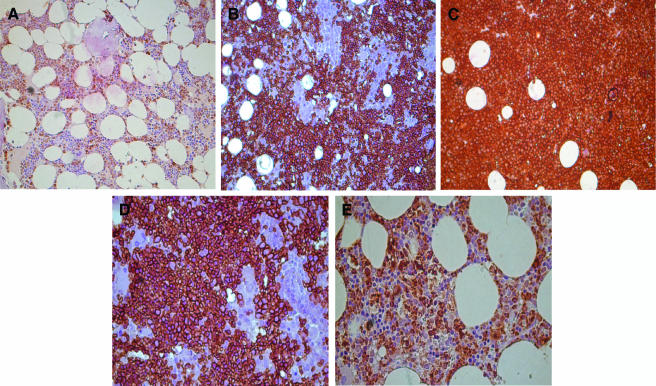
In silico analysis of the proteins that were differentially expressed after siRNA treatment identified Annexin-II as the most prominent protein to be involved in certain hematological disorders, such as MDS. We have previously reported that IRF-1 mRNA is either diminished or inactivated through alternative splicing in human myelodysplasia [23]. We have further reported that TLR-4 is upregulated in hemopoietic progenitor cells of MDS patients, contributing to the increased apoptosis of their bone marrow progenitor cells [24]. In this study, LPS stimulation upregulated TLR-4 expression and increased ICAM.1 expression in MDS marrow and in THP-1 cells that were used as controls [24]. Moreover, TLR-4 upregulation was found to be tumor necrosis factor-mediated both in MDS and in THP-1 cells, whereas apoptosis was similarly increased after tumor necrosis factor and LPS treatment [24]. In the present study, the expression of Annexin-II following the siIRF-1 treatment of THP-1 cells prompted us to further investigate Annexin-II expression in the bone marrow of MDS patients with or without full-length IRF-1 mRNA, as well as in the bone marrow of three patients with acute promyelocytic leukemia, the latter being used as positive controls. The MDS patients without full-length IRF-1 transcript presented a higher Annexin-II expression than the MDS patients with IRF-1 full-length transcript. A gradual increase in the immunoreactive index was observed among normal controls (0.04 ± 0.02), MDS patients displaying IRF-1 full-length transcript (0.40 ± 0.16), and MDS patients with an absence of the IRF-1 full-length transcript (0.78 ± 0.11) (P < .05). The highest score was noted in the acute promyelocytic leukemia group of patients (0.96 ± 0.39) (Figure 5).
Figure 5.
Expression of Annexin II on bone marrow biopsies by immunohistochemistry. Panels A, B, and C are derived from a control, an MDS patient without IRF-1 full-length, and an acute promyelocytic leukemia, respectively (magnification, x20), whereas panels D and E are derived from MDS patients with and without IRF-1, respectively, under higher magnification (x40). (A) Normal donor characterized by the presence of the IRF-1 full-length transcript. The expression of Annexin-II is limited only to the cytoplasm of the myeloid cells. The immunoreactivity in this group (n = 3) is estimated at 0.04 ± 0.02. (B) MDS patient characterized by the absence of the IRF-1 full-length transcript. Annexin-II positive staining was detected in all myeloid cells and partially in the erythroid cells. The immunoreactivity in the MDS patients characterized by the absence of the IRF-1 full-length transcript (n = 5) was evaluated at 0.78 ± 0.11. (C) Representative of an acute promyelocytic leukemia patient. In this group (n = 3), the Annexin-II staining was universal and the immunoreactivity percentage reached 0.96 ± 0.039. Interestingly, this type of leukemia is characterized by the absence of the IRF-1 full-length transcript. (D) MDS patient characterized by the absence of IRF-1 full-length transcript. The Annexin-II (as assessed by staining) is concentrated in the membrane of the cell. (E) MDS patient characterized by the presence of IRF-1 full-length transcript. The Annexin-II (as assessed by staining) is localized in both the membrane and the cytoplasm. In the MDS patients characterized by the presence of the IRF-1 full-length transcript (n = 5), the immunoreactivity is estimated at 0.40 ± 0.16.
