Abstract
Background
Cancer anemia causes fatigue and correlates with poor treatment outcome. Erythropoietin has been introduced in an attempt to correct these defects. However, five recent clinical trials reported a negative impact of erythropoietin on survival and/or tumor control, indicating that experimental evaluation of a possible direct effect of erythropoietin on cancer cells is required. Cancer recurrence is thought to rely on the proliferation of cancer initiating cells (CICs). In breast cancer, CICs can be identified by phenotypic markers and their fate is controlled by the Notch pathway.
Methods
In this study, we investigated the effect of erythropoietin on CICs in breast cancer cell lines. Levels of erythropoietin receptor (EpoR), CD24, CD44, Jagged-1 expression, and activation of Notch-1 were assessed by flow cytometry. Self-renewing capacity of CICs was investigated in sphere formation assays.
Results
EpoR expression was found on the surface of CICs. Recombinant human Epo (rhEpo) increased the numbers of CICs and self-renewing capacity in a Notch-dependent fashion by induction of Jagged-1. Inhibitors of the Notch pathway and PI3-kinase blocked both effects.
Conclusions
Erythropoietin functionally affects CICs directly. Our observation may explain the negative impact of recombinant Epo on local control and survival of cancer patients with EpoR-positive tumors.
Keywords: CD24-/low/CD44+ breast cancer cells, breast cancer-initiating cells, rhEpo, Notch, Epor
Introduction
Cancer-related and chemotherapy-induced anemia negatively impacts the quality of life of many cancer patients and is accompanied by a poor prognosis [1–5]. Therefore, anemia is frequently corrected by blood transfusions or application of erythropoiesis-stimulating agents (ESAs) [7]. However, three randomized clinical studies reported a negative impact of ESAs on overall survival and local tumor control in breast [8], head and neck [9], and non-small cell lung cancer patients [10]. More recently, two large clinical trials (DAHANCA 10 and Anemia of Cancer, study by Amgen) were stopped after interim analyses showed increased death rates in patients treated with ESAs. Whereas some of the patients treated with ESAs experienced thrombo-embolic events that could be easily related to changes in blood rheology, a negative impact of ESAs on local tumor control most likely resulted from more complex interactions.
We have recently demonstrated that overexpression of the erythropoietin receptor (EpoR) increased the clonogenicity of cancer cells [11] and that local control and overall survival in patients receiving recombinant human erythropoietin (rhEpo) were only negatively affected if the cancers stained positive using an anti-EpoR antibody [12]. A better understanding of the mechanisms underlying these rhEpo-related effects may allow rational selection of patients for treatment and uncover novel targets to improve cancer treatment outcome.
One view of cancer is that it arises from and is maintained by a small number of cancer stem cells (CSCs), which have the ability to self-renew whereas their progenies do not [13]. CSCs can now be identified prospectively in brain tumors [14], breast cancer [15], prostate cancer [16], cancer of the head and neck [6], pancreatic cancer [17], and melanoma [18]. The hypothesis is that CSCs are responsible for the regrowth and metastatic spread of a tumor and the efficacy of any given treatment depends on the killing of this population of cells [13]. In breast cancer, a CD44+/CD24-/low cell population can be isolated from patient tumor samples or established cell lines that are highly enriched for the putative breast cancer stem cell population exhibiting a 3-log increased tumorigenicity [15].
Furthermore, Dontu et al. [19] demonstrated that mammary development relies on the developmental Notch signaling pathway that regulates the fate of mammary stem cells. On binding of Notch ligands, the Notch receptors undergo intramembranous cleavage by the γ-secretase protease complex. This releases the intracellular domain of the receptor (Notch-ICD) for translocation into the nucleus, where it switches the function of CBF-1 from a transcriptional repressor into an activator. Function of γ-secretase can be blocked by specific inhibitors, which are already in clinical trials for patients suffering from Alzheimer's disease [20].
The stem cell in breast cancer that is capable of repopulating a tumor from a single cell has not yet been firmly characterized. Therefore, we will join others in calling the population of CD44+/CD24-/low putative stem cells, breast cancer-initiating cells (BCICs). Adopting a technique for the propagation of normal mammary stem cells [21], Ponti et al. [22] recently demonstrated that BCICs can be propagated as mammospheres in vitro. BCICs derived from MCF-7 breast cancer cells mimicked the phenotype and tumorigenicity of BCICs derived from primary estrogen receptor-positive breast cancers. This offers an invaluable tool to study the treatment responses of BCICs directly and to compare them to vast literature gained using breast cancer cell lines in the past. Using the same techniques as Ponti et al., we were able to enrich mammospheres to contain up to 40% of CD44+/CD24-/low cells and demonstrate that BCICs are a radio-resistant subpopulation of cancer cells. Remarkably, the number of BCICs increased after sublethal fractionated irradiation in vitro. Radiation activated the developmental Notch signaling pathway and inhibition of this pathway prevented the radiation-induced increase in the number of BCICs [23]. The relevance of this cell population was underlined by two other studies demonstrating that BCICs have enhanced invasive properties [24] and that the most early disseminated cells in the bone marrow of breast cancer patients exhibit the CD44+/CD24-/low phenotype [25]. Such cells are also resistant to conventional cancer treatment [23,26,27].
