Abstract
Overexpression of the epidermal growth factor receptor (EGFR) in epithelial tumors is associated with poor prognosis and is the target for a number of cancer therapeutics. Monoclonal antibody (mAb) 806 is a novel anti-EGFR antibody with significant therapeutic efficacy in tumor models when used as a single agent, and displays synergistic antitumor activity in combination with other EGFR therapeutics. Unlike other EGFR antibodies, mAb 806 is selective for tumor cells and does not bind to normal tissue, making it an ideal candidate for generation of radioisotope or toxin conjugates. Ideally, antibodies suited to these therapeutic applications must bind to and actively internalize their cognate receptor. We investigated the intracellular trafficking of fluorescently tagged mAb 806 in live cells and analyzed its biodistribution in a tumor xenografted nude mouse model. Following binding to EGFR, mAb 806 was internalized through dynamin-dependent, clathrin-mediated endocytosis. Internalized mAb 806 localized to early endosomes and subsequently trafficked to and accumulation in lysosomal compartments. Furthermore, biodistribution analysis in nude mice showed specific uptake and retention of radiolabeled mAb 806 to human tumor xenografts. These results highlight the potential use of mAb 806 for generation of conjugates suitable for diagnostic and therapeutic use in patients with EGFR-positive malignancies.
Keywords: Internalization, epidermal growth factor receptor, intracellular trafficking, dynamin, monoclonal antibody
Introduction
The epidermal growth factor receptor (EGFR) is overexpressed in a variety of epithelial malignancies and, therefore, has become an attractive target for numerous anti-cancer therapeutics [1]. These have included tyrosine kinase inhibitors and antibodies [2,3], with the first anti-EGFR therapeutic, Cetuximab, being recently approved for use in colorectal cancer patients [4]. Whereas these agents continue to show promising efficacy in a number of advanced clinical trials, targeting of normal tissue such as the liver, skin, and gastrointestinal tract, limits their use and restricts antibodies to naked therapy, whereby antibody alone elicits a therapeutic effect [5–7].
We have previously described a novel anti-EGFR antibody, monoclonal antibody (mAb) 806, which targets the EGFR deletion variant, de2-7 EGFR as well as wild-type (wt) EGFR expressed in cells overexpressing the receptor [8–15]. MAb 806 does not bind to normal tissue expressing physiological levels of the receptor, including skin and liver, as demonstrated by extensive immunohistochemistry studies, as well as our first-in-man clinical trial of ch806 [11,16]. This unique specificity profile of mAb 806 arises from its ability to recognize an epitope that is exposed only during the transition of the receptor between a tethered inactive conformation to an untethered active conformation [17,18]. In addition, when the EGFR is overexpressed, untethering appears to occur at an increased rate, possibly due to changes in glycosylation [14]. In cells expressing the truncated de2-7 EGFR, the epitope is continuously exposed and therefore mAb 806 is able to bind constitutively. As such, this epitope is different to all other epitopes targeted by current anti-EGFR antibodies and is therefore not accessible in normal human tissues when the receptor is expressed at physiological levels [17,18].
In vivo therapeutic evaluation of mAb 806 alone and in combination with other anti-EGFR agents also shows substantial antitumor effects in de2-7 EGFR expressing and wt EGFR overexpressing tumors. No activity against cells expressing normal levels of EGFR were detected [8,12,15]. Given the unique specificity of mAb 806 and its ability to elicit a significant antitumor response, we investigated its potential for targeted drug delivery by assessing its internalization profile and biodistribution in tumor-bearing mice.
Indeed, tumor-specific antibodies which are able to illicit downregulation of receptor from the cell surface, thereby potentially attenuating receptor activity, may have greater efficacy than those that do not [19,20]. We have recently shown that treatment of de2-7 EGFR expressing tumors with mAb 806 in combination with another prototypical anti-EGFR antibody (mAb 528) results in substantial receptor downregulation, leading to a significant antitumor response [15]. Coupled with the unique specificity of mAb 806, its ability to internalize following binding to the receptor can be used to generate immunoconjugates which would allow for targeted delivery of radiation or toxins to tumor cells without toxicity to normal tissue [21–24]. Such a procedure would not be possible with current therapeutic agents targeting the wt EGFR, such as Cetuximab, due to extensive uptake and subsequent toxicity in organs such as the liver, skin, and gastrointestinal tract.
This study investigates the mechanism of mAb 806 internalization following binding to EGFR in cells overexpressing the wt receptor, as well as its intracellular trafficking profile. Biodistribution analysis with two different radioisotopes was also performed to ascertain tissue uptake and tumor cell retention.
Materials and methods
Cell Lines and Reagents
The epidermoid carcinoma cell line, A431 [10], and squamous carcimona cell line, HN5 [14,25], have been described previously. The mAbs 806 and 528 have also been described previously and were produced in the Biological Production Facility (Ludwig Institute for Cancer Research, Melbourne, Australia). Other monoclonal antibodies against CD107a (also known as lysosomal-associated membrane protein 1 (LAMP1)) and early endosome autoantigen 1 (EEA1) were purchased from Pharmingen (San Diego, CA) and Transduction Laboratories (San Diego, CA), respectively. Cy2-conjugated anti-mouse secondary antibody and unlabeled goat anti-mouse blocking Fab fragment were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Biotinylated epidermal growth factor complexed to Alexa 488 and transferrin (Tfn) labeled with fluorescein isothiocyanate (FITC) were purchased from Molecular Probes (Eugene, OR).
Immunofluorescence
MAb 806 or 528 was directly labeled with cyanine 3 (Cy3) dye using the Cy3 Monoclonal Antibody Labeling kit (Amersham Pharmacia Biotech UK Ltd, Buckinghamshire, UK) according to the manufacturer's instructions. Successful labeling of antibody was determined through flow cytometry analysis of binding to A431; all analyses were performed in triplicate. Immunofluorescence was conducted on A431 cells grown on 12-mm glass coverslips or 12-mm Biocoat Cell Environments poly-d-lysine coverslips (Becton Dickinson Labware, Bedford, MA). Cy3-conjugated mAb 806 and 528 were used at concentrations of 5 and 2 ™g/ml, respectively, and surface labeling was carried out at 4°C for 20 minutes. Internalization of surface-bound antibody was initiated by incubation in prewarmed (37°C) serum-free media. At the appropriate time points, coverslips were removed, washed in ice-cold BSA/PBS before fixation in 4% paraformaldehyde (PFA). Cells were then permeabilized with 0.1% Triton X-100 and incubated with unlabelled goat anti-mouse Fab fragment to block all existing mouse binding sites. Samples were then incubated with the appropriate antibody to intracellular organelles (i.e., LAMP1 or EEA1) followed by labeling with Cy2-conjugated secondary antibody. Samples were subsequently mounted in Fluoromount G (Southern Biotechnology, Birmingham, AL) and analyzed with an epifluorescent microscope (Olympus America Inc., Melville, NY) using appropriate wavelength settings.
Cellular Transfection
DNA vectors for green fluorescent protein (GFP)-tagged lysosomal glycoprotein 120 (lgp-120-GFP) and dominantnegative dynamin K44A (DynK44A-GFP) were kindly provided by Prof. I. Mellman and Prof. P. De Camilli, from the Department of Cell Biology, Yale University School of Medicine. Cells grown in glass-bottom microwell dishes (MatTek Corp., Ashland, MA) were transfected overnight using a reagent (Lipofectamine; Invitrogen Life Technologies, Victoria, Australia) following the manufacturer's instructions. Epifluorescent imaging was performed 24 hours after successful transfection.
