Abstract
Hypoxic preconditioning (HP) is a rapid and reversible pro-adaptive response to mild hypoxic exposure that protects cells from subsequent hypoxic or ischemic insult[1, 2]. HP mechanisms are of great interest because of their therapeutic potential and insight into metabolic adaptation and cell death. HP has been widely demonstrated in the vertebrate subphylum but not in invertebrates[2]. Here we report that the nematode Caenorhabditis elegans has a potent HP mechanism that protects the organism as well as its neurons and myocytes from hypoxic injury. The time course of C. elegans HP was consistent with vertebrate delayed HP, appearing 16 hrs after preconditioning and lasting at least 36 hrs. The apoptosis pathway has been proposed as either a trigger or target of HP. Testing of mutations in the canonical C. elegans apoptosis pathway showed that in general the genes in this pathway are not required for HP. However, loss-of-function mutations in ced-4, which encodes an Apaf-1 homolog, completely blocked HP. RNAi silencing of ced-4 in adult animals immediately preceding preconditioning blocked HP, indicating that CED-4 is required in adults during and/or after preconditioning. CED-4/Apaf-1 is essential for HP in C. elegans and acts through a mechanism independent of the classical apoptosis pathway.
Results and Discussion
All aerobic animals require oxygen for survival. However, they and their cells vary dramatically in oxygen requirements and abilities to survive after hypoxia. Certain mammalian cells, most notably cardiac myocytes and neurons, have the ability to alter their hypoxic sensitivities following a sublethal ischemic or hypoxic incubation. This adaptive phenomenon is called ischemic or hypoxic preconditioning (HP) and has been demonstrated in a large variety of mammalian cell types [1, 2]. Cellular protection by HP occurs in two temporally distinct phases, early and late. Hypoxic protection by early preconditioning occurs within minutes, peaks in about an hour, and lasts for about 4 hours. Protection by late preconditioning does not become evident until 12-24 hours after the preconditioning stimulus, but can last several days. The mechanisms of early and late preconditioning appear to be distinct with late preconditioning, but not early, requiring new transcription and translation. In particular, the mechanisms of late preconditioning are poorly understood. Because HP has not been reported in an invertebrate, one outstanding question is whether this adaptive response to hypoxia is restricted to vertebrates or is also present in invertebrates and, if so, might the mechanism require evolutionarily conserved components.
Hypoxic Preconditioning in C. elegans
The nematode Caenorhabditis elegans is sensitive to hypoxic injury [3-5]. C. elegans neurons, myocytes, and the whole animal are injured and killed by hypoxic exposure [5], and genes have been identified that regulate cellular and whole animal hypoxic sensitivity [3-7]. We tested whether C. elegans has an HP mechanism that can protect from whole animal death. Indeed, a 4 hour hypoxic exposure followed by 20 hour recovery resulted in protection from a subsequent 20 hr hypoxic incubation. After recovery from the hypoxic incubation, HP-treated animals were not only alive but moving relatively normally compared to the paralyzed or dead No HP controls (Figures 1A–1B). The hypoxic preconditioning incubation was performed by transferring animals from their normal growth conditions, normoxia at 20° on agar plates seeded with bacteria, to liquid buffer in the hypoxia chamber at 26°. We tested whether the hypoxia was responsible for the preconditioning as opposed to higher temperature or incubation in the liquid. Higher temperature, liquid, nor their combination resulted in any significant hypoxia protection against animal death while hypoxic incubation on agar plates resulted in the same amount of protection, albeit more variable, as liquid hypoxic incubations (Figures 1C). A four hour hypoxic incubation at 25°, a common temperature for culture of C. elegans, also induced significant preconditioning (Figure S1). Thus, hypoxia at a more physiological temperature is still capable of inducing a protective response. We also examined whether hypoxic preconditioning provided protection from thermal insults. C. elegans is killed by prolonged incubation at 37°. We observed no protection by HP from the lethality of a 37° incubation (Figure S2). Thus, despite the 26° preconditioning temperature, certainly a cellular stress in its on right for C. elegans, our results find no evidence for cross-tolerance to hyperthermia. However, our data does not rule out the possibility of a synergy between the stresses of higher temperature and hypoxia resulting in preconditioning.