We hypothesized that rhEpo might act on BCICs to increase the size of the BCICs' population and compromise tumor control in breast cancer patients receiving rhEpo treatment. We addressed our hypothesis using our established in vitro BCIC system with MCF-7, T47D, and MDA-MB-231 breast cancer cell lines, which account for two thirds of all experimental literature on breast cancer [28].
We found that pharmacological concentrations of rhEpo increased the number of putative BCICs in established breast cancer cell lines. The increase was mediated by the activation of the Notch signaling pathway, could be blocked by inhibiting this pathway, and mimicked by overexpression of a constitutively active Notch-1 receptor. Notch activation occurred through the induction of the Notch receptor ligand Jagged-1 in a phosphoinositide-3 kinase (PI3K)-dependent fashion and could be blocked by a PI3K inhibitor.
Methods
Cell Culture
MCF-7, T47D, and MDA-MB-231 breast cancer cells were purchased from the American Type Culture Corporation (Manassas, VA) and cultured in log-growth phase in minimum essential medium (MCF-7 and T47D) (Cellgro, Kansas City, MO) (supplemented with 0.1 mM nonessential amino acids and 1 mM sodium pyruvate) and Dulbecco's modified Eagle's medium (DMEM) (MDA-MB-231) (Cellgro), respectively, after supplementing with 10% heat-inactivated fetal calf serum and 0.01 mg/ml bovine insulin (Sigma, St. Louis, MO) at 37°C in a humidified atmosphere (5% CO2). Mammosphere cultures were established as described by Ponti et al. [22] under serum-free conditions in phenol red-free DMEM/F12, supplemented with 0.4% bovine serum albumin, 20 ng/ml basic fibroblast growth factor (Sigma), and 10 ng/ml epidermal growth factor (Sigma). Cultures were fed with fresh growth factors every 3 days.
Transfection
All plasmid DNA were prepared using a commercial DNA extraction and isolation kit (Midiprep; Quiagen, Valencia CA). The Notch-ICD plasmid [29] was a gift from Dr. L. Miele (Loyola University Medical Center). The pNICD plasmid was constructed by cloning the intracellular domain of Notch-1 (5309–7655 bp) into the expression vector pcDNA3 (Invitrogen, Carlsbad, CA). The empty pcDNA3 vector was used as a control. MCF-7 cells were transfected with the pNICD plasmid or the pcDNA3 control vector using Lipofectamine2000 (Invitrogen) and OptiMEM (Invitrogen). After 24 hours, the cells were replated and maintained under 1 mg/ml neomycin (Sigma) selection. Individual clones were selected, grown in the presence of 1 mg/ml neomycin, and tested for the expression of intracellular Notch-1 (Notch-1-ICD). Clones overexpressing intracellular Notch-1 were expanded under neomycin selection to generate stable expression of MCF-7-pNICD and MCF-7-pcDNA3 cell lines.
Drug Treatment
RhEpo (1 IU/ml) treatment of monolayer cultures of MCF-7 cells was performed from day 2 to 4 after plating. Cells were harvested on day 5 when flow cytometry was performed to assess the cells' phenotypes. The γ-secretase inhibitor (GSI), GSI XVII (InSolution; Calbiochem, San Diego, CA), was added at a final concentration of 5 µM (0.1% final concentration of DMSO). LY294002 (Calbiochem) was dissolved in DMSO and added at a final concentration of 10 µM. Control cells received 0.1% DMSO only. Cells were serum-starved for 5 hours before the start of the experiment to prevent EpoR internalization through binding of fetal calf serum-derived erythropoietin (Epo). Serum starvation in long-term experiments in which cells were treated with rhEpo for 3 consecutive days was not tolerated by the cells and was therefore omitted.
