Abstract
Two adjacent regions within the transactivation domain of p53 are sufficient to support sequence-specific transactivation when fused to a heterologous DNA binding domain. It has been hypothesized that these two subdomains of p53 may contribute to the expression of distinct p53-responsive genes. Here we have used oligonucleotide microarrays to identify transcripts induced by variants of p53 with point mutations within subdomains 1, 2, or 1 and 2 (QS1, QS2, and QS1/QS2, respectively). The expression of 254 transcripts was increased in response to wild-type p53 expression but most of these transcripts were poorly induced by these variants of p53. Strikingly, a number of known p53-regulated transcripts including TNFRSF10B, BAX, BTG2, and POLH were increased to wild-type levels by p53QS1 and p53QS2 but not p53QS1/QS2, indicating that either subdomain 1 or 2 is sufficient for p53-dependent expression of a small subset of p53-responsive genes. Unexpectedly, there was no evidence for p53QS1- or p53QS2-specific gene expression. Taken together, we found heterogeneity in the requirement for transactivation subdomains 1 and 2 of p53 without any subdomain-specific contribution to p53-induced gene expression.
Keywords: p53, gene expression, microarray, transcription factor, apoptosis
Introduction
The p53 tumor suppressor plays a pivotal role in preventing oncogenic transformation [1]. More than half of all human cancers is associated with alterations in p53 [1]. Decreased p53 activity is associated with hereditary cancers [2] and p53 nullizygous mice are cancer-prone [3]. The p53 protein is a sequence-specific transcription factor that can regulate the expression of a plethora of genes [1]. This protein is activated and accumulates in cells in response to a variety of cellular stresses and thus is an important regulator of stress gene regulation [1].
The p53 protein is a modular protein with several well-characterized functional domains. The C-terminus of p53 is required for oligomerization and contains sequence-independent DNA, DNA damage, and RNA binding activities [4]. This region is dispensable for p53 to function as a transcriptional activator [5,6]. The central third of p53 contains the sequence-specific DNA binding domain required for p53 to function as a transcriptional activator [7]. The majority of tumor-associated p53 mutations fall within the DNA binding domain [8]. The N-terminus of p53 contains an activation domain (AD) that is also required for sequence-specific transcriptional activation [9,10].
The N-terminal 73 amino acids of p53 expressed as a fusion protein with the DNA binding domain of the yeast GAL4 protein functions as an activator of GAL4-dependent gene expression [10]. The minimal transactivation domain was subsequently localized to the N-terminal 42 amino acids of p53 [9] and critical hydrophobic amino acids (Leu-22 and Trp-23) within this acidic region were found to be important for transactivation [5,11,12]. The mutation of these residues (L22Q/W23S) decreased the ability of the N-terminal 42 amino acids of p53 to function as an AD [5,11,12]. The p53L22Q/W23S variant and the murine equivalent (p53L25Q/W26S) are commonly used as transactivation-deficient versions of p53 [13–17]. Intriguingly, the L22Q/W23S variant (hereafter referred to as the QS1 variant) reportedly retains some p53 activity despite a profound transactivation defect [13,18-20]. Specifically, the QS1 variant retains the ability to induce apoptosis in some cellular contexts but is unable to induce G1 arrest [13,14,19,20]. Intriguingly, the homozygous QS1 knock-in mice undergo embryonic lethality although p53 is not required for embryonic development [14,21]. The QS1 variant is not equivalent to the complete loss of p53.
A second functional transactivation subdomain in the N-terminus of p53 has also been identified through a similar strategy. Amino acids 43 to 73 of p53 fused to the DNA binding domain of GAL4 were able to drive Gal4-dependent reporter gene expression and two critical hydrophobic amino acids (Trp-53 and Phe-54) were again critical for this activity [5,11,20]. Like the QS1 variant of p53, the W53Q/F54S variant (hereafter referred to as the QS2 variant) is defective in sequence-specific transactivation, when the expression of a small number of well-characterized p53 target genes was assessed [16,18–20]. Intriguingly, the QS2 variant of p53 was reported to retain the ability to induce p53-dependent G1 arrest but not p53-dependent apoptosis [20]. Therefore, despite the fact that the QS1 and QS2 variants of p53 have defects in sequence-specific transactivation, they exhibit some distinct biologic activities. This has led several laboratories to hypothesize that these domains function independently in regulating distinct subsets of p53 target genes [14,16,19–21].
Before this study, the relative contribution of these two AD subdomains to p53-mediated gene expression had not been assessed. Here we used recombinant adenoviruses expressing wild-type p53, p53QS1, p53QS2, and p53QS1/QS2 to drive p53-dependent gene expression in colorectal carcinoma cell lines in which endogenous p53 expression had been abolished by gene targeting. Gene expression was assessed using Affymetrix Oligonucleotide microarrays containing over 50,000 features. The expression of 254 transcripts was increased in response to Adp53wt infection and approximately 10% of these transcripts was also induced by the QS1 and QS2 variants but not the compound mutant. A small number of these genes were induced to wild-type levels by the QS variants; however, the fold increase in expression of the transcripts induced by the QS1 and QS2 variants was strongly correlated. These results indicate that the two subdomains cooperate to activate transcription of most p53 target genes. Our work also identified another subgroup of p53 target genes that appear to use either subdomain interchangeably.