Discussion
IRF-1 plays a central role in immune and inflammatory responses. Being a key transcriptional activator of the IFNα/β genes in virus-infected cells, it orchestrates fundamental processes of innate immunity [26]. Recent studies have demonstrated that IRFs are also involved in the regulation of TLR expression [20,27]. With this report, we demonstrate that IRF-1 silencing through RNA interference, leads to an overexpression of TLR-4 in THP-1 cells. THP-1 cells activate NF-κB and other transcription factors in response to TLR ligands and cytokines. Unlike other cell lines, which are engineered to respond to TLR agonists, THP-1 cells naturally express the TLR genes and others involved in TLR-signal cascade, thereby constituting a useful and convenient model system for TLR studies [28]. Consequently, the use of THP-1 cells to investigate the association of IRF-1 and TLR-4 is apt.
A composite binding site for both the IRF family members and PU.1, an Ets transcription factor, has been located in the promoter of TLR-4 adjacent to the inner purine-rich motif [12]. A similar site has been identified and examined in the promoter of the CYBB gene that encodes for gp91phox, a subunit of the phagocyte respiratory burst oxidase catalytic unit [29]. It has been demonstrated that PU.1 is essential for the formation of the multiprotein transcription complexes in which IRF-1 and ICSBP can participate either solely or together. ICSBP binds to its target DNA sequence following association with either IRF-1 or IRF-2, through a conserved domain known as the IRF-1 association domain [30]. While being structurally similar and recognizing the same DNA sequences, the IRF-1 and IRF-2 are functionally distinct, being competitive inhibitors of each other at the transcriptional level [31]. Furthermore, the 8-hour half-life of IRF-2 engenders long-lasting transcription complexes, in contrast to IRF-1 characterized by 30 minutes of half-life. The precise mechanism, by which IRF-1 interferes with TLR-4 inducion, remains elusive. TLR-4 overexpression in siIRF-1 THP-1 cells could be attributed to the formation of an altered transcription complex, comprising either PU.1 and ICSBP alone or the substitution of IRF-1 by the more stable IRF-2.
Although IRF-1 has been directly implicated in the regulation of the TLR-4 gene, as yet no direct relationship between IRF-1 and TLR-4 activation has been reported. In our study, we show that TLR-4 not only presented elevated levels in siIRF-1 THP-1 cells compared to the control group, but it was also characterized by activation, with ICAM.1 being significantly upregulated in IRF-1 silenced cells. Proteomic analysis revealed an up-modulation of Annexin-II protein in siIRF-1 THP-1 cells. Annexin-II, a phospholipid-binding protein, serves as a platform on the cell surface for the binding of both plasminogen and tissue plasminogen activator [32]. By anchoring both these molecules in close proximity to each other, Annexin-II provides an environment that enhances plasmin production by protecting the fibrinolytic enzymes from their inhibitors. Furthermore, the elevated expression of Annexin-II in leukemic cells correlates with their in vitro ability to generate plasmin. This results in increased fibrinolysis, production of fibronectin fragments, and plasmin-mediated proteolytic activation of metalloproteinases [24,33]. The upmodulation of Annexin-II in IRF-1 silenced cells could lead to an elevated plasmin activity that gives rise to an abundance of fibronectin fragments. Such fragments are well-known TLR-4 ligands and could account for their activation.