Flow Cytometry
For analysis of CD24 and CD44 expression, cells were labeled using a mouse anti-human CD24-fluorescein isothiocyanate (BD Pharmingen, San Jose, CA) and a mouse anti-human CD44-phycoerythrin (PE) (BD Pharmingen). In experiments investigating EpoR expression, cells were serum-starved for 5 hours. For detecting EpoR expression, cells were labeled with a monoclonal fluorescein isothiocyanate-conjugated mouse anti-human EpoR antibody (FAB307F; R&D Systems, Minneapolis, MN), a PE/Cy5-conjugated mouse anti-human CD44 antibody (BioLegend, San Diego, CA), and a PE-conjugated mouse anti-CD24 antibody (Beckman Coulter, Fullerton, CA) using standard protocols. For analysis of Jagged-1 expression and Notch activation, cells were harvested, fixed in 4% paraformaldehyde for 10 minutes at room temperature, permeabilized with 0.5% saponin, and incubated with a rabbit anti-human Jagged-1 (Santa Cruz Biotechnology, Santa Cruz, CA) or a rabbit anti-human cleaved Notch-1 antibody (Cell Signaling Inc., Danvers, MA) and fluorochrome-conjugated secondary antibodies using standard protocols. Flow cytometry was performed on a FACScalibur flow cytometer using the CellQuest software package (BD Biosciences, San Jose, CA).
Primary Sphere Formation Assay
The frequency of cells in the nonadherent population of monolayer cultures that were capable of initiating sphere formation was assessed by harvesting, washing, and resuspending cells in phenol red-free DMEM/F12 medium (supplemented with 0.4% bovine serum albumin, 20 ng/ml basic fibroblast growth factor, and 10 ng/ml epidermal growth factor), and passing them through a 40-mm sieve. Cells were then counted, diluted, and plated at clonal density into 96-well plates. Mammospheres were counted on day 5.
Results
rhEpo Increases the Size of the Putative BCIC Population
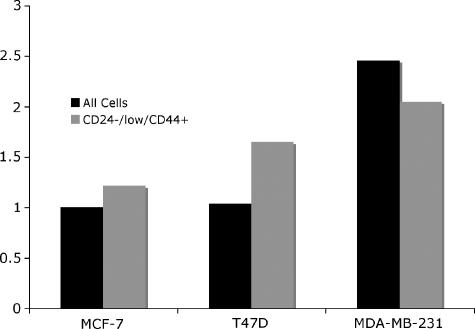
The expression of EpoR on breast cancer cells has been described by others [30], but not its expression on CD44+/CD24-/low cells. MCF-7, T47D, and MDA-MB-231 CD44+/CD24-/low cells were found to express levels of EpoR comparable to levels of EpoR on unselected cells (Figure 1).
Figure 1.
MCF-7, T47D, and MDA-MB-231 monolayer cells were serum-starved for 5 hours, trypsinized, and analyzed for EpoR-expression on the cell surface by flow cytometry. EpoR was expressed on CD44+/CD24-/low cells, the putative stem cell population (gray) as well as on unselected cells (black).
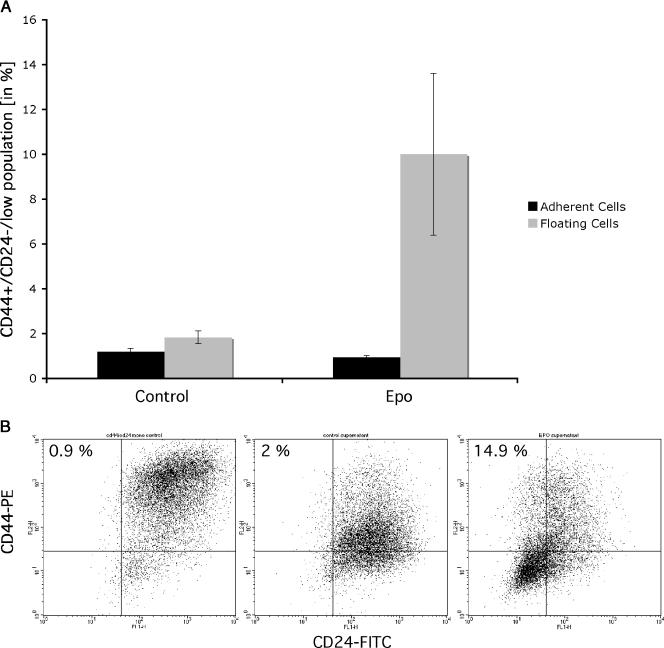
To test if rhEpo modulates the size of the CD44+/CD24-/low population,MCF-7 monolayer cultures were treated with 1 IU/ml rhEpo for 3 consecutive days (days 2–4 of culture). About 24 hours later, monolayer cells and cells floating in the supernatant were analyzed for CD24 and CD44 expression. Cells were stained with antibodies against CD24 and CD44 and the size of the CD44+/CD24-/low population of cells was analyzed by flow cytometry. Treatment with rhEpo did not change the number of CD44+/CD24-/low in the adherent cell population but increased the number of CD44+/CD24-/low cells in the supernatant almost 5-fold from 2% (± 0.3%) to 9.5% (± 1.8%) (means ± SEM; P < .01, Student's t test) (Figure 2, A and B).