Materials and Methods
Cell Culture and UV Treatment
The HCT116 p5-/- cell line was kindly provided by Dr. Bert Vogelstein (John's Hopkins University). Cells were maintained in McCoy's 5A media supplemented with 10% fetal bovine serum (Wisent, St. Bruno, Quebec, Canada). Adenovirus constructs expressing p53wt, p53QS1, p53QS2, and p53QS1/QS2 were kindly provided by Dr. Ruth Slack (University of Ottawa, Ontario, Canada). The adenovirus Ad-BHGΔE1ΔE3 (Ad-empty) control was generously provided by Dr. Frank Graham (McMaster University, Canada). Viruses were propagated using human embryonic kidney (HEK293) cells and cesium chloride gradient purification [22]. Virus titers were determined in HEK293 cells by standard methods [22] and titers are expressed as plaque-forming units per milliliter (pfu/ml). Cell lines were routinely tested for mycoplasma contamination.
RNA Isolation and Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
HCT116 p53-/- cells at 70% to 80% confluence were infected at a multiplicity of infection of 25 with indicated adenovirus in serum-free media for 1 hour. Growth medium containing 10% fetal bovine serum was replaced and cells were returned to the incubator for the indicated time. Infected cells were collected and total RNA was isolated using the RNeasy RNA isolation kit (Qiagen, Valencia, CA) according to manufacturer's specifications. Five micrograms of total RNA was reverse-transcribed using a first-strand cDNA synthesis kit (MBI Fermentas, Burlington, ON, Canada). Quantitative RT-PCR was performed using the SYBR Green Fluorescent DNA stain (Invitrogen, Burlington, ON, Canada), a LightCycler 2 quantitative PCR machine (Roche Diagnostics, Mannheim, Germany), and LightCycler software version 3 (Roche Diagnostics). The primers used were ACTB (GGGCATGGGTCAGAAGGAT and GTGGCCATCTCTTGCTCGA), APAF1 (CAACGGGAGATGACAATG and CTGGAGAAAAGCAAAGGTC), BAK1 (GCCATCAGCAGGAACAGGAG and ACACCCAGAACCACCAGCAC), BTG2 (CACAGAGCACTACAAACACC and ACAAGACGCAGATGGAGC), CASP6 (GCTTTGTGTGTGTCTTCC and CTCAGTTATGTTGGTGTCC), CDKN1A (CCTCAAATCGTCCAGCGACCTT and CATTGTGGGAGGAGCTGTGAAA), TNFRSF6 (CTCATCTTAATGGCCTAATGCA and GCTTCAGTTTATAACTATCTTCAC), TNFRSF10B (GGCATCATCATAGGAGTCAC and GTCAAAGGGCACCAAGTC), TP53I3 (TCTCTATGGTCTGATGGG and TTGCCTATGTTCTTGTTG), and MafB (TGCTGAGAGAGAGAACCGAGAG and CACCACCAAGAACTCTTCCTAC).
Microarrays
Total RNA was collected from HCT116p53-/- cells infected for 16 hours with 25 pfu/cell of Ad-BHGΔE1ΔE3, Adp53wt, Adp53QS1, Adp53QS2, or Adp53QS1/QS2 using the RNeasy Mini kit (Qiagen). Human Genome U133plus2.0 oligonucleotide arrays (Affymetrix, Santa Clara, CA) were used for expression analysis. Experimental procedures were performed according to the manufacturer specifications at the Ottawa Genomics Innovation Centre Affymetrix Gene-Chip Facility (Ottawa, Ontario, Canada). Affymetrix Microarray Suite 6.0 (MAS6.0) software was used to analyze the microarray data. MAS6.0 software uses a nonparametric Wilcoxon signed rank test to determine whether statistically meaningful differences in probe cell intensities were detected between samples (change calls were determined using γ1H and γ1L values of 0.0025). Genes were considered to be induced if and only if they were statistically (P ≤ .0025) increased in all experiments compared to Ad-BHGΔE1ΔE3 infected controls by an average of two-fold.
Western Blot Analysis
Total protein was extracted from cells using 1% sodium dodecyl sulfate and brief sonication. Protein samples were run on 4% to 12% Bis-Tris acrylamide gels, transferred to nitrocellulose membrane (Hybond-C; Amersham, Piscataway, NJ) and blocked with 5%skim milk-phosphate-buffered saline with 1% Tween 20 (TBS-T). Monoclonal antibodies raised against p53 were DO-1 (Ab6; Calbiochem, San Diego, CA), Pab1801 (Ab2; Calbiochem), and Pab421 (Ab1; Calbiochem). Additional antibodies were raised against p21WAF1 (Ab1; Calbiochem), PUMA (Ab1; Calbiochem), MDM2 (SMP14; Santa Cruz Biotechnology, Santa Cruz, CA) and MafB (P-20; Santa Cruz Biotechnology). Anti-mouse immunoglobulin (IgG) conjugated with horseradish peroxidase was used as a secondary antibody (Calbiochem), and protein bands were detected using the SuperSignal WestPico Chemiluminescent Substrate kit (Pierce, Rockford, IL) after being exposed to a film (X-OMAT; Kodak, Rochester, NY).
Immunoprecipitation and Mass Spectrometry
HCT116 p53-/- cells were infected with a multiplicity of infection of 25 of Adp53wt. Twenty-four hours postinfection cells were washed twice with PBS and scraped into PBS on ice. Cells were then treated as per manufacturer's instructions for the use of Protein A-agarose beads (Roche Diagnostics). Protein lysates were immunoprecipitated with p53 antibody Pab421. Precipitated protein extracts were run on a 4% to 12% Bis-Tris polyacrylamide gel and subsequently treated with GelCode Blue Stain Reagent according to manufacturer's specifications (Pierce). Bands of interest were excised and subjected to trypsin digestion. Matrix-assisted laser desorption/ionization time of flight tandem mass spectrometry (MALDI-TOF MS/MS) was performed at the Ontario Genomics Innovation Centre Proteomics Facility at the Ottawa Health Research Institute (Ottawa, Ontario, Canada). Peptides were identified using Mascot [23].