Time-Lapse Microscopy
Time-lapse series were acquired at 37°C on an inverted microscope (Olympus 1X70; Olympus America Inc., Melville, NY), equipped with a 60x oil immersion objective, NA 1.45, and a 16-bit charge-coupled device camera (Till Photonics LLC, Pleasanton, CA) using 1 x 1 or 2 x 2 binning. A dual dichroic GFP/Texas red filter (Chroma, Rockingham, VT) allowed for simultaneous detection of GFP and/or red fluorescent protein (RFP). Switching between the excitation wavelengths was controlled by TILLvisION software (Till Photonics LLC). Exposure times were 200 and 400 ms for GFP and RFP channels, respectively. Time-lapse sequences corresponding to roughly one frame per 7 seconds (for 1 hour) were subsequently acquired and merged as RGBs using TILLvisION. Series were exported as tagged image file format and processed in Adobe Photoshop (Adobe Systems Inc., San Jose, CA). Videos of representative experiments are in the Supplemental data.
Quantification of Mean Ratio of Fluorescence Intensity
Using Andor IQ image analysis software (Andor Technology, South Windsor, CT), regions of interest corresponding to labeled antibody were selected from the intracellular region and the plasma membrane of each cell. Mean intensity values for both areas of interest over the course of the 60-minute time-lapse movies were monitored every minute and the ratio of intracellular to membrane fluorescence intensity was calculated. The average ratio at each time point was subsequently calculated for four to six cells per treatment and plotted over time.
Electron Microscopy
Cells were preincubated with mAb 806 antibody on ice before induction of internalization at 37°C for 0, 5, 10, and 30 minutes. Samples were subsequently fixed in 4% PFA in 0.25 M Hepes (pH 7.4), for 1 hour at room temperature, followed by fixation in 8% PFA in the same buffer, overnight at 4°C. Cell pellets were frozen in liquid nitrogen and cryosectioned using an ultramicrotome (Leica Ultracut UCT; Leica Microsystems Inc., Bannockburn, IL). Sections were labeled using a rabbit anti-mouse antibody, followed by protein A conjugated to 5-nm gold particles. Sections were embedded in 1.8% methyl cellulose and 0.5% uranyl acetate, air-dried, examined in a transmission electron microscope (Tecnai 12 Biotwin; FEI Company, Hillsboro, OR), and photographed.
Biodistribution of 125I- and 111In-CHX-A″-DTPA-Labeled mAb 806 In Vivo
A comparison of the biodistribution and localization of 125I- and 111In-CHX-A″-DTPA-[26] labeled mAb 806 was performed using a radiolabeling procedure previously described [27]. Radiolabeling was performed on the day of injection into mice. Before injection, radiopurity, specific activity, binding ability, and number of antibody molecules bound per cell for the final radiolabeled product were tested.
Biodistribution analysis was conducted in BALB/c nude mice bearing established (70 mm3) subcutaneous A431 tumors (n = 24). Animals were injected IV through tail vein injection with either mAb 806 or mAb 100–310 (an isotypematched mAb directed to human A33 antigen) labeled with 5 ™Ci of both 125I- and 111In-CHX-A″-DTPA (total, 10 ™g of antibody and 10 ™Ci of radioactivity) suspended in 100 ™l of sterile PBS. Groups of four tumor-bearing mice injected with radiolabeled mAb 806 were sacrificed at 4, 24, 48, 72, and 168 hours after injection of radiolabeled antibody. One group of four mice injected with radiolabeled 100–310 was sacrificed 24 hours after antibody injection. Mice were bled through cardiac puncture and tumors and organs (brain, lung, liver, stomach, spleen, and kidney) were immediately removed, blotted dry, and weighed. All samples were counted in a dual chamber gamma scintillation counter (Cobra II, Auto-gamma; Packard Instruments, Canberra, Australia) using a dual tracer program with standard windows set for each isotope, 15 to 75 keV for 125I and 120 to 460 keV for 111In. Standards prepared from the injected material were counted each time with tissues and tumors, enabling calculations to be corrected for the physical decay of the isotopes. Results of labeled antibody distribution over time were expressed as the percentage injected dose/gram (% ID/g) [(cpm tissue sample / cpm standard) x 100 /weight in grams] and as tumor/blood ratios.
Results
Binding of Cy3 Dye-Conjugated mAb 806 and 528 to Cell Lines
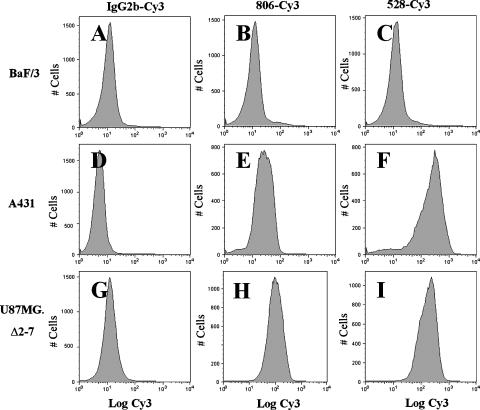
The binding specificity of mAb 806 and 528 conjugated with Cy3 dye to wt EGFR and de2-7 EGFR was assessed using A431 and U87MG.Δ2-7 cells, respectively. Binding to U87MG.Δ2-7 cells was included as a positive control for mAb 806 given its relatively low binding to A431 cells. FACS analysis shows that both antibodies retained their specificity following conjugation to Cy3. Low but highly reproducible binding of mAb 806-Cy3 to A431 cells was observed by FACS analysis (Figure 1E) and was consistent with previous observations where mAb 806 bound ∼ 10% of the 2 x 106 EGFRs/cell [8,10]. As expected, the anti-EGFR 528-Cy3 antibody showed strong binding to A431 cells (Figure 1F). An isotype-matched IgG2b antibody (100–310) conjugated to Cy3 was used as a negative control (Figure 1, A, D, and G). Neither antibody bound to the negative control murine hemopoietic cell line, BaF/3 (Figure 1, B and C), which does not express endogenous wt or de2-7 EGFR [28]. These results are consistent with those obtained with unconjugated antibody [8], suggesting that direct conjugation with fluorescent tags do not alter the specificity of these antibodies.
Figure 1.
Flow cytometric analysis of BaF/3, A431, and U87MG.Δ2–7 cell lines. Cells were incubated with either an irrelevant IgG2b, mAb 806, or mAb 528 by methods previously described [17] following conjugation with Cy3 dye. (A–C) BaF/3 cells were directly labeled with IgG2b/Cy3, mAb 806/Cy3, and mAb 528/Cy3, respectively; binding was visualized by flow cytometry. (D and E) A431 cells were directly labeled with IgG2b/Cy3, mAb 806/Cy3, and mAb 528/Cy3, respectively; binding was visualized by flow cytometry. (G and H) U87MG.Δ2–7 cells were directly labeled with IgG2b/Cy3, mAb 806/Cy3, and mAb 528/Cy3, respectively; binding was visualized by flow cytometry. All analyses were performed in triplicate; data are representative of binding observed in all samples.