Figure 2.
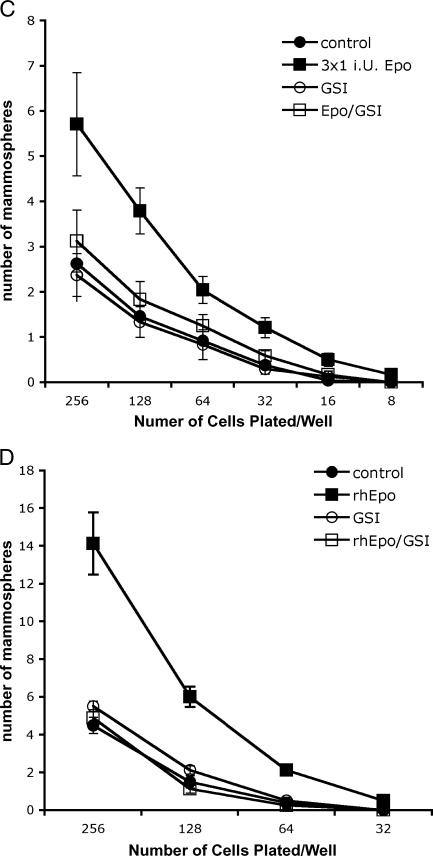
(A) FACS analysis detecting the size of the CD44+/CD24-/low cell population in monolayers and supernatant of MCF-7 cells. Treatment with rhEpo (3 x 1 IU/ml) increased the size of this population 4.8-fold from 2 ± 0.08% to 9.5 ± 1.8% (P < .01, Student's t test). Data from four independent experiments are shown. (B) Representative FACS analysis of MCF-7 monolayer cultures treated with rhEpo (3 x 1 IU/ml) for 3 consecutive days. About 0.9% of the cell in the adherent cell population was CD44+/CD24-/low (left panel). Treatment with rhEpo increased the frequency of CD44+/CD24-/low cells in the supernatant from 2% (middle panel) to 14.9% (right panel). (C and D) Primary sphere formation assay of MCF-7 (C) or MDA-MB-231 (D) cells treated with rhEpo (3 x 1 IU/ml) for 3 consecutive days. RhEpo increased the rate of primary spheres formation (MCF-7: 1 ± 0.4% for untreated cells, 2.9 ± 0.6% for Epo-treated cells; MDA-MB-231: 1.2 ± 0.6 for untreated cells, 4.5 ± 1.1% for Epo-treated cells, P < .01, two-sided Student's t test; means ± SEM). Preincubation (30 minutes) with GSI (5 µM) treatment prevented the rhEpo-induced increase in primary sphere formation (MCF-7: GSI-treated cells, 1 ± 0.4%; Epo + GSI-treated cells, 1.5 ± 0.4%; MDA-MB-231: GSI-treated cells, 1.5 ± 0.7%; Epo + GSI - treated cells, 1.1 ± 0.8%).
The effect of rhEpo was also demonstrated by its ability to increase in primary sphere formation by cells taken from the supernatant of MCF-7 or MDA-MB-231 monolayer cultures and plated at clonal densities into 96-well plates. Cells derived from cultures treated with 1 IU/ml rhEpo on 3 consecutive days (days 2–4 of culture) showed a significant increase in primary sphere formation (MCF-7: 1 ± 0.4% for untreated cells, 2.9 ± 0.6% for Epo-treated cells; MDA-MB-231: 1.2 ± 0.6 for untreated cells, 4.5 ± 1.1% for Epo-treated cells, P < .01, two-sided Student's t test; means ± SEM) (Figure 2, C and D).
rhEpo Increases the Number of BCICs in a Notch-Dependent Fashion
We have previously shown that activation of the Notch signaling pathway increases the number of BCICs [23]. To investigate if blocking activation of the Notch-1 pathway would prevent Epo-induced increases in the number of BCICs, monolayer cultures were pretreated with GSI, 30 minutes before each daily treatment with rhEpo. GSI treatment prevented the rhEpo-induced increase in primary sphere formation (MCF-7: GSI-treated cells, 1 ± 0.4%; Epo + GSI-treated cells, 1.5 ± 0.4%; MDA-MB-231: GSI-treated cells, 1.5 ± 0.7%; Epo + GSI-treated cells, 1.1 ± 0.8%) (Figure 2, C and D).