Results
The QS1 and QS2 Variants of p53 Are Impaired in p53-Dependent Gene Expression
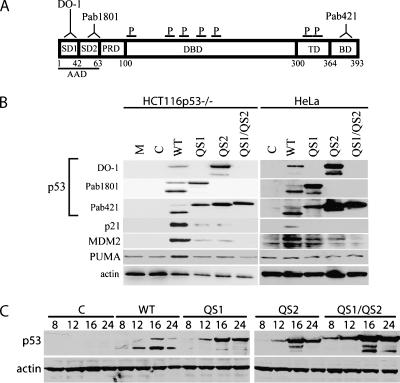
HCT116 cells in which p53 had been inactivated by gene targeting (HCT116p53-/-) [24] were infected with recombinant adenoviruses expressing wild-type, QS1, QS2, or QS1/QS2 variants of p53. Cell lysates were collected for immunoblot analysis at various times following infection using a panel of anti-p53 antibodies. The use of this panel of antibodies allowed us to distinguish between the variant forms of p53 in all experiments (Figure 1A). Immunoblot analysis revealed the presence of two immunoreactive bands that migrated at approximately 47 and 53 kDa (Figure 1B). Wild-type p53 was immunoprecipitated with Pab421 and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The bands were gel excised and analyzed by MALDI-TOF mass spectrometry. The seven peptides identified were located within the DNA binding domain of p53 (Figure 1A and Table W1). These peptides coupled with our panel of anti-p53 antibodies indicated that both bands represent full-length p53 (Figure 1A). The two forms likely correspond to differentially modified forms. However, the N- and C-termini of p53, containing the known modification sites, were not represented among the identified peptides and thus the specific modifications were not ascertained. The increased expression of these variants relative to wild-type p53 was expected because the QS variants do not induce mdm2 expression (Figure 1B), the ubiquitin ligase responsible for the rapid turnover of wild-type p53 [25].
Figure 1.
Expression of transactivation subdomain variants of p53. (A) Schematic representation of epitopes recognized by the indicated monoclonal antibodies (DO-1, Pab1801, and Pab421), peptides (P) identified by mass spectroscopy (see Table W1), and p53 functional domains. SD1 and SD2 denote subdomains 1 and 2 within the acidic AD. PRD, proline-rich domain; DBD, DNA binding domain; TD, tetramerization domain; BD, basic domain. Numbers below indicate the amino acid position. (B) Immunoblot analysis of p53 expression 16 hours following infection of either HCT116p53-/- or HeLa cells with the indicated recombinant adenovirus, using three different anti-p53 monoclonal antibodies. M and C represent mock- and control virus-infected samples whereas WT, QS1, QS2, and QS1/QS2 denote the wild-type and variant forms of p53. Similar blots were obtained with cell lysates derived from HCT116 and MDAH041 cells (data not shown). (C) Samples were collected at 8, 16, or 24 hours and subsequently analyzed by immunoblot analysis with the Pab421 monoclonal antibody.
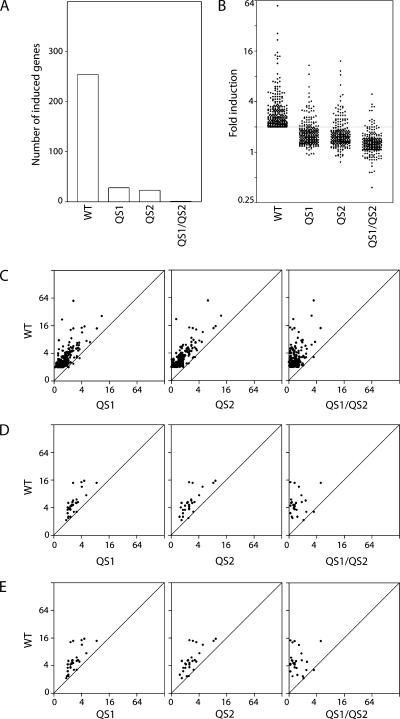
To identify transcripts induced in response to Adp53wt, Adp53QS1, Adp53QS2 and Adp53QS1/QS2 infection, microarray analysis was performed using total RNA collected from HCT116p53-/- cells 16 hours post infection because maximal p53 levels were achieved within this time frame (Figure 1C). The expression of 254 transcripts increased significantly following infection with the Adp53wt virus compared to control virus infection (Table W2). Of these, only 28, 23, and 1 were induced by Adp53QS1, Adp53QS2, and Adp53QS1/QS2, respectively (Figure 2A and Table W3). The mean induction of the Adp53wt-induced transcripts was significantly higher than the fold increase in expression due to the expression of any of the QS variants (Figure 2B). In fact, very few of the Adp53wt-induced genes appeared to be induced to wild-type levels by either the QS1 or QS2 variants (Figure 2C). Infection with the Adp53wt virus resulted in a greater increase in gene expression even when examining genes determined to be induced in response to either Adp53QS1 or Adp53QS2 infection (Figure 2, D and E). Therefore, the majority of WT-, QS1-, and QS2-regulated genes were poorly induced by the QS variants.
Figure 2.