Internalization of mAb 806 following Binding to a Subset of EGFR Expressed in A431 and HN5 Cells
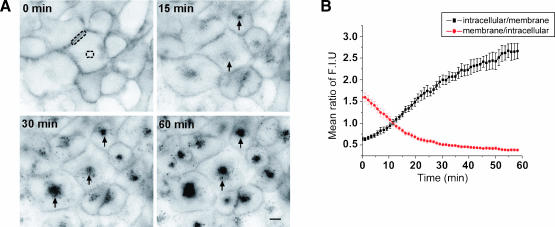
MAb 806-Cy3 internalization, following binding to 10% of wt EGFR recognized by this antibody in A431 (Figure 2) and HN5 cells (Figure W1), was assessed. Internalization of mAb 806 was also compared to that of mAb 528-Cy3 that binds to the bulk of the wt EGFR. Internalization of antibody preincubated at 4°C was observed by time-lapse microscopy for 1 hour at 37°C (Figure 2A and Figure W1A). Rapid internalization of mAb 806-Cy3 was indicated by the appearance of small intracellular punctuate structures (Figure 2A and Figure W1A; 15 minutes; arrows). Over time, these structures increased in number and accumulated intracellularly. Quantification of the mean fluorescence intensity ratio between the intracellular region versus the cell surface and vice versa, over the time course of 60 minutes was calculated (Figure 2B and Figure W1B). This ratio steadily increased over time, highlighting the gradual accumulation of internalized antibody within intracellular compartments (Figure 2B and Figure W1B; intracellular/membrane). Concurrently, the ratio of membrane to intracellular fluorescence was seen to decrease with time, consistent with the progressive removal of antibody from the cell surface and its concomitant intracellular accumulation (Figure 2B and Figure W1B). Isotype control antibody tagged with Cy3 did not bind to cells and was not internalized (data not shown).
Figure 2.
Time-lapse microscopy of antibody internalization in A431 cells. (A) Cells were preincubated with mAb 806-Cy3 at 4°C (0 minute). Internalized antibody is indicated by arrows in sequential images representing 15, 30, and 60 minutes of incubation at 37°C. (B) Mean ratio of fluorescence intensity between the intracellular region and cell surface. The mean fluorescence of the intracellular region (dashed circle) and cell membrane (dashed rectangle) were calculated (n = 5) and the average ratio of intracellular to membrane (black plot) and membrane to intracellular (red plot) fluorescence ± SEM plotted over time (n = 5). Scale bar, 20 µm. Refer to Supplemental data for Video W1.
Dynamin-Dependent Internalization of mAb 806
The dependence of mAb 806 internalization on the presence of functional dynamin, a GTPase essential for the fission of endocytic vesicles from the plasma membrane [29,30], was assessed in A431 cells expressing dominant-negative dynamin K44A tagged with GFP (DynK44A-GFP) [31,32].
DynK44A-GFP-transfected and -nontransfected cells were continuously imaged for 60 minutes following incubation with mAb 806-Cy3 (Figure 3). In nontransfected cells, internalized mAb 806-Cy3 showed a characteristic intracellular accumulation (Figure 3A). This is also shown by the steady increase over time in the ratio of intracellular to membrane fluorescence (and concurrent decrease in membrane to intracellular fluorescence ratio; Figure 3B). When endocytosis was blocked by the expression of dominantnegative dynamin K44A (Figure 3, asterisk), there was no net intracellular accumulation of fluorescence. Furthermore, the constant membrane to intracellular fluorescence ratio indicates a failure in the clearance of cell surface-localized antibody. This result indicates that 806 internalization is dependent on the presence of functional dynamin, a key regulator of scission of clathrin-coated vesicles.
Figure 3.
Time-lapse of mAb 806 internalization in A431 cells expressing dynamin K44A-GFP. (A) Cells were transiently transfected with DynK44A-GFP (red asterisk). Panels representing images at 5, 10, 20, 30, and 60 minutes after mAb 806-Cy3 internalization are shown. (B) Mean ratio of intracellular to membrane (black) and membrane to intracellular (red) fluorescence of untransfected wt cells ± SEM (n = 4), over time. Untransfected cells show gradual increase in intracellular fluorescence with a concurrent decrease in membrane associated fluorescence. (C) Mean ratio of intracellular to membrane (black) and membrane to intracellular (red) fluorescence of DynK44A-transfected cells ± SEM (n = 6), over time. DynK44A-transfected cells show little accumulation of intracellular fluorescence while sustaining high membrane fluorescence intensity. Scale bar, 20 µm. Refer to Supplemental data for Video W2.
Endosomal Localization of mAb 806 in A431 Cells
We initially compared the internalization of mAb 806 following binding to the wt EGFR in A431 cells to that of Tfn. The recycling of Tfn in A431 cells has been extensively characterized previously [33,34].
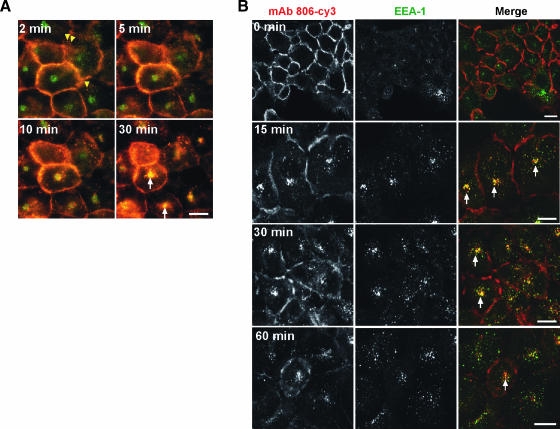
Cells simultaneously preincubated with mAb 806-Cy3 and Tfn-FITC were allowed to internalize at 37°C (Figure 4A; 2 minutes). Labeled mAb 806 (red) was present intracellularly as small vesicles (yellow arrowheads), as well as on the cell surface. FITC-labeled Tfn (green) is present both at the plasma membrane, where it colocalizes with membrane-bound mAb 806 (areas of yellow signal) and intracellularly (green signal).
Figure 4.
(A) Time-lapse microscopy of internalization and colocalization of mAb 806-Cy3 (red) and transferrin-FITC (green) in A431 cells. Internalization of mAb 806 at 2 minutes is represented by yellow arrowheads. Colocalization is indicated by white arrows. Scale bar, 20 µm. Refer to Supplemental data for Video W3. (B) Colocalization of mAb 806-Cy3 (red) and EEA1 (green) in A431 cells. Following internalization of mAb 806, cells were fixed at different time intervals and stained for EEA1 localization. Colocalization is indicated by arrows in the merged images. Scale bar, 20 µm.
Longer incubation at 37°C, increased the number of mAb 806 punctate moving into the cytoplasm which colocalized with Tfn-FITC, suggesting they at least transiently localize to endocytic vesicles and/or compartments (Figure 4A; 30 minutes; arrows indicating yellow signal). Colocalization between these two agents suggests that mAb 806 is internalized through similar mechanisms to that of Tfn and localized within similar intracellular structures.