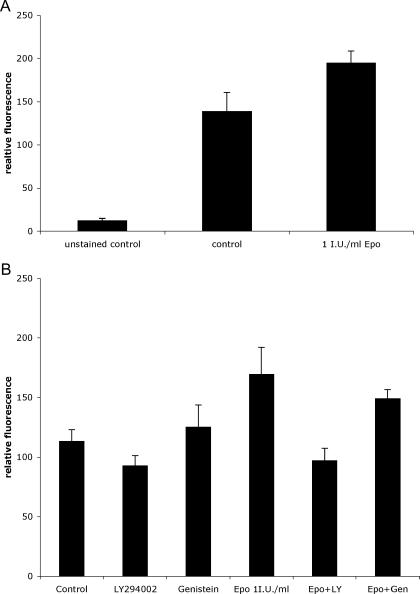
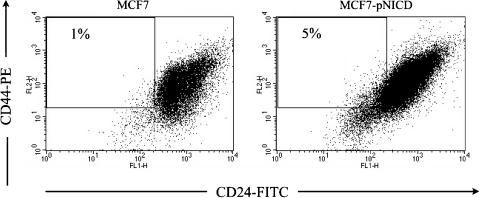
To confirm that rhEpo increased the number of BCICs in a Notch-dependent fashion, we treated MCF-7 cells for 2 or 4 hours with 1 IU/ml rhEpo and stained cells with antibodies against Notch-ICD, or Jagged-1, and analyzed expression by flow cytometry. Treatment with rhEpo caused a 1.4 ± 0.16-fold (mean ± SEM, P = .041, n = 3, two-sided paired Student's t test) induction of Jagged-1 expression (Figure 3A) and a 1.5 ± 0.19-fold (mean ± SEM, P = .049, n = 3, two-sided paired Student's t test) activation of Notch-1 after 2 hours (Figure 3B). To confirm that activated Notch signaling truly increased the number of putative breast cancer stem cells, we stably transfected MCF-7 cells with an expression vector for a constitutively active Notch-ICD. When MCF-7-pNICD cells were stained with antibodies against CD24 and CD44, analyzed by flow cytometry, and compared to mock-transfected cells, the number of CD44+/CD24-/low cells was increased in MCF-7-pNICD cells, consistent with our other data (Figure 4).
Figure 3.
(A) Treatment of MCF-7 monolayer culture with 1 IU/ml rhEpo for 2 hours caused a 1.4 ± 0.16-fold (mean ± SEM, P = .041, n = 3, two-sided paired Student's t test) induction of Jagged-1 expression. (B) Treatment of MCF-7 monolayer culture with 1 IU/ml rhEpo for 2 hours caused a 1.5 ± 0.19-fold (mean ± SEM; P = .049, n = 3, two-sided paired Student's t test) activation of Notch-1 after 2 hours. Treatment with the PI3K inhibitor LY294002 but not the JAK2 inhibitor genistein prevented rhEpo-induced activation of Notch-1.
Figure 4.
Representative FACS analysis of MCF-7 cells, stable transfected with an expression vector coding for constitutive active Notch, or an empty vector (n = 2). Expression of constitutive active Notch increased the population of BCICs.
rhEpo Activates Notch Signaling through PI3K Pathways
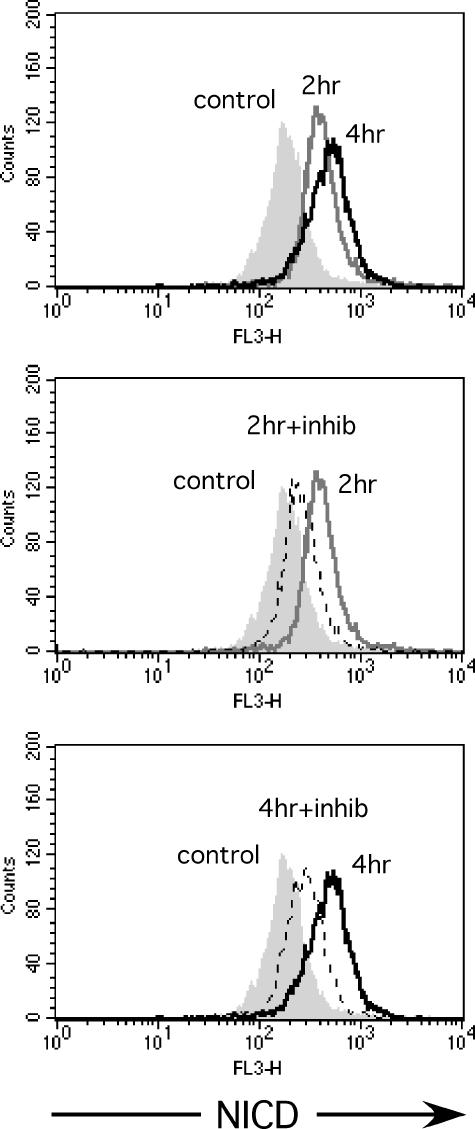
Signaling in response to rhEpo through the Epo receptor is rapid and involves at least three major pathways: Janus kinase 2 (JAK2)-dependent activation of STAT5, activation of the Ras/Raf pathway, and PI3K/Akt-dependent/JAK2-independent activation of nuclear factor-kappa B (NF-κB) [31]. We previously reported activation of NF-κB in cancer cells after treatment with rhEpo that was dependent on EpoR expression levels [11]. Because NF-κB is known to induce Jagged-1 expression [32], we repeated our experiment using the PI3K inhibitor LY294002 and the JAK2 inhibitor genistein. As expected, treatment with LY294002 but not genistein prevented rhEpo-induced activation of Notch-1 (Figure 3B). As expected, pretreatment of the cells with the GSI inhibited activation of Notch-1 (Figure 5).