LPS binding to TLR-4, triggers activation of the cellular signaling pathways resulting in nuclear translocation of NF-κB and apoptosis [34]. The molecular mechanism that links the upstream NF-κB signaling to the recruitment and activation of caspases remains unclear. TLR triggering has been linked to excessive programmed cell death through the Fas-associated death domain pathway [35–37]. TLR-4 and its respective intracellular binding partners, MyD88, Mal/TIRAP, along with the extrinsic Fas-associated death domain-caspase 8 pathway, have been shown to mediate LPS-induced apoptosis. However, questions remain as to how this signaling pathway activates the effector proteases of apoptosis [38,39]. It has been suggested that members of the Bcl-2 family play the role of mediator in LPS-induced apoptosis. LPS upregulates the expression of the proapoptotic Bcl-2 family members Bax, Bad, and Bak, and downregulates the levels of the antiapoptotic members, such as Bcl-2 and Bcl-xL [40,41]. In addition, the vascular endothelial growth factor that inhibits LPS-induced upregulation of the proapoptotic Bcl-2 members provides protection against LPS-induced endothelial cell apoptosis [42]. Our results further support these data, showing that overexpression and activation of TLR-4 in IRF-1 silenced cells are accompanied by increased apoptosis. Although the mechanism by which TLR-4-elicited apoptosis remains unknown, our data implicate a role for Bcl-2 family proteins in determining THP-1 apoptosis after TLR-4 activation.
We have previously reported that the bone marrow hematopoietic cells of MDS patients either abolish their IRF-1 expression or present spliced variants with functional deregulation [23,43]. We further reported that TLR-4 is not only significantly upregulated in CD34+ cells in MDS patients but is also involved in promoting apoptosis, possibly contributing to MDS cytopenia [24]. In the latter study, the response of the THP-1-stimulated cell line used as control was similar to that of bone marrow cells derived from MDS patients following identical stimulation with the TLR-4 ligand LPS. This prompted us to investigate the presence of Annexin-II in bone marrow biopsies of MDS patients with or without full-length IRF-1 mRNA expression. Indeed, immunohistochemistry revealed the abundant presence of Annexin-II in MDS patients with absence of full-length IRF-1 mRNA. This is in accordance with the overexpression of Annexin-II in siIRF-1 THP-1 cells. Whether this abundance of Annexin-II in the MDS bone marrow environment is pathogenetically implicated in the TLR-4 overexpression and activation in these patients warrants further investigation.
Acknowledgements
We express our thanks to Haralampos M. Moutsopoulos for his guidance and encouragement. We also thank M.N. Manoussakis and E. Stea for their comments and suggestions.
Abbreviations
- IFN
interferon
- IRF-1
interferon regulatory factor-1
- LPS
lipopolysaccharide
- MDS
myelodysplastic syndrome
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- PCR
polymerase chain reaction
- siRNA
small interfering RNA
- TLR-4
Toll-like receptor-4
Footnotes
The present study was supported by research funding from the General Secretariat of Research and Development (PENED-01ED263) and Boehringer-Ingelheim Hellas Co.
References
- 1.Mamane Y, Heylbroeck C, Génin P, Algarté M, Servant MJ, LePage C, DeLuca C, Kwon H, Lin R, Hiscott J. Interferon regulatory factors: the next generation. Gene. 1999;237(1):1–14. doi: 10.1016/s0378-1119(99)00262-0. [DOI] [PubMed] [Google Scholar]
- 2.Kroger A, Koster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. J Interferon Cytokine Res. 2002;22(1):5–14. doi: 10.1089/107999002753452610. [DOI] [PubMed] [Google Scholar]