The intracellular compartments to which endocytosed mAb 806 localized to was identified through dual labeling with an antibody against EEA1. After 15 and 30 minutes of incubation at 37°C, internalized mAb 806 was visualized as small punctuate, cytoplasmic vesicular structures, which partially colocalized with EEA1 (Figure 4B; 15 and 30 minutes; arrows), suggesting that these structures containing internalized mAb 806 are predominantly early endosomes. Following 60 minutes incubation at 37°C, the extent of colocalization between mAb 806 and EEA1 decreased (Figure 4B; 60 minutes). This suggests that the majority of fluorescently labeled antibody may have moved out of early endocytic compartments by this time. Similar results were also observed for mAb 528 (data not shown). There was no evidence of endoplasmic reticulum/Golgi involvement in mAb 806 internalization.
Lysosomal Localization of Internalized mAb 806 in A431 Cells
Lysosomal localization of internalized mAb 806 was also assessed following binding to the wt EGFR in A431 cells. Immunofluorescence staining with a lysosomal-specific antibody (anti-LAMP1) showed specific cytoplasmic staining which did not overlap with noninternalized mAb 806 (Figure 5A; 0 minutes merge). Following incubation at 37°C for 60 minutes, mAb 806 was observed to colocalize with LAMP1 as indicated in the merged images (Figure 5A; 60 minutes).
Figure 5.
Lysosomal localization of mAb 806-Cy3 in A431 Cells. (A) Preincubated mAb 806-Cy3 (red) was allowed to internalize for 60 minutes at 37°C. Cells were subsequently fixed and stained for LAMP1 (green). Colocalization is indicated by arrows in the merged images. Scale bar, 20 µm. (B) Time-lapse microscopy of internalization and colocalization of mAb 806-Cy3 (red) and lgp-120-GFP (green) in A341 cells. Sequential images of transfected A431 cells are shown at 0, 30, 45, and 60 minutes. Colocalization is indicated by the arrows. Scale bar, 20 µm. Refer to Supplemental data for Video W4.
Time-lapse imaging of mAb 806 targeting to lysosomal compartments was also investigated in A431 cells by assessing the extent of mAb 806 colocalization with the lysosomal glycoprotein 120 (lgp-120), an integral membrane protein located in lysosomal compartments [35]. A431 cells were transfected with lgp-120-GFP and positively transfected cells were imaged live at 37°C following preincubation with mAb 806-Cy3 (Figure 5B). At time 0 (Figure 5B; 0 minutes), mAb 806-Cy3 (red) is seen only on the cell surface as expected, whereas cells positively transfected for lgp-120-GFP displayed intracellular staining for lysosomal compartments (Figure 5B; 0 minutes; green fluorescence). At 30 minutes, mAb 806, seen as small punctate structures, rapidly migrated in an inward centrifugal motion toward the perinuclear region in transfected and untransfected cells (Figure 5B; 30 minutes). Initially, these structures did not colocalize with lgp-120-GFP (Figure 5B; 30 minutes). However, following longer incubation at 37°C, areas of colocalization between mAb 806-Cy3 and lgp-120-GFP were observed (Figure 5B; arrows), with maximal colocalization occurring at 60 minutes in this experiment.
Electron Microscopic Analysis of mAb 806 Internalization and Intracellular Trafficking
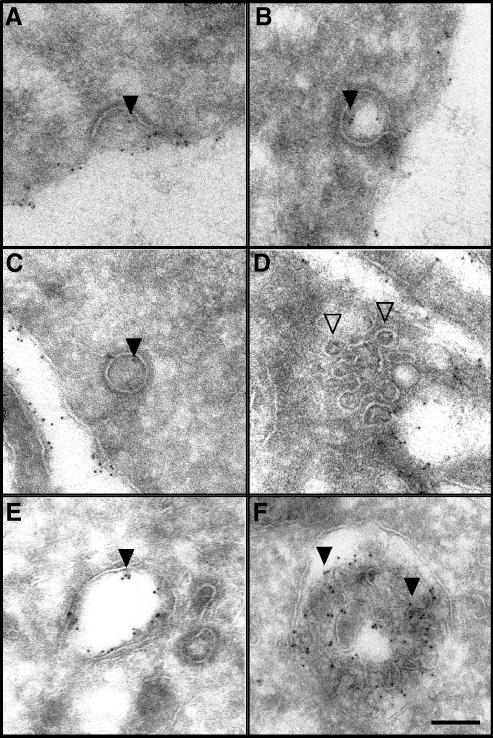
To support the immunofluorescence data, we also investigated the localization of internalized mAb 806 following binding to the wt EGFR on A431 cells by electron microscopy. Following 5 minutes of incubation at 37°C, gold particles, corresponding to mAb 806, were observed in structures resembling clathrin-coated pits (Figure 6, A and B; closed arrowheads). Gold particles were also detected in free clathrin-coated vesicles after antibody was allowed to internalize for 10 minutes (Figure 6C). No gold particles were served in structures resembling caveolae (Figure 6D; open arrowheads).
Figure 6.
Electron microscopic analysis of mAb 806 internalization and intracellular trafficking following binding to amplified EGFR in A431 cells. (A–B) Gold particles (arrowheads) were detected in deeply invaginated clathrincoated pits after 5 minutes at 37°C. (C) Gold particles were also localized in free clathrin-coated vesicles within the cytoplasm. (D) No gold particles were present in structures resembling caveolae (open arrowheads) at 5 minutes. (E) Gold particles were also detected in tubular-vesicular structures resembling early endosomes after 10 to 15 minutes at 37°C. (F) After 30 minutes, gold particles were localized to multivesicular bodies. Scale bar, 100 nm.
Following longer incubation at 37°C, mAb 806 gold particles were observed in large tubular-vesicular structures resembling early endocytic compartments (Figure 6E). After 30 minutes, mAb 806 was localized to structures resembling multivesticular endosomes (Figure 6F). Gold particles were readily observed on the internal vesicles of multivesticular endosomes, suggesting eventual localization and degradation in lysosomal compartments. These observations are therefore consistent with the immunofluorescence microscopy data that indicated colocalization of mAb 806 initially with EEA1 and subsequently with LAMP1 and lgp-120 between 30 and 60 minutes.
Biodistribution of 111In/125I-Labeled mAb 806 in A431 Tumor-Bearing Nude Mice
MAb 806 was successfully trace-radiolabeled with both 111In and 125I, and the quality of the radioconjugates was assessed before commencement of the biodistribution study. The instant thin-layer chromatography-determined radiochemical purity of 125I- and 111In-labeled mAb 806 was 99.6% and 99.5%, respectively. The specific activity for each radioconjugate was 0.99 and 0.73 mCi/mg for 125I- and 111In-labeled mAb 806, respectively. To confirm retention of immunoreactivity following radiolabeling, binding of 125I- and 111In-labeled mAb 806 to antigen-positive A431 cells was determined. The number of molecules of radiolabeled antibody bound to A431 cells was approximately equivalent for 125I- and 111In-labeled mAb 806 (3.14 x 105 and 3.89 x 105, respectively) and comparable to that previously published [10]. 125I- and 111In-labeled control isotype-matched mAb 100–310 demonstrated no immunoreactivity with A431 cells, as expected.
The in vivo biodistribution and targeting of radiolabeled antibody was investigated in A431 tumor-bearing mice. Groups of mice (n = 4) bearing A431 tumors (70 mm3) were injected IV with radiolabeled mAb 806 before harvest of normal tissue, blood, and tumor 4, 24, 48, 72, and 168 hours postinjection. Table 1 presents the % ID/g of 125I- and 111In-labeled mAb 806 in A431 tumors and blood for each time point.