Figure 5.
Representative FACS analysis of Notch-1 activation in MCF-7 cells. Treatment with 1 IU/ml rhEpo for 2 or 4 hours (upper panel: gray line and black line; filled histogram, control) caused activation of Notch-1. This activation was inhibited by pretreatment with a GSI for 30 minutes at 2 and 4 hours (middle and lower panels: gray line, rhEpo; dashed line, rhEpo + GSI; filled histogram, control).
Discussion
The use of ESAs to correct chemotherapy-induced anemia or cancer anemia is currently under debate after five large placebo-controlled clinical trials showed increased cancer-related death rates for patients treated with ESAs [8,10,33] (DAHANCA 10 and Anemia of Cancer, both unpublished). These findings could not be explained by elevated hemoglobin levels, suggesting a direct effect of ESAs on cancer cells. However, experimental evidence for a direct effect of ESAs on cancer cells in the literature is inconclusive. Results from studies investigating direct effects of ESAs and cancers using a broad variety of in vitro models, in vivo models, and ESAs doses range from chemo- and radiosensitizing effects to chemo- and radioprotective effects, whereas others reported no effect at all [34–42].
EpoR was detected on cells derived from all three breast cancer cell lines confirming previous studies [30,43]. Here we report for the first time that EpoR is expressed on the surface of the BCIC population. It is important to note that the specificity of anti-EpoR antibodies is currently under debate [44] but it has been clearly demonstrated that the EpoR signals on rhEpo binding in non-erythroid cells, including cancer cells [45].
Using a pharmacological concentration of rhEpo (1 IU/ml), we found that the size of the CD44+/CD24-/low nonadherent population of BCICs was increased after treatment with rhEpo. The increase in the number of BCICs observed after rhEpo treatment was significant and the cells were not only viable but, more importantly, exhibited an increased self-renewal capacity as demonstrated by primary in vitro sphere formation.
We have previously demonstrated that activation of the Notch signaling pathway is part of the cellular stress response to clinical doses of ionizing radiation [23]. Interestingly, this effect was mediated by increased expression of the Notch receptor ligand Jagged-1 in the non-BCIC population that activated Notch signaling in BCICs. As for radiation [23], we have shown in this study that rhEpo treatment activated Notch signaling in BCICs. It further opens the possibility that EpoR expression may not be essential for BCICs to respond to rhEpo directly, but indirectly as they are the receiving part in the Notch signaling cascade, as long as the surrounding cells in a tumor express the receptor and respond to rhEpo with induction of Notch ligand expression. The role of Notch signaling for the rhEpo-induced increase in BCICs was further underlined by the observations that expression of constitutive active Notch increased the number of BCICs. As in our previous study [23], the number of BCICs was increased in the population of cells floating on top of the adherent cells. Comparable results were initially reported by Ponti et al. [22] and this may reflect the fact that this population usually contains not only dead cells but also cells undergoing mitosis and therefore detach from the adherent population.
We have previously shown that treatment of EpoR-expressing cancer cells activates the transcription factor NF-κB [11]. This activation is independent of JAK2 [31], mediated through the PI3K pathway [46], and Jagged-1 is a known downstream target gene of NF-κB [32]. Consistent with these previous findings, inhibition of the PI3K but not JAK2 prevented the increase in BCICs in response to rhEpo. Remarkably, GSI alone did not affect the number of mammospheres formed, which may indicate that GSIs are not toxic for BCICs, rather they only interfere with the switch to symmetric cell division while sphere formation remains intact.
Conclusions
Taken together, our study demonstrates that rhEpo mediates an increase in the number of BCICs in MCF-7 and MDA-MB-231 cultures, which could be blocked by inhibition of Notch signaling. With putative stem cells now identified in other cancers [14,16–18], it will be interesting to investigate if ESAs generally affect the growth of these cells also. Our study provides a possible explanation for the unexpected negative clinical effects of ESAs on local control and survival in cancer patients. Clearly, using established cell lines, our study has limitations and our observations need to be confirmed in primary tumor cell lines derived from patient samples.