Most p53 target genes are poorly induced by the QS variants. (A) Two hundred and fifty-four genes were induced by Adp53wt. Of these, only 28, 23, and 1 were induced by QS1, QS2, and QS1/QS2, respectively. (B) The fold increase in expression of these 254 genes was determined following infection of cells with adenoviruses expressing wild-type, QS1, QS2, or QS1/QS2 variant of p53. The fold increase in the expression following infection with Adp53QS1, Adp53QS2, or Adp53QS1/QS2 was less than the fold increase in response to Adp53wt infection (one-way analysis of variance followed by a Tukey's Multiple Comparisons test, P ≤ .001). (C–E) The fold increase in expression due to Adp53wt expression was compared to the fold increase in expression due to indicated transactivation subdomain variant of p53 for Adp53wt-, Adp53QS1-, and Adp53QS2-induced genes (C, D, and E, respectively). The 254, 28, and 23 genes induced by Adp53wt, Adp53QS1, and Adp53QS2 are listed in Tables W2 and W3.
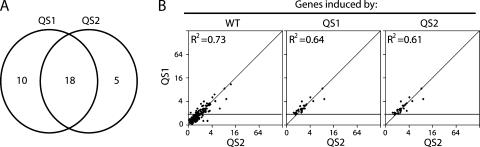
Correlation between Genes Induced By the QS1 and QS2 Variants
Having determined that most Adp53wt-induced genes were poorly induced by the variants, we sought to determine whether distinct subgroups of p53 target genes were preferentially responsive to the QS variants of p53. The fold change in p53 target gene expression in response to Adp53QS1 infection was plotted with respect to Adp53QS2 infection (Figure 3B). We observed a very striking linear correlation between the fold change in expression induced by the QS1 and QS2 variants of p53 regardless of whether the expression of Adp53wt-, Adp53QS1-, or Adp53QS2-induced genes were considered (Figure 3B; R2 values were 0.73, 0.64, and 0.61, respectively). Therefore, the Adp53QS1- and Adp53QS2-induced genes were induced to a similar extent by both variants (Figure 3B and Table W3). These results indicate that the disruption of either subdomain of p53 similarly affected the overall pattern of p53 transcriptional activation. We interpret these results to indicate that the contribution of transactivation subdomains 1 and 2 to p53-mediated gene expression was heterogeneous but not subdomain-specific.
Figure 3.
Correlation between Adp53QS1- and Adp53QS2-induced genes. (A) A Venn diagram is used to represent the overlap between Adp53QS1- and Adp53QS2-induced genes, as defined in the Materials and Methods section. (B) The effect of Adp53QS1 and Adp53QS2 infection on the expression of the 254 Adp53wt-, 28 Adp53QS1-, and 23 Adp53QS2-induced genes was determined. The genes induced by Adp53wt, Adp53QS1, and Adp53QS2 are listed in Tables W2 and W3. A very tight correlation (R2 values are inset) between Adp53QS1- and Adp53QS2-induced gene expression was observed within the subset of target genes.
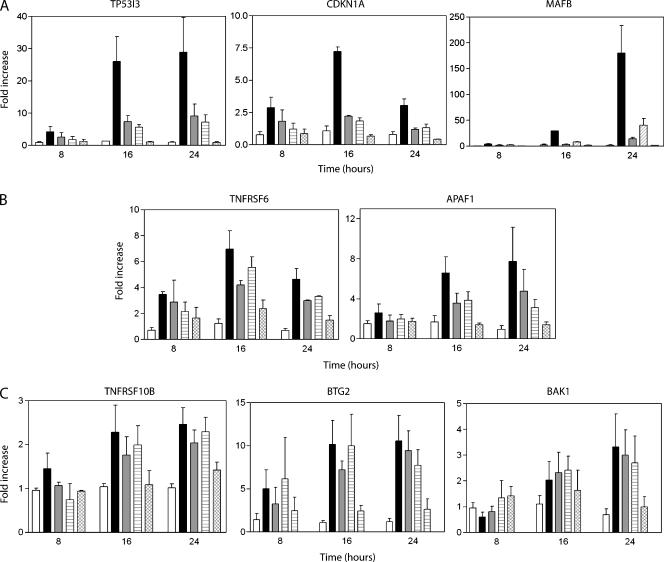
Based on our definition of induced genes (see Materials and Methods section), the expression of 18 genes increased in response to both QS variants but 10 and 5 wild-type p53-induced transcripts appeared to be increased in response to either QS1 or QS2, respectively (Figure 3A). To determine whether these apparently p53QS1- and p53QS2-specific genes were in fact specifically and preferentially upregulated by one of the variants, the pattern of allele-specific gene expression was confirmed by quantitative RT-PCR for 11 different transcripts at several different times following viral infection. The majority of p53 target genes were poorly induced by the QS1 and QS2 variants (Figure 4A). Several p53 target genes were significantly induced by the QS variants but were induced more strongly by wild-type p53 (Figure 4B). Lastly, a few target genes were induced to near wild-type levels by p53QS1 and p53QS2 (Figure 4C). The quantitative RT-PCR data correlated well with the microarray analysis and none of the p53-upregulated transcripts examined displayed a subdomain-specific pattern of gene expression (Figure 4 and Tables W3 and W4). The expression of several p53-regulated proteins was assessed by immunoblot in independent cell lines and no subdomain-specific differences in protein expression were detected (Figure 1B). Collectively, we interpret our results to indicate that the apparently p53QS1- and p53QS2-specific targets were not specifically induced by a single variant. Therefore, the response of p53-induced transcripts to the QS variants was heterogeneous but not subdomain-specific.
Figure 4.
Representative transcripts induced by wild-type p53. (A–C) Expression of the indicated transcript was determined by real-time RT-PCR using samples collected at the indicated time following virus infection (8, 16, or 24 hours). Expression of β-actin was used to normalize all RT-PCR results. Open, black, grey, hatched, and crosshatched bars represent control, Adp53wt-, Adp53QS1-, Adp53QS2-, and Adp53QS1/QS2-infected samples. Each value represents the mean fold increase in expression (± SEM) determined from a minimum of three independent experiments.