Table 1.
Biodistribution of 125I- and 111In-CHX-A″-DTPA-Labeled mAb 806 in A431 Xenografted BALB/c Nude Mice*.
| Time† (Hours) | n‡ | % ID/g§ in A431 Tumor (Mean ± SD) | % ID/g in Blood (Mean ± SD) | ||
| 111In | 125I | 111In | 125I | ||
| 4 | 4 | 21.8 ± 6.1 | 13.7 ± 3.3 | 30.9 ± 1.9 | 29.8 ± 1.7 |
| 24 | 4 | 36.4 ± 10.0 | 9.9 ± 3.5 | 19.3 ± 2.3 | 18.6 ± 2.2 |
| 48 | 4 | 44.9 ± 11.4 | 7.2 ± 2.3 | 12.6 ± 1.7 | 12.5 ± 1.7 |
| 72 | 4 | 36.6 ± 8.2 | 4.5 ± 1.2 | 13.2 ± 1.9 | 10.7 ± 1.2 |
| 168 | 4 | 23.6 ± 6.3 | 2.5 ± 0.9 | 5.2 ± 1.7 | 4.7 ± 1.6 |
Mice bearing A431 tumors received tail vein injections containing 5 µCi of both 125I- and 111In-CHX-A±-DPTA-labeled mAb 806 (total, 10 µg of antibody; 10 µCi) on day 0. Groups of four mice were sacrificed at the indicated times. The blood was collected, tumors removed, blotted dry, and weighed. Radioactivity was measured.
Time in hours after injection of antibody.
Number of mice per time point.
% ID/g was calculated. Data are presented as mean ± SD.
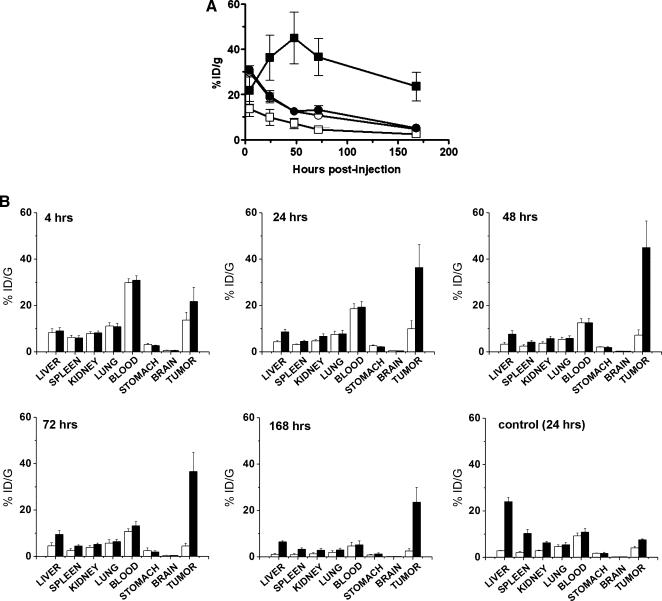
Following injection, the % ID/g of 111In-mAb 806 in A431 xenografts gradually increased before peaking at 48 hours after antibody injection (44.9%) (Figure 7A). Subsequently, the level of 111In-mAb 806 detected in the tumor slowly decreased over the course of the study and, at 168 hours postinjection, the% ID/g was 24%. In contrast, the maximum uptake of 125I-mAb 806 in tumors (13.7%) occurred 4 hours after antibody injection and gradually decreased with time (2.5% at 168 hours postinjection) (Figure 7A). Tumor uptake was superior for the 111In-labeled mAb 806, at all time points studied. For example, at 48 hours, the % ID/g of 111In was more than 6-fold higher than 125I (44.9% vs 7.2% respectively, P < .02). Furthermore, as much as a 9-fold greater uptake of 111In-labeled mAb 806 compared to 125I-labeled mAb 806 was detected 72 hours postinjection (36.6% vs 4.4%, respectively) (P < .003) (Figure 7A). The blood levels of both isotopes closely approximated each other throughout the study, declining progressively over time (Figure 7A), indicating that antibody serum half life had no effect on tumor targeting. The tumor/blood ratio for 111In-labeled mAb 806 peaked at 4.67 ± 0.66 at 168 hours, compared with 0.56 ± 0.11 at 24 hours for 125I-mAb 806.
Figure 7.
Biodistribution of mAb 806. (A) Biodistribution (% ID/g) of 111In-CHX-A″-DTPA- (closed symbols) and 125I- (open symbols) labeled mAb 806 in A431 tumor xenografts and blood from BALB/c nude mice. Twenty mice received IV injections of a total of 10 µg (10 µCi) of radiolabeled mAb 806. Means for each time point consisting of data from four mice are shown. Bars, SD. Data are presented as: 111In-blood (●); 111In-A431 tumor (■); 125I-blood (○); 125I-A431 tumor (□). (B) Biodistribution of 125I- (white bar) and 111In-CHX-A″-DTPA- (black bar) labeled mAb 806 in normal murine tissues, A431 tumors, and blood at 4, 24, 48, 72, and 168 hours after IV administration (10 µCi, 10 µg total mAb 806) of BALB/c nude mice. The biodistribution of 125I- (white bar) and 111In-CHX-A″-DTPA- (black bar) labeled control mAb 100–310 was also assessed at 24 hours postinjection. Data from groups of four mice are expressed as mean % ID/g. Bars, SD.
The biodistribution of radiolabeled mAb 806 in normal tissue was also analyzed and results at 4, 24, 48, 72, and 168 hours after injection of the antibody are presented in Figure 7B. The %ID/g in all normal tissues analyzed was less than 10% at all time points. The percentage uptake of 111In-labeled mAb 806 was higher in the liver, spleen, and kidney than those of 125I-labeled mAb 806 at all time points. This may reflect differences in catabolism and biochemical processing of iodotyrosine derivatives versus 111In-DTPA-labeled metabolites. Normal tissue distribution of the control isotype-matched 100–310 antibody labeled with 125I and 111In was also assessed at 24 hours postinjection (Figure 7B). Higher levels of 111In-mAb 100–310 uptake were noted in both the liver and the spleen. This most likely represents accumulation of free 111In in these organs indicating that radiolabeled mAb 100–310 may be less stable. Importantly, uptake of 111In-mAb 100–310 was only 7.5% in tumor tissue compared to 36% uptake of 111In-labeled mAb 806, 24 hours postinjection (Figure 7, A and B). Tumor/blood ratios of 0.7 and 0.4 for 111In- and 125I-labeled mAb 100–310, respectively, were detected 24 hours postinjection, further indicating nonspecific tumor tissue uptake of this antibody.