Abbreviations
- BCIC
breast cancer - initiating cell
- CSC
cancer stem cell
- Epo
erythropoietin
- ESA
erythropoiesis-stimulating agent
- GSI
γ-secretase inhibitor
- JAK2
Janus kinase 2
- NF-κB
nuclear factor - kappa B
- rhEpo
recombinant human erythropoietin
Footnotes
Support for this research was provided by University of California, Los Angeles Jonsson Cancer Center Foundation to F.P. T.P. and E.V. were supported by training grants from the National Institute of Biomedical Imaging and BioEngineering (5 T32 EB002101 to T.P. and 2 T32 EB002101-31 to E.V.).
References
- 1.Henke M, Sindlinger F, Ikenberg H, Gerds T, Schumacher M. Blood hemoglobin level and treatment outcome of early breast cancer. Strahlenther Onkol. 2004;180:45–51. doi: 10.1007/s00066-004-1123-7. [DOI] [PubMed] [Google Scholar]
- 2.Fein DA, Lee WR, Hanlon AL, Ridge JA, Langer CJ, Curran WJ, Jr, Coia LR. Pretreatment hemoglobin level influences local control and survival of T1–T2 squamous cell carcinomas of the glottic larynx. J Clin Oncol. 1995;13:2077–2083. doi: 10.1200/JCO.1995.13.8.2077. [DOI] [PubMed] [Google Scholar]
- 3.Lee WR, Berkey B, Marcial V, Fu KK, Cooper JS, Vikram B, Coia LR, Rotman M, Ortiz H. Anemia is associated with decreased survival and increased locoregional failure in patients with locally advanced head and neck carcinoma: a secondary analysis of RTOG 85-27. Int J Radiat Oncol Biol Phys. 1998;42:1069–1075. doi: 10.1016/s0360-3016(98)00348-4. [DOI] [PubMed] [Google Scholar]
- 4.Dubray B, Mosseri V, Brunin F, Jaulerry C, Poncet P, Rodriguez J, Brugere J, Point D, Giraud P, Cosset JM. Anemia is associated with lower local-regional control and survival after radiation therapy for head and neck cancer: a prospective study. Radiology. 1996;201:553–558. doi: 10.1148/radiology.201.2.8888257. [DOI] [PubMed] [Google Scholar]
- 5.Frommhold H, Guttenberger R, Henke M. The impact of blood hemoglobin content on the outcome of radiotherapy. The Freiburg experience. Strahlenther Onkol. 1998;174(Suppl 4):31–34. [PubMed] [Google Scholar]
- 6.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, Trelle S, Weingart O, Bayliss S, Brunskill S, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD003407.pub4. CD003407. [DOI] [PubMed] [Google Scholar]
- 8.Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4:459–460. doi: 10.1016/s1470-2045(03)01163-x. [DOI] [PubMed] [Google Scholar]
- 9.Henke M, Laszig R, Rübe C, Schäfer U, Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;262:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 10.Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, Szechtman B, Roa W, Mulroy L, Rudinskas L, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25(9):1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- 11.Pajonk F, Weil A, Sommer A, Suwinski R, Henke M. The erythropoietin-receptor pathway modulates survival of cancer cells. Oncogene. 2004;23:8987–8991. doi: 10.1038/sj.onc.1208140. [DOI] [PubMed] [Google Scholar]
- 12.Henke M, Mattern D, Pepe M, Bezay C, Weissenberger C, Werner M, Pajonk F. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol. 2006;24:4708–4713. doi: 10.1200/JCO.2006.06.2737. [DOI] [PubMed] [Google Scholar]
- 13.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 14.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 15.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 18.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 19.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siemers ER, Quinn JF, Kaye J, Farlow MR, Porsteinsson A, Tariot P, Zoulnouni P, Galvin JE, Holtzman DM, Knopman DS, et al. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006;66:602–604. doi: 10.1212/01.WNL.0000198762.41312.E1. [DOI] [PubMed] [Google Scholar]
- 21.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 23.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 24.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R, Jr, Badve S, Nakshatri H. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH, Cote RJ. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 26.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, Rosen JM. Wnt/β-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120:468–477. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]
- 28.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 29.Curry CL, Reed LL, Nickoloff BJ, Miele L, Foreman KE. Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest. 2006;86:842–852. doi: 10.1038/labinvest.3700442. [DOI] [PubMed] [Google Scholar]