- 3.Fujita T, Kimura Y, Miyamoto M, Barsoumian EL, Taniguchi T. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature. 1989;337(6204):270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- 4.Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, Furia A, Miyata T, Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 6.Harada H, Kondo T, Ogawa S, Tamura T, Kitagawa M, Tanaka N, Lamphier MS, Hirai H, Taniguchi T. Accelerated exon skipping of IRF-1 mRNA in human myelodysplasia/leukemia; a possible mechanism of tumor suppressor inactivation. Oncogene. 1994;9(11):3313–3320. [PubMed] [Google Scholar]
- 7.Liebermann DA, Hoffman B. Interferon regulatory factor-1 myelodysplasia and leukemia. Leuk Res. 2006;30(9):1069–1071. doi: 10.1016/j.leukres.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka N, Taniguchi T. The interferon regulatory factors and oncogenesis. Semin Cancer Biol. 2000;10(2):73–81. doi: 10.1006/scbi.2000.0310. [DOI] [PubMed] [Google Scholar]
- 9.Tzoanopoulos D, Speletas M, Arvanitidis K, Veiopoulou C, Kyriaki S, Thyphronitis G, Sideras P, Kartalis G, Ritis K. Low expression of interferon regulatory factor-1 and identification of novel exons skipping in patients with chronic myeloid leukaemia. Br J Haematol. 2002;119(1):46–53. doi: 10.1046/j.1365-2141.2002.03829.x. [DOI] [PubMed] [Google Scholar]
- 10.Willman CL, Sever CE, Pallavicini MG, Harada H, Tanaka N, Slovak ML, Yamamoto H, Harada K, Meeker TC, List AF, et al. Deletion of IRF-1, mapping to chromosome 5q31.1, in human leukemia and preleukemic myelodysplasia. Science. 1993;259(5097):968–971. doi: 10.1126/science.8438156. [DOI] [PubMed] [Google Scholar]
- 11.Abdollahi A, Lord KA, Hoffman-Liebermann B, Liebermann DA. Interferon regulatory factor 1 is a myeloid differentiation primary response gene induced by interleukin 6 and leukemia inhibitory factor: role in growth inhibition. Cell Growth Differ. 1991;2(8):401–407. [PubMed] [Google Scholar]
- 12.Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B. PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem. 2000;275(13):9773–9781. doi: 10.1074/jbc.275.13.9773. [DOI] [PubMed] [Google Scholar]
- 13.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 15.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., 3rd The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276(13):10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 16.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4. J Immunol. 2001;167(5):2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 17.Andreakos E, Foxwell B, Feldmann M. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunol Rev. 2004;202:250–265. doi: 10.1111/j.0105-2896.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 18.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 19.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van't Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002;168(3):1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 20.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6(9):644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 21.Cioca DP, Aoki Y, Kiyosawa K. RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Ther. 2003;10(2):125–133. doi: 10.1038/sj.cgt.7700544. [DOI] [PubMed] [Google Scholar]
- 22.Donze O, Picard D. RNA interference in mammalian cells using siRNAs synthesized with T7 RNA polymerase. Nucleic Acids Res. 2002;30(10):e46. doi: 10.1093/nar/30.10.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maratheftis CI, Bolaraki PE, Giannouli S, Kapsogeorgou EK, Moutsopoulos HM, Voulgarelis M. Aberrant alternative splicing of interferon regulatory factor-1 (IRF-1) in myelodysplastic hematopoietic progenitor cells. Leuk Res. 2006;30(9):1177–1186. doi: 10.1016/j.leukres.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Maratheftis CI, Andreakos E, Moutsopoulos HM, Voulgarelis M. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clin Cancer Res. 2007;13(4):1154–1160. doi: 10.1158/1078-0432.CCR-06-2108. [DOI] [PubMed] [Google Scholar]
- 25.Menell JS, Cesarman GM, Jacovina AT, McLaughlin MA, Lev EA, Hajjar KA. Annexin II and bleeding in acute promyelocytic leukemia. N Engl J Med. 1999;340(13):994–1004. doi: 10.1056/NEJM199904013401303. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi T, Lamphier MS, Tanaka N. IRF-1: the transcription factor linking the interferon response and oncogenesis. Biochim Biophys Acta. 1997;1333(1):M9–M17. doi: 10.1016/s0304-419x(97)00014-0. [DOI] [PubMed] [Google Scholar]