Discussion
The use of mAbs that target growth factor receptors such as immunotherapeutic agents has been the subject of intensive investigation by numerous groups. Indeed, many such antibodies are currently being assessed in advanced clinical trials or, in the case of Herceptin (anti-ErbB2) [36], Erbitux, and Vectabix (anti-EGFR) [4], have been approved for treatment of patients with advanced metastatic disease. Furthermore, there is a growing body of data highlighting the potential use of antibodies as carriers for radioisotopes and toxins [21–24]. These conjugates have demonstrable antitumor activity in experimental models and thereby warrant further investigation as to their suitability for clinical use. Binding of such antibody constructs to their cognate receptor would potentially elicit both receptor inhibition and the induction of cytotoxic effects attributable to the toxin or radioisotope. Indeed, the inhibition of receptor activity may in fact enhance the cytotoxicity of the compound used if chosen correctly. The ability of an antibody to modulate the activity of its target receptor is critical to its function, likewise, the type of radioisotope or drug/toxin best suited to a particular antibody is predominantly determined by its fate following binding to its cognate receptor. Accordingly, antibody internalization and intracellular retention are important criteria for assessing the potential of a therapeutic agent being considered for conjugation with a toxin or radioisotope conjugate.
The unique specificity of mAb 806, and its proven efficacy in animal models against xenografts expressing either the de2-7 EGFR or wt EGFR, makes it an ideal reagent for translation into a clinical setting. This study presents the internalization and intracellular trafficking of mAb 806 in A431 cells, a well-characterized cell line that overexpresses the wt EGFR, as the main aim of this study was to determine the intracellular trafficking pathway of mAb 806 that could be exploited for therapeutic payload delivery. The A431 cell line has been extensively studied as a model system for the study of wt EGFR biology and for preclinical evaluation of wt EGFR therapeutics [1,2,3,31]. Using live cell fluorescent microscopy, we found that mAb 806 undergoes rapid internalization following binding to the wt EGFR overexpressed on A431 cells. Internalization of mAb 806 was reproducible in the HN5 squamous carcinoma cell line. Internalization of mAb 806 was dynamin-dependent as was also recently shown for anti-ErbB2 antibody internalization [37]. Indeed, dynamin is required for a number of endocytic processes such as clathrin-mediated endocytosis, endocytosis through caveolae, phagocytosis, and macropinocytosis [38,39]. However, immunoelectron microscopy clearly identified mAb 806 to be localized in structures morphologically resembling clathrin-coated pits and vesicles. Following internalization, mAb 806 was detected in early endocytic compartments and at later time points (30 and 60 minutes) in lysosomal compartments. Indeed, for an antibody to act as a carrier for delivery of increased quantities of radioisotope or toxin to the tumor site over time, its ability to internalize, traffic, and accumulate in lysosomal compartments is crucial. Therefore, the retention of mAb 806 within the target tumor cells makes it an ideal candidate for radioisotope or toxin conjugation.
Given that mAb 806 is known to recognize a transitional form of the EGFR that exists as it switches from the tethered inactive form to the untethered form [17,18], our data showing rapid mAb 806 internalization also suggests that the inactive intermediate conformation of the EGFR can undergo internalization. This unique specificity of mAb 806 arises from its recognition of an epitope buried at the CR1/CR2 interface in the active dimer form of the EGFR, which is partially buried in the inactive tethered configuration [18]. Given that this epitope is buried in both conformations, binding of mAb 806 to either the tethered or the untethered conformation is prevented. MAb 806 binding only occurs during the transition from the tethered inactive form of the EGFR to the untethered form [18]. Moreover, in this intermediate untethered state, the mAb 806 epitope is exposed, allowing antibody binding [17]. This transitional state accounts for only 5% to 10% of total EGFR on the cell surface at a given time, which corresponds to the proportion of receptor recognized by mAb 806 in cells such as A431 that overexpress the EGFR. Furthermore, it is likely that over longer periods of time, a greater proportion of the cell surface EGFR may be targeted by mAb 806 as the receptor continues to untether. This cumulative uptake of mAb 806 by tumor cells has been shown in animal model studies, and in human tumors in our first-in-man clinical trial [16,40]. Therefore, mAb 806 binding almost certainly traps the EGFR in this transitional state preventing dimerization and subsequent activation. Indeed, a recent study investigating the antitumor activity of the ErbB2 antibody, 2C4, showed that this antibody is capable of blocking formation of ErbB2 homo- or heterodimers [41]. Following binding of 2C4 to ErbB2 monomers, this antibody was able to cause significant downregulation of ErbB2, indicating that monomeric receptors are also capable of undergoing successful internalization [41]. This downregulation of monomeric ErbB2, in turn, has been linked to the significant efficacy of mAb 2C4 in vivo against ErbB2 expressing breast and prostate cancer xenografts [42]. Therefore, our data suggest that transient configurations of the EGFR are also capable of undergoing successful internalization even when inactive, which can subsequently lead to an antitumor response.
Because internalizing antibodies can be used as diagnostic and therapeutic tools, and having established that mAb 806 is able to internalize following binding to a proportion of the EGFR, its potential as a carrier for radioisotopes/toxins was evaluated in an in vivo A431 tumor model. The %ID/g of 111In-labeled mAb 806 in A431 tumor xenografts was significantly greater than that for 125I-labeled mAb 806 at all time points. Whereas initial targeting of both mAb 806 conjugates is presumably identical in this tumor model, the differences in tumor retention of 111In and 125I indicate that 111In is a more accurate reflection of true uptake and retention of mAb 806 in tumor xenografts. This data would also indicate that 111In (or a nonresidualizing tracer) would be a better candidate for conjugation to mAb 806 for diagnostic purposes, such as the identification of occult tumors. The reasons for the difference in tumor retention of each isotope are well established. Whereas the metabolites of iodine moieties are rapidly excreted from the cell [43], slower catabolism of the 111In-DTPA-labeled metabolites results in accumulation and retention in lysosomal compartments for longer periods [44]. Therefore, the ability of mAb 806 to rapidly internalize following binding to a transient conformation of the EGFR can successfully be translated into an in vivo setting resulting in specific targeting of tumor tissue. In addition, the uptake and retention of mAb 806 in wt EGFR tumor is comparable to the maximum uptake of antibodies that target all wt EGFR on the surface of the cell (and block ligand binding) such as Cetuximab. This highlights the importance of normal wt EGFR turnover on the surface of cancer cells in vivo, which increases the available transitional form of the EGFR that can bind to mAb 806 with time.
Whereas gamma-emitting isotopes such as 111In are not routinely used for therapeutic purposes, they remain important predictive agents for the behavior of other radiometals such as Yttrium-90 (90Y) or Lutetium-177 (177Lu). For example, 90Y is a high-energy beta emitter with a particle range of up to 12 mm and has been extensively used for radioimmunotherapy with several antibody-antigen systems in patients with a variety of cancers [45–49]. 90Y also has a long residence time in tumors, thereby enabling delivery of a large radiation dose to the tumor cell. Accordingly, 111In is often used as a surrogate gamma-imaging isotope to predict suitable therapeutic doses for other radiometals such as 90Y, as it possesses comparable biodistribution and clearance rates [27]. Therefore, the specific targeting of 111In-labeled mAb 806 to tumor tissue indicates that similar targeting of a 90Y or 177Lu conjugate of mAb 806 may enable delivery of a sufficient therapeutic dose to produce effective tumor cell killing and subsequent tumor regression with negligible side effects to normal tissue. Such targeted delivery of antibody-radioisotope conjugates is not possible with currently approved anti-EGFR or -ErbB2 antibodies such as Cetuximab, Panitumumab, and Herceptin, as these agents can bind to receptor expressed in normal tissue.