- 30.Acs G, Zhang PJ, Rebbeck TR, Acs P, Verma A. Immunohistochemical expression of erythropoietin and erythropoietin receptor in breast carcinoma. Cancer. 2002;95:969–981. doi: 10.1002/cncr.10787. [DOI] [PubMed] [Google Scholar]
- 31.Bittorf T, Buchse T, Sasse T, Jaster R, Brock J. Activation of the transcription factor NF-kappaB by the erythropoietin receptor: structural requirements and biological significance. Cell Signal. 2001;13:673–681. doi: 10.1016/s0898-6568(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 32.Bash J, Zong WX, Banga S, Rivera A, Ballard DW, Ron Y, Gelinas C. Rel/NF-kappaB can trigger the Notch signaling pathway by inducing the expression of Jagged1, a ligand for Notch receptors. EMBO J. 1999;18:2803–2811. doi: 10.1093/emboj/18.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, Makhson A, Roth A, Dodwell D, Baselga J, et al. Maintaining normal hemoglobin levels with Epoetin Alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23(25):5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 34.Yasuda Y, Musha T, Tanaka H, Fujita Y, Fujita H, Utsumi H, Matsuo T, Masuda S, Nagao M, Sasaki R, et al. Inhibition of erythropoietin signalling destroys xenografts of ovarian and uterine cancers in nude mice. Br J Cancer. 2001;84:836–843. doi: 10.1054/bjoc.2000.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selzer E, Wacheck V, Kodym R, Schlagbauer-Wadl H, Schlegel W, Pehamberger H, Jansen B. Erythropoietin receptor expression in human melanoma cells. Melanoma Res. 2000;10:421–426. doi: 10.1097/00008390-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Thews O, Koenig R, Kelleher DK, Kutzner J, Vaupel P. Enhanced radiosensitivity in experimental tumours following erythropoietin treatment of chemotherapy-induced anaemia. Br J Cancer. 1998;78:752–756. doi: 10.1038/bjc.1998.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westphal G, Niederberger E, Blum C, Wollman Y, Knoch TA, Rebel W, Debus J, Friedrich E. Erythropoietin and G-CSF receptors in human tumor cells: expression and aspects regarding functionality. Tumori. 2002;88:150–159. doi: 10.1177/030089160208800214. [DOI] [PubMed] [Google Scholar]
- 38.Ribatti D, Marzullo A, Nico B, Crivellato E, Ria R, Vacca A. Erythropoietin as an angiogenic factor in gastric carcinoma. Histopathology. 2003;42:246–250. doi: 10.1046/j.1365-2559.2003.01581.x. [DOI] [PubMed] [Google Scholar]
- 39.Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, Tazaki A, Onozaki M, Hashimoto M, Musha T, Ogawa K, et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis. 2003;24:1021–1029. doi: 10.1093/carcin/bgg060. [DOI] [PubMed] [Google Scholar]
- 40.Belenkov AI, Shenouda G, Rizhevskaya E, Cournoyer D, Belzile JP, Souhami L, Devic S, Chow TY. Erythropoietin induces cancer cell resistance to ionizing radiation and to cisplatin. Mol Cancer Ther. 2004;3:1525–1532. [PubMed] [Google Scholar]
- 41.Carvalho G, Lefaucheur C, Cherbonnier C, Metivier D, Chapel A, Pallardy M, Bourgeade MF, Charpentier B, Hirsch F, Kroemer G. Chemosensitization by erythropoietin through inhibition of the NF-kappaB rescue pathway. Oncogene. 2005;24:737–745. doi: 10.1038/sj.onc.1208205. [DOI] [PubMed] [Google Scholar]
- 42.Stuben G, Pottgen C, Knuhmann K, Schmidt K, Stuschke M, Thews O, Vaupel P. Erythropoietin restores the anemia-induced reduction in radiosensitivity of experimental human tumors in nude mice. Int J Radiat Oncol Biol Phys. 2003;55:1358–1362. doi: 10.1016/s0360-3016(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 43.Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–3565. [PubMed] [Google Scholar]
- 44.Brown WM, Maxwell P, Graham AN, Yakkundi A, Dunlop EA, Shi Z, Johnston PG, Lappin TR. Erythropoietin receptor expression in non-small cell lung carcinoma: a question of antibody specificity. Stem Cells. 2007;25:718–722. doi: 10.1634/stemcells.2006-0687. [DOI] [PubMed] [Google Scholar]
- 45.Dunlop EA, Percy MJ, Boland MP, Maxwell AP, Lappin TR. Induction of signalling in non-erythroid cells by pharmacological levels of erythropoietin. Neurodegener Dis. 2006;3:94–100. doi: 10.1159/000092099. [DOI] [PubMed] [Google Scholar]
- 46.Digicaylioglu M, Garden G, Timberlake S, Fletcher L, Lipton SA. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc Natl Acad Sci USA. 2004;101:9855–9860. doi: 10.1073/pnas.0403172101. [DOI] [PMC free article] [PubMed] [Google Scholar]