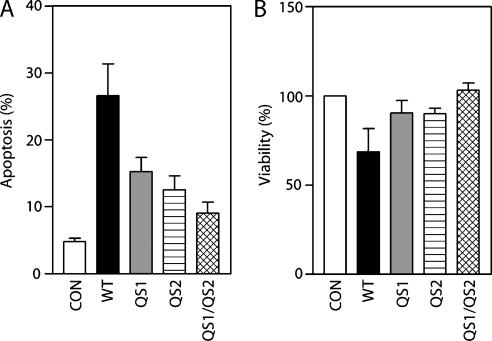
The 254 Adp53wt-induced genes were subjected to gene ontology (GO) analysis (http://www.geneontology.org/). Several genes were associated with the GO terms apoptosis (GO:0006915), cell cycle (GO:0007049), and DNA repair (GO:0006281) (Table 1), consistent with known p53 biology [26]. Of these terms, only apoptosis was statistically overrepresented (P < .01) based on analysis using the web-based GOstat software (http://gostat.wehi.edu.au/). Consistent with the preponderance of proapoptotic genes, Adp53wt infection resulted in a significant increase in the proportion of apoptotic cells (Figure 5, A and B). Both QS variants were reduced in their capacity to induce cell death and there was no significant difference in their ability to induce apoptosis in these cells (Figure 5, A and B). Most of the apoptosis annotated genes were poorly induced by the QS1 and QS2 variants of p53 compared to wild-type p53 (Table 1). Therefore, decreased p53-dependent gene expression correlated with decreased p53-dependent apoptosis in these cells. Similarly, the cell cycle-annotated genes were poorly induced by all variants (Table 1). Interestingly, two of the three genes associated with DNA repair (BTG2 and POLH) were induced to near wild-type levels by the QS1 and QS2 variants but not the QS1/QS2 variant (Table 1 and Figure 4C). Due to the limited number of repair-related genes, the significance of this specific observation remains unclear. Overall, our results suggest that there is substantial heterogeneity in the contribution of subdomains 1 and 2 to p53-mediated gene expression but there are no subdomain-specific effects.
Table 1.
Adp53wt-Induced Genes Involved in Apoptosis, Cell Cycle, and/or DNA Repair.
| GO Term* | Locus | p53 Variant | |||
| WT | QS1 | QS2 | QS1/QS2 | ||
| Apoptosis (GO:0006915) | TP53I3 | 3.9† | 2.0 | 1.3 | 0.1 |
| TP53INP1 | 3.8 | 1.4 | 1.5 | 0.4 | |
| APAF1 | 2.8 | 1.8 | 1.8 | 1.0 | |
| TNFRSF6 | 2.4 | 1.8 | 1.7 | 0.9 | |
| CASP6 | 1.7 | 0.8 | 0.7 | 0.4 | |
| TNFRSF10B | 1.5 | 0.9 | 0.8 | 0.5 | |
| MDM2 | 1.4 | 0.3 | 0.1 | 0.0 | |
| BAX | 1.4 | 1.2 | 0.9 | 0.2 | |
| AKTIP | 1.2 | 0.3 | 0.4 | 0.4 | |
| AMID | 1.2 | 0.4 | 0.2 | 0.2 | |
| BID | 1.1 | 0.4 | 0.3 | 0.3 | |
| CARD10 | 1.1 | 0.5 | 0.4 | 0.4 | |
| BAK1 | 1.0 | 0.5 | 0.4 | 0.3 | |
| TRAF4 | 1.0 | 0.4 | -0.1 | -0.2 | |
| Cell Cycle (GO:0007049) | CDKN1A | 2.4 | 1.1 | 0.8 | -0.8 |
| SESN1 | 2.0 | 0.9 | 0.8 | 0.1 | |
| GAS2L1 | 1.4 | 0.8 | 0.6 | 0.4 | |
| MDM2 | 1.4 | 0.3 | 0.1 | 0.0 | |
| RB1 | 1.3 | 0.8 | 0.6 | 0.8 | |
| RHOB | 1.2 | 0.4 | 0.3 | 0.5 | |
| PARD6G | 1.2 | 0.7 | 0.6 | 0.4 | |
| SFN | 1.2 | 0.4 | 0.3 | -0.3 | |
| SESN2 | 1.1 | 0.3 | 0.2 | 0.0 | |
| LATS2 | 1.1 | 0.5 | 0.4 | 0.4 | |
| HRAS | 1.0 | 0.5 | 0.4 | 0.2 | |
| DNA Repair (GO:0006281) | BTG2 | 2.9 | 2.4 | 2.0 | 0.9 |
| POLH | 2.2 | 1.8 | 1.9 | 0.9 | |
| DDB2 | 1.1 | 0.4 | 0.3 | -0.3 | |
Genes involved in apoptosis, cell cycle and DNA repair were identified using the Gene Ontology database (http://geneontology.org/). Of these GO terms, only apoptosis (GO:0006915) and related GO terms were significantly over represented among the Adp53wt-induced genes based on GOstat analysis (http://gostat.wehi.edu.au/).
The mean fold increase in expression (log2) determined from microarray experiments, as described in the Materials and Methods section.
Figure 5.