Collectively, these studies establish that mAb 806 is able to rapidly internalize, following binding to a transient form of the EGFR. Accumulation of mAb 806 in lysosomal compartments makes it an ideal candidate for construction of mAb 806 conjugates linked to radioisotopes, drugs, or toxins. Furthermore, mAb 806 has no detectable binding to wt EGFR expressed on normal tissues possessing physiological levels of receptor such as the liver, skin, and gastrointestinal tract and, therefore, would be unlikely to cause normal tissue toxicity, which is dose-limiting for current anti-wt EGFR antibodies and kinase inhibitors. We have generated a chimeric version of this antibody [40], and this antibody has recently completed a Phase I first-in-man trial, confirming the tumor selectivity of ch806 and lack of normal tissue binding [16]. These characteristics along with the unique specificity of mAb 806 make it an ideal therapeutic agent that can be used for both diagnostic and therapeutic purposes in patients with EGFR-positive malignancies.
Supplementary Material
Acknowledgements
We thank our colleagues in the Johns, Scott, and Toomre laboratories for helpful input.
Conflict of interest statement: Terrance G. Johns, Lloyd J. Old, and Andrew M. Scott are coinventors of a patent for monoclonal antibody 806. Andrew M. Scott is a consultant to Life Science Pharmaceuticals, which has the license for mAb 806. Lloyd J. Old is a noncompensated board member of Life Science Pharmaceuticals.
Abbreviations
- Cy3
cyanine 3
- EEA1
early endosome autoantigen 1
- EGFR
epidermal growth factor receptor
- FITC
fluorescein isothiocyanate
- GFP
green fluorescent protein
- LAMP1
lysosomal-associated membrane protein 1
- mAb
monoclonal antibody
- PFA
paraformaldehyde
- Tfn
transferrin
Footnotes
This work was partly supported by the National Health and Medical Research Council of Australia (Program Grant 280912), the Human Frontier Science Program (Young Investigator Award RGY40/2003), Bayer Pharmaceuticals Scholar Award, the Ludwig Institute for Cancer Research (to D.T.), and a Boehringer Ingelheim Fonds PhD Scholarship (to R.Z.).
This article refers to supplementary materials, which are designated by Figure W1 and Videos W1 to W5 and are available online at www.neoplasia.com.
References
- 1.de Bono JS, Rowinsky EK. The ErbB receptor family: a therapeutic target for cancer. Trends Mol Med. 2002;8:S19–S26. doi: 10.1016/s1471-4914(02)02306-7. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Langer CJ. Epidermal growth factor receptors as a target for cancer treatment: the emerging role of IMC-C225 in the treatment of lung and head and neck cancers. Semin Oncol. 2002;29:27–36. doi: 10.1053/sonc.2002.31525. [DOI] [PubMed] [Google Scholar]
- 3.Wakeling AE. Epidermal growth factor receptor tyrosine kinase inhibitors. Curr Opin Pharmacol. 2002;2:382–387. doi: 10.1016/s1471-4892(02)00183-2. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RM. Cetuximab. Nat Rev Drug Discov. 2005;(Suppl):S10–S11. doi: 10.1038/nrd1728. [DOI] [PubMed] [Google Scholar]
- 5.Divgi CR, Welt S, Kris M, Real FX, Yeh SD, Gralla R, Merchant B, Schweighart S, Unger M, Larson SM, et al. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J Natl Cancer Inst. 1991;83:97–104. doi: 10.1093/jnci/83.2.97. [DOI] [PubMed] [Google Scholar]
- 6.Busam KJ, Capodieci P, Motzer R, Kiehn T, Phelan D, Halpern AC. Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol. 2001;144:1169–1176. doi: 10.1046/j.1365-2133.2001.04226.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Doorn R, Kirtschig G, Scheffer E, Stoof TJ, Giaccone G. Follicular and epidermal alterations in patients treated with ZD1839 (Iressa), an inhibitor of the epidermal growth factor receptor. Br J Dermatol. 2002;147:598–601. doi: 10.1046/j.1365-2133.2002.04864.x. [DOI] [PubMed] [Google Scholar]
- 8.Luwor RB, Johns TG, Murone C, Huang HJ, Cavenee WK, Ritter G, Old LJ, Burgess AW, Scott AM. Monoclonal antibody 806 inhibits the growth of tumor xenografts expressing either the de2-7 or amplified epidermal growth factor receptor (EGFR) but not wild-type EGFR. Cancer Res. 2001;61:5355–5361. [PubMed] [Google Scholar]
- 9.Mishima K, Johns TG, Luwor RB, Scott AM, Stockert E, Jungbluth AA, Ji XD, Suvarna P, Voland JR, Old LJ, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 2001;61:5349–5354. [PubMed] [Google Scholar]
- 10.Johns TG, Stockert E, Ritter G, Jungbluth AA, Huang HJ, Cavenee WK, Smyth FE, Hall CM, Watson N, Nice EC, et al. Novel monoclonal antibody specific for the de2-7 epidermal growth factor receptor (EGFR) that also recognizes the EGFR expressed in cells containing amplification of the EGFR gene. Int J Cancer. 2002;98:398–408. doi: 10.1002/ijc.10189. [DOI] [PubMed] [Google Scholar]
- 11.Jungbluth AA, Stockert E, Huang HJ, Collins VP, Coplan K, Iversen K, Kolb D, Johns TJ, Scott AM, Gullick WJ, et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci USA. 2003;100:639–644. doi: 10.1073/pnas.232686499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johns TG, Luwor RB, Murone C, Walker F, Weinstock J, Vitali AA, Perera RM, Jungbluth AA, Stockert E, Old LJ, et al. Antitumor efficacy of cytotoxic drugs and the monoclonal antibody 806 is enhanced by the EGF receptor inhibitor AG1478. Proc Natl Acad Sci USA. 2003;100:15871–15876. doi: 10.1073/pnas.2036503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luwor RB, Zhu HJ, Walker F, Vitali AA, Perera RM, Burgess AW, Scott AM, Johns TG. The tumor-specific de2-7 epidermal growth factor receptor (EGFR) promotes cells survival and heterodimerizes with the wild-type EGFR. Oncogene. 2004;23:6095–6104. doi: 10.1038/sj.onc.1207870. [DOI] [PubMed] [Google Scholar]
- 14.Johns TG, Mellman I, Cartwright GA, Ritter G, Old LJ, Burgess AW, Scott AM. The antitumor monoclonal antibody 806 recognizes a high-mannose form of the EGF receptor that reaches the cell surface when cells over-express the receptor. FASEB J. 2005;19:780–782. doi: 10.1096/fj.04-1766fje. [DOI] [PubMed] [Google Scholar]
- 15.Perera RM, Narita Y, Furnari FB, Gan HK, Murone C, Ahlkvist M, Luwor RB, Burgess AW, Stockert E, Jungbluth AA, et al. Treatment of human tumor xenografts with monoclonal antibody 806 in combination with a prototypical epidermal growth factor receptor-specific antibody generates enhanced antitumor activity. Clin Cancer Res. 2005;11:6390–6399. doi: 10.1158/1078-0432.CCR-04-2653. [DOI] [PubMed] [Google Scholar]