- 27.Moynagh PN. TLR signalling and activation of IRFs: revisiting old friends fromthe NF-kappaB pathway. Trends Immunol. 2005;26(9):469–476. doi: 10.1016/j.it.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Li T, Hu J, Li L. Characterization of Tollip protein upon lipopolysaccharide challenge. Mol Immunol. 2004;41(1):85–92. doi: 10.1016/j.molimm.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Eklund EA, Jalava A, Kakar R. PU.1, interferon regulatory factor 1, and interferon consensus sequence-binding protein cooperate to increase gp91(phox) expression. J Biol Chem. 1998;273(22):13957–13965. doi: 10.1074/jbc.273.22.13957. [DOI] [PubMed] [Google Scholar]
- 30.Dror N, Alter-Koltunoff M, Azriel A, Amariglio N, Jacob-Hirsch J, Zeligson S, Morgenstern A, Tamura T, Hauser H, Rechavi G, et al. Identification of IRF-8 and IRF-1 target genes in activated macrophages. Mol Immunol. 2007;44(4):338–346. doi: 10.1016/j.molimm.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Harada H, Willison K, Sakakibara J, Miyamoto M, Fujita T, Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990;63(2):303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- 32.Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator: I. Identity with Annexin II. J Biol Chem. 1994;269(33):21191–21197. [PubMed] [Google Scholar]
- 33.Gilmore WS, Olwill S, McGlynn H, Alexander HD. Annexin A2 expression during cellular differentiation in myeloid cell lines. Biochem Soc Trans. 2004;32(Pt 6):1122–1123. doi: 10.1042/BST0321122. [DOI] [PubMed] [Google Scholar]
- 34.Daun JM, Fenton MJ. Interleukin-1/Toll receptor family members: receptor structure and signal transduction pathways. J Interferon Cytokine Res. 2000;20(10):843–855. doi: 10.1089/10799900050163217. [DOI] [PubMed] [Google Scholar]
- 35.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. Embo J. 2000;19(13):3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan W, Ha T, Li Y, Ozment-Skelton T, Williams DL, Kelley J, Browder IW, Li C. Overexpression of TLR2 and TLR4 susceptibility to serum deprivation-induced apoptosis in CHO cells. Biochem Biophys Res Commun. 2005;337(3):840–848. doi: 10.1016/j.bbrc.2005.09.123. [DOI] [PubMed] [Google Scholar]
- 37.Haase R, Kirschning CJ, Sing A, Schröttner P, Fukase K, Kusumoto S, Wagner H, Heesemann J, Ruckdeschel K. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J Immunol. 2003;171(8):4294–4303. doi: 10.4049/jimmunol.171.8.4294. [DOI] [PubMed] [Google Scholar]
- 38.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11(1):115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 39.Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, Blenis J, Tschopp J. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol. 2000;2(4):241–243. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 40.Haendeler J, Zeiher AM, Dimmeler S. Vitamin C and E prevent lipopolysaccharide-induced apoptosis in human endothelial cells by modulation of Bcl-2 and Bax. Eur J Pharmacol. 1996;317(2–3):407–411. doi: 10.1016/s0014-2999(96)00759-5. [DOI] [PubMed] [Google Scholar]
- 41.Messmer UK, Briner VA, Pfeilschifter J. Tumor necrosis factor-alpha and lipopolysaccharide induce apoptotic cell death in bovine glomerular endothelial cells. Kidney Int. 1999;55(6):2322–2337. doi: 10.1046/j.1523-1755.1999.00473.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim KY, Shin HK, Choi JM, Hong KW. Inhibition of lipopolysaccharide- induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther. 2002;300(2):709–715. doi: 10.1124/jpet.300.2.709. [DOI] [PubMed] [Google Scholar]
- 43.Giannouli S, Tzoanopoulos D, Ritis K, Kartalis G, Moutsopoulos HM, Voulgarelis M. Autoimmune manifestations in human myelodysplasia: a positive correlation with interferon regulatory factor-1 (IRF-1) expression. Ann Rheum Dis. 2004;63(5):578–582. doi: 10.1136/ard.2003.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]