Effect of QS variants of p53 on cell viability and apoptosis. Apoptosis and cell viability were assessed 48 hours following infection with either control adenovirus or adenoviruses expressing the indicated variants of p53. Apoptosis was assessed by subdiploid DNA content (A) and viability was assessed by Trypan blue exclusion (B). Each point represents the mean (±SEM) determined from three independent experiments. Adp53wt induced more apoptosis than the variants (one-way analysis of variance followed by Tukey's Multiple Comparisons test, P ≤ .01 for apoptosis and P ≤ .05 for viability). No significant difference in viability or apoptosis was observed when comparing QS1 and QS2 variants.
Discussion
The p53 protein can act as both a positive and negative regulator of gene expression but p53 is best understood as a transcriptional activator. The p53 protein is a positive regulator of several hundred genes and this is mediated by the sequence-specific binding of p53 to consensus elements found in promoters, enhancer regions, introns, or the 5′ untranslated regions of these genes [1]. Transcriptional activation further requires the p53-dependent recruitment of the histone acetyl transferases CBP/p300, general transcription factors, and RNA polymerase II to the promoter of target genes [27–30]. The N-terminal AD is required for the recruitment of these proteins and subsequent p53-dependent gene activation [11,30]. Amino acids 1 to 42 were found to function as a minimal transcriptional AD [9,10]. However, it was subsequently shown that this minimal region was part of a larger AD with each of the two subdomains capable of supporting sequence-specific transactivation when expressed as a fusion protein with a heterologous DNA binding domain [5,11].
The relative contribution of subdomains 1 and 2 to p53 activity has been examined at the cell biologic level by two laboratories. Zhu et al. [20] reported that p53QS1 is unable to induce G1 arrest but retains the ability to induce apoptosis in tumor cell lines [18]. In contrast, p53QS2 was reportedly able to induce cell cycle arrest but was impaired in its ability to induce apoptosis in these same cell lines [20]. Cregan et al. [16] reported that overexpression of either p53QS1 or p53QS2 in neuronal cells led to similar levels of apoptosis but that forced expression of the p53QS2 variant in p53 nullizygous neuronal cells led to significantly more apoptosis than the p53QS1 variant when these cells were subsequently treated with camptothecin. Based on these studies, it was hypothesized that the p53 transactivation subdomains contribute to the regulation of distinct subsets of p53 target genes that affect the biologic activity of these variants. However, the relative contribution of the two distinct subdomains in the AD to p53-dependent transcriptional activity had remained untested.
Here we found that infection of these cells with recombinant adenoviruses expressing p53QS1, p53QS2, and p53QS1/QS2 resulted in the induction of far fewer p53 target genes than Adp53wt infection. Approximately 10% of the Adp53-induced genes were also increased on Adp53QS1 and Adp53QS2 infection. The identity and fold increase in expression of the p53QS1- and p53QS2-upregulated genes were strongly correlated, indicating that these subdomains do not contribute to the expression of distinct subsets of genes. The majority of p53-regulated genes were induced poorly by p53QS1, p53QS2, and p53QS1/QS2, indicating that both subactivation domains are required to increase the expression of most p53 target genes. Conversely, a relatively small number of p53-target genes including TNFRSF10B, BAX, BTG2, and POLH were induced to near wild-type levels by p53QS1 and p53QS2, but were poorly induced by p53QS1/QS2. Therefore, the p53-dependent induction of this subgroup of p53 target genes requires a functional transactivation domain but subdomain 1 or 2 appears to be sufficient and used interchangeably for p53 target gene expression within this group of genes. We did not detect any subdomain-specific p53 target genes.
Like many other transactivation domains, the N-terminus of p53 is not highly conserved overall at the level of amino acid sequence; however, there is a high level of sequence conservation among rodents and primates within subdomain 1 (34% identity; see Figure W1). The region of highest homology includes amino acids 13 through 26 (93% identity), a region of p53 termed box 1 [31]. The N-terminus of p53 is rich in acidic residues characteristic of acidic ADs and is mostly unstructured under physiological conditions [32]. Box 1 contains a number of hydrophobic residues and nuclear magnetic resonance studies indicate that amino acids 18 to 26 within box 1 form a helix within the context of the larger disordered transactivation domain [32]. Mutation of Leu-22 and Trp-23 within the helical region in the QS1 variant is predicted to disrupt this region of limited secondary structure [32]. The high level of conservation between mouse and human p53 has made it possible to generate knock-in mice expressing the QS1 variant from the endogenous p53 locus [14], as discussed later. It is likely that this secondary structure is important for the activity of subdomain 1.
Subdomain 2, within the transactivation domain of p53, is well conserved among primate species but is poorly conserved when the sequence comparison is extended to rodent versions of p53 (Figure W1). The limited homology between mouse and human p53 makes the QS2 variant more difficult to model in mice. Much like subdomain 1, hydrophobic residues within subdomain 2 of human p53 give rise to localized secondary structure within the mostly unstructured acidic AD (nascent turns between Met-40 and Met-44 and between Asp-48 and Trp-53) [32–34]. Regions of limited secondary structure within mostly unstructured ADs are common among transcriptional activators [12,30,34,35]. The second subdomain of p53 reportedly binds to many proteins known to interact with subdomain 1, such as mdm2, RPA, TFIID, TFIIH, and p300 [11,27,30,34,36–44]. The QS2 point mutations are thought to disrupt the localized secondary structure and would be expected to disrupt protein-protein interactions important for transcriptional activation [12,30,32–34].