- 16.Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, Skrinos E, Murone C, Saunder TH, Chappell B, et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci USA. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johns TG, Adams TE, Cochran JR, Hall NE, Hoyne PA, Olsen MJ, Kim YS, Rothacker J, Nice EC, Walker F, et al. Identification of the epitope for the EGFR-specific monoclonal antibody 806 reveals that it preferentially recognizes an untethered form of the receptor. J Biol Chem. 2004;279:30375–30381. doi: 10.1074/jbc.M401218200. [DOI] [PubMed] [Google Scholar]
- 18.Walker F, Orchard SG, Jorissen RN, Hall NE, Zhang HH, Hoyne PA, Adams TE, Johns TG, Ward C, Garrett TP, et al. CR1/CR2 interactions modulate the functions of the cell-surface epidermal growth factor receptor. J Biol Chem. 2004;279:22387–22398. doi: 10.1074/jbc.M401244200. [DOI] [PubMed] [Google Scholar]
- 19.Guan H, Jia SF, Zhou Z, Stewart J, Kleinerman ES. Herceptin down-regulates HER-2/neu and vascular endothelial growth factor expression and enhances taxol-induced cytotoxicity of human Ewing's sarcoma cells in vitro and in vivo. Clin Cancer Res. 2005;11:2008–2017. doi: 10.1158/1078-0432.CCR-04-0777. [DOI] [PubMed] [Google Scholar]
- 20.Austin CD, De Maziere AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15:5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldenberg DM. Advancing role of radiolabeled antibodies in the therapy of cancer. Cancer Immunol Immunother. 2003;52:281–296. doi: 10.1007/s00262-002-0348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross JS, Gray K, Gray GS, Worland PJ, Rolfe M. Anticancer antibodies. Am J Clin Pathol. 2003;119:472–485. doi: 10.1309/Y6LP-C0LR-726L-9DX9. [DOI] [PubMed] [Google Scholar]
- 23.Scott AM, Larson SM. Tumor imaging and therapy. Radiol Clin North Am. 1993;31:859–879. [PubMed] [Google Scholar]
- 24.Rustamzadeh E, Low WC, Vallera DA, Hall WA. Immunotoxin therapy for CNS tumor. J Neurooncol. 2003;64:101–116. doi: 10.1007/BF02700025. [DOI] [PubMed] [Google Scholar]
- 25.Modjtahedi H, Affleck K, Stubberfield C, Dean C. EGFR blockade by tyrosine kinase inhibitor or monoclonal antibody inhibits growth, directs terminal differentiation and induces apoptosis in the human squamous cell carcinoma HN5. Int J Oncol. 1998;13:335–342. doi: 10.3892/ijo.13.2.335. [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Kobayashi H, Sun B, Yoo TM, Paik CH, Gansow OA, Carrasquillo JA, Pastan I, Brechbiel MW. Stereochemical influence on the stability of radio-metal complexes in vivo. Synthesis and evaluation of the four stereoisomers of 2-(p-nitrobenzyl)-trans-CyDTPA. Bioorg Med Chem. 1997;5:1925–1934. doi: 10.1016/s0968-0896(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 27.Clarke K, Lee FT, Brechbiel MW, Smyth FE, Old LJ, Scott AM. In vivo biodistribution of a humanized anti-Lewis Y monoclonal antibody (hu3S193) in MCF-7 xenografted BALB/c nude mice. Cancer Res. 2000;60:4804–4811. [PubMed] [Google Scholar]
- 28.Palacios R, Steinmetz M. IL-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 29.Slepnev VI, De Camilli P. Accessory factors in clathrindependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- 30.Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopkins CR. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983;35:321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- 34.Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis V, Green SA, Marsh M, Vihko P, Helenius A, Mellman I. Glycoproteins of the lysosomal membrane. J Cell Biol. 1985;100:1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenner TL, Adams VR. First MAb approved for treatment of metastatic breast cancer. J Am Pharm Assoc (Wash) 1999;39:236–238. [PubMed] [Google Scholar]
- 37.Friedman LM, Rinon A, Schechter B, Lyass L, Lavi S, Bacus SS, Sela M, Yarden Y. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc Natl Acad Sci USA. 2005;102:1915–1920. doi: 10.1073/pnas.0409610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones SM, Howell KE, Henley JR, Cao H, McNiven MA. Role of dynamin in the formation of transport vesicles from the trans- Golgi network. Science. 1998;279:573–577. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- 39.Henley JR, Cao H, McNiven MA. Participation of dynamin in the biogenesis of cytoplasmic vesicles. FASEB J. 1999;13(Suppl 2):S243–S247. doi: 10.1096/fasebj.13.9002.s243. [DOI] [PubMed] [Google Scholar]
- 40.Panousis C, Rayzman VM, Johns TG, Renner C, Liu Z, Cartwright G, Lee FT, Wang D, Gan H, Cao D, et al. Engineering and characterisation of chimeric monoclonal antibody 806 (ch806) for targeted immunotherapy of tumours expressing de2-7 EGFR or amplified EGFR. Br J Cancer. 2005;92:1069–1077. doi: 10.1038/sj.bjc.6602470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 42.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 43.Geissler F, Anderson SK, Press O. Intracellular catabolism of radiolabeled anti-CD3 antibodies by leukemic T cells. Cell Immunol. 1991;137:96–110. doi: 10.1016/0008-8749(91)90060-o. [DOI] [PubMed] [Google Scholar]
- 44.Duncan JR, Welch MJ. Intracellular metabolism of indium-111-DTPA-labeled receptor targeted proteins. J Nucl Med. 1993;34:1728–1738. [PubMed] [Google Scholar]
- 45.Riva P, Franceschi G, Riva N, Casi M, Santimaria M, Adamo M. Role of nuclear medicine in the treatment of malignant gliomas: the locoregional radioimmunotherapy approach. Eur J Nucl Med. 2000;27:601–609. doi: 10.1007/s002590050549. [DOI] [PubMed] [Google Scholar]
- 46.Paganelli G, Grana C, Chinol M, Cremonesi M, De Cicco C, De Braud F, Robertson C, Zurrida S, Casadio C, Zoboli S, et al. Antibody-guided three-step therapy for high grade glioma with yttrium-90 biotin. Eur J Nucl Med. 1999;26:348–357. doi: 10.1007/s002590050397. [DOI] [PubMed] [Google Scholar]
- 47.Behr TM, Salib AL, Liersch T, Behe M, Angerstein C, Blumenthal RD, Fayyazi A, Sharkey RM, Ringe B, Becker H, et al. Radioimmunotherapy of small volume disease of colorectal cancer metastatic to the liver: preclinical evaluation in comparison to standard chemotherapy and initial results of a phase I clinical study. Clin Cancer Res. 1999;5:3232s–3242s. [PubMed] [Google Scholar]
- 48.Richman CM, DeNardo SJ, O′Donnell RT, Goldstein DS, Shen S, Kukis DL, Kroger LA, Yuan A, Boniface GR, Griffith IJ, et al. Dosimetrybased therapy in metastatic breast cancer patients using 90Y monoclonal antibody 170H.82 with autologous stem cell support and cyclosporin A. Clin Cancer Res. 1999;5:3243s–3248s. [PubMed] [Google Scholar]
- 49.Epenetos AA, Hird V, Lambert H, Mason P, Coulter C. Long term survival of patients with advanced ovarian cancer treated with intraperitoneal radioimmunotherapy. Int J Gynecol Cancer. 2000;10:44–46. doi: 10.1046/j.1525-1438.2000.99510.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.