As indicated above, knock-in mice expressing the QS1 variant from the endogenous p53 locus have been generated [14]. Homozygous p53QS1 expression results in embryonic lethality; however, homozygous p53QS1-targeted mouse embryonic fibroblasts (MEFs) were obtained [14]. Using these MEFs, the induction of five of the six p53 target genes tested was reduced in the QS1-expressing MEFs compared to control cell lines following doxorubicin treatment [14]. The single gene induced by the QS1 variant of murine p53 was BAX [14] and we similarly found that the human QS1 variant was able to upregulate BAX expression. Unexpectedly, we found that the QS1 and QS2 variants similarly increased the expression of BAX along with three other known p53 target genes (TNFRSF10B, BTG2, and POLH). Therefore, only one of the two subdomains appears to be necessary and sufficient for p53-dependent gene expression of this subset of p53 target genes. We interpret the heterogenous requirement for subdomains 1 and 2 to indicate that the requirement for specific protein-p53 AD interactions must vary in a p53 target gene-specific manner.
In summary, our results suggest that compound mutations of critical hydrophobic amino acids in either subdomain 1 or 2 decrease the affinity of the AD for cofactors or other components of basal transcription apparatus that are rate-limiting for p53-dependent gene expression, at most p53-induced promoters. Surprisingly, the induction of a small subset of p53-responsive genes, including BAX, TNFRSF10B, BTG2, and POLH, is not limited by mutations in either of the subdomains alone. Therefore, this latter group of genes appears to have a less stringent requirement for as yet unidentified protein-protein interactions. Importantly, we did not find any genes that were preferentially induced by any single AD variant. Our results support a model in which the transcription activation subdomains of p53 contribute equally to p53-dependent target gene expression.
Supplementary Material
Acknowledgements
We thank Ruth Slack (University of Ottawa) and Frank Graham (McMaster University) for the recombinant adenoviruses. We acknowledge the assistance of Microarray Core Facility of StemCore Laboratories in the Sprott Center for Stem Cell Research at the Ottawa Health Research Institute.
Abbreviations
- MALDI-TOF
matrix-assisted laser desorption/ionization time of flight
- AD
activation domain
- MEF
mouse embryonic fibroblast
Footnotes
This work was initially supported by the Cancer Research Society and, more recently, by the National Cancer Institute of Canada with Funds from the Terry Fox Run. J.M.S. was supported by an Ontario Graduate Scholarship in Science and Technology. L.J.S. was supported by an Ontario Premier's Research Excellence Award to B.C.M. B.C.M. is a Research Scientist of the National Cancer Institute of Canada funded through the Canadian Cancer Society.
This article refers to supplementary materials, which are designated by Tables W1, W2, W3, and W4 and Figure W1 and are available online at www.neoplasia.com.
References
- 1.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 2.Iwakuma T, Lozano G, Flores ER. Li-Fraumeni syndrome: a p53 family affair. Cell Cycle. 2005;4:865–867. doi: 10.4161/cc.4.7.1800. [DOI] [PubMed] [Google Scholar]
- 3.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 4.Ahn J, Prives C. The C-terminus of p53: the more you learn the less you know. Nat Struct Biol. 2001;8:730–732. doi: 10.1038/nsb0901-730. [DOI] [PubMed] [Google Scholar]
- 5.Candau R, Scolnick DM, Darpino P, Ying CY, Halazonetis TD, Berger SL. Two tandem and independent sub-activation domains in the amino terminus of p53 require the adaptor complex for activity. Oncogene. 1997;15:807–816. doi: 10.1038/sj.onc.1201244. [DOI] [PubMed] [Google Scholar]
- 6.Unger T, Mietz JA, Scheffner M, Yee CL, Howley PM. Functional domains of wild-type and mutant p53 proteins involved in transcriptional regulation, transdominant inhibition, and transformation suppression. Mol Cell Biol. 1993;13:5186–5194. doi: 10.1128/mcb.13.9.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992;1:45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- 8.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 9.Unger T, Nau MM, Segal S, Minna JD. p53: a transdominant regulator of transcription whose function is ablated by mutations occurring in human cancer. EMBO J. 1992;11:1383–1390. doi: 10.1002/j.1460-2075.1992.tb05183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields S, Jang SK. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990;249:1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- 11.Chang J, Kim DH, Lee SW, Choi KY, Sung YC. Transactivation ability of p53 transcriptional activation domain is directly related to the binding affinity to TATA-binding protein. J Biol Chem. 1995;270:25014–25019. doi: 10.1074/jbc.270.42.25014. [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Chen J, Elenbaas B, Levine AJ. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994;8:1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 13.Haupt Y, Rowan S, Shaulian E, Vousden KH, Oren M. Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 1995;9:2170–2183. doi: 10.1101/gad.9.17.2170. [DOI] [PubMed] [Google Scholar]
- 14.Johnson TM, Hammond EM, Giaccia A, Attardi LD. The p53QS transactivation-deficient mutant shows stress-specific apoptotic activity and induces embryonic lethality. Nat Genet. 2005;37:145–152. doi: 10.1038/ng1498. [DOI] [PubMed] [Google Scholar]
- 15.Attardi LD, Lowe SW, Brugarolas J, Jacks T. Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J. 1996;15:3693–3701. [PMC free article] [PubMed] [Google Scholar]
- 16.Cregan SP, Arbour NA, Maclaurin JG, Callaghan SM, Fortin A, Cheung EC, Guberman DS, Park DS, Slack RS. p53 activation domain 1 is essential for PUMA upregulation and p53-mediated neuronal cell death. J Neurosci. 2004;24:10003–10012. doi: 10.1523/JNEUROSCI.2114-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimenez GS, Nister M, Stommel JM, Beeche M, Barcarse EA, Zhang XQ, O'Gorman S, Wahl GM. A transactivation-deficient mouse model provides insights into Trp53 regulation and function. Nat Genet. 2000;26:37–43. doi: 10.1038/79152. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Ko LJ, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Zhou W, Jiang J, Chen X. Identification of a novel p53 functional domain that is necessary for mediating apoptosis. J Biol Chem. 1998;273:13030–13036. doi: 10.1074/jbc.273.21.13030. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Zhang S, Jiang J, Chen X. Definition of the p53 functional domains necessary for inducing apoptosis. J Biol Chem. 2000;275:39927–39934. doi: 10.1074/jbc.M005676200. [DOI] [PubMed] [Google Scholar]
- 21.Johnson TM, Attardi LD. p53QS: an old mutant teaches us new tricks. Cell Cycle. 2005;4:731–734. doi: 10.4161/cc.4.6.1696. [DOI] [PubMed] [Google Scholar]
- 22.Ng P, Graham FL. Construction of first-generation adenoviral vectors. Methods Mol Med. 2002;69:389–414. doi: 10.1385/1-59259-141-8:389. [DOI] [PubMed] [Google Scholar]
- 23.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 25.Dai MS, Jin Y, Gallegos JR, Lu H. Balance of Yin and Yang: ubiquitylation-mediated regulation of p53 and c-Myc. Neoplasia. 2006;8:630–644. doi: 10.1593/neo.06334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 27.Espinosa JM, Emerson BM. Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol Cell. 2001;8:57–69. doi: 10.1016/s1097-2765(01)00283-0. [DOI] [PubMed] [Google Scholar]
- 28.Espinosa JM, Verdun RE, Emerson BM. p53 functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. Mol Cell. 2003;12:1015–1027. doi: 10.1016/s1097-2765(03)00359-9. [DOI] [PubMed] [Google Scholar]
- 29.Gomes NP, Bjerke G, Llorente B, Szostek SA, Emerson BM, Espinosa JM. Gene-specific requirement for P-TEFb activity and RNA polymerase II phosphorylation within the p53 transcriptional program. Genes Dev. 2006;20:601–612. doi: 10.1101/gad.1398206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teufel DP, Freund SM, Bycroft M, Fersht AR. Four domains of p300 each bind tightly to a sequence spanning both transactivation subdomains of p53. Proc Natl Acad Sci USA. 2007;104:7009–7014. doi: 10.1073/pnas.0702010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu WL, Midgley C, Stephen C, Saville M, Lane DP. Biological significance of a small highly conserved region in the N terminus of the p53 tumour suppressor protein. J Mol Biol. 2001;313:711–731. doi: 10.1006/jmbi.2001.5082. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Mok KH, Muhandiram R, Park KH, Suk JE, Kim DH, Chang J, Sung YC, Choi KY, Han KH. Local structural elements in the mostly unstructured transcriptional activation domain of human p53. J Biol Chem. 2000;275:29426–29432. doi: 10.1074/jbc.M003107200. [DOI] [PubMed] [Google Scholar]
- 33.Kaustov L, Yi GS, Ayed A, Bochkareva E, Bochkarev A, Arrowsmith CH. p53 transcriptional activation domain: a molecular chameleon? Cell Cycle. 2006;5:489–494. doi: 10.4161/cc.5.5.2489. [DOI] [PubMed] [Google Scholar]
- 34.Chi SW, Lee SH, Kim DH, Ahn MJ, Kim JS, Woo JY, Torizawa T, Kainosho M, Han KH. Structural details on mdm2-p53 interaction. J Biol Chem. 2005;280:38795–38802. doi: 10.1074/jbc.M508578200. [DOI] [PubMed] [Google Scholar]
- 35.Mapp AK, Ansari AZ. A TAD further: exogenous control of gene activation. ACS Chem Biol. 2007;2:62–75. doi: 10.1021/cb600463w. [DOI] [PubMed] [Google Scholar]
- 36.Buschmann T, Lin Y, Aithmitti N, Fuchs SY, Lu H, Resnick-Silverman L, Manfredi JJ, Ronai Z, Wu X. Stabilization and activation of p53 by the coactivator protein TAFII31. J Biol Chem. 2001;276:13852–13857. doi: 10.1074/jbc.M007955200. [DOI] [PubMed] [Google Scholar]
- 37.Farmer G, Colgan J, Nakatani Y, Manley JL, Prives C. Functional interaction between p53, the TATA-binding protein (TBP), and TBP-associated factors in vivo. Mol Cell Biol. 1996;16:4295–4304. doi: 10.1128/mcb.16.8.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leveillard T, Andera L, Bissonnette N, Schaeffer L, Bracco L, Egly JM, Wasylyk B. Functional interactions between p53 and the TFIIH complex are affected by tumour-associated mutations. EMBO J. 1996;15:1615–1624. [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Miller CW, Koeffler PH, Berk AJ. The p53 activation domain binds the TATA box-binding polypeptide in Holo-TFIID, and a neighboring p53 domain inhibits transcription. Mol Cell Biol. 1993;13:3291–3300. doi: 10.1128/mcb.13.6.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin DW, Munoz RM, Subler MA, Deb S. p53 binds to the TATA-binding protein-TATA complex. J Biol Chem. 1993;268:13062–13067. [PubMed] [Google Scholar]
- 41.Thut CJ, Chen JL, Klemm R, Tjian R. p53 transcriptional activation mediated by coactivators TAFII40 and TAFII60. Science. 1995;267:100–104. doi: 10.1126/science.7809597. [DOI] [PubMed] [Google Scholar]
- 42.An W, Kim J, Roeder RG. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell. 2004;117:735–748. doi: 10.1016/j.cell.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 44.Lill NL, Grossman SR, Ginsberg D, DeCaprio J, Livingston DM. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.