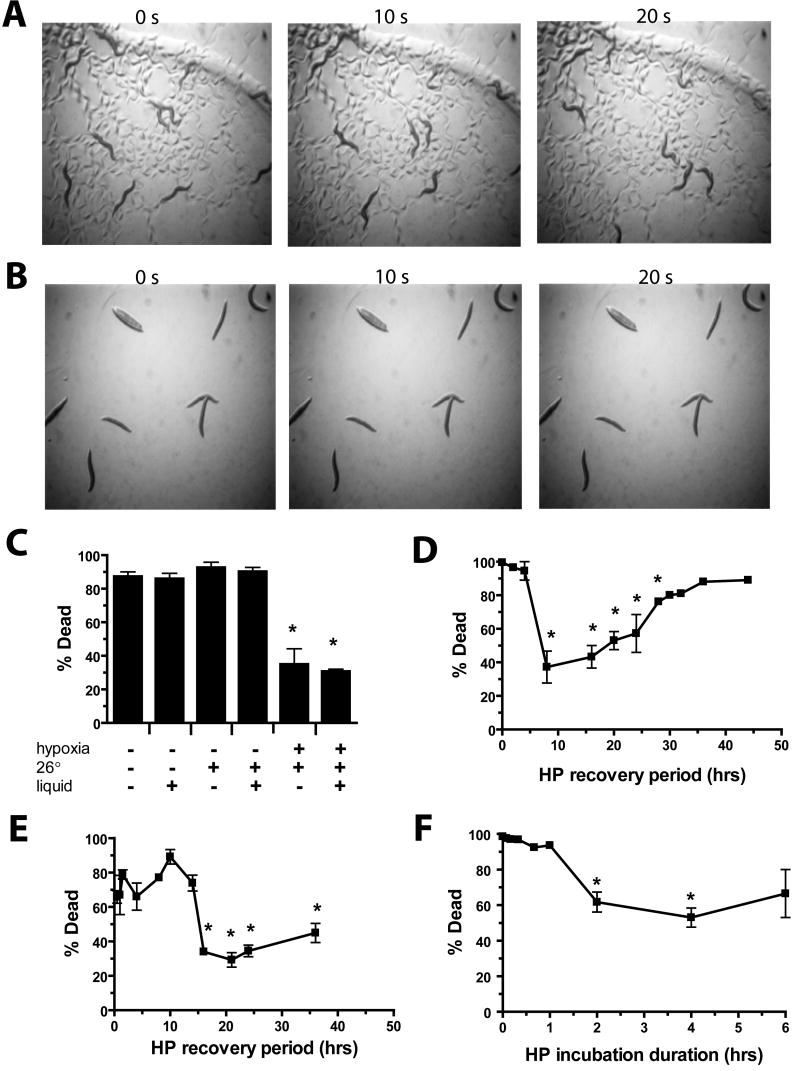
Figure 1. Hypoxic preconditioning protects C. elegans from lethal hypoxic insults.
(A–B) Time lapse images of (A) hypoxia preconditioned versus (B) nonpreconditioned adult worms following a 20 hr recovery from a 20 hr hypoxic incubation (pO2 < 0.3%). Note well-moving preconditioned animals and immobile non-preconditioned animals without tracks in the bacterial lawn.
(C) Preconditioning requires hypoxia, not just higher temperature or buffer. Preconditioning for 4 hrs followed by 20 hr recovery were varied by normoxic or hypoxic, 20° or 26°, in liquid or on agar incubations as noted. Bars represent mean +/- sem of three trials (> 50 animals/trial). * p < 0.01 versus normoxic, 20°, agar condition (two-tailed t-test).
(D–E) Onset and duration of protection by hypoxic preconditioning from hypoxic (D), or azide (E) killing. Following a 4 hr HP incubation, animals were allowed to recover for varying times in room air at 20° on agar prior to a 20 hr hypoxic or 1 hr 0.5M azide killing incubation. * p < 0.01 versus zero HP recovery time (two-tailed t-test).
(F) Length of hypoxic incubation time required to produce HP. The length of the hypoxic preconditioning at 26° in buffer was varied and was followed 20 hrs later by a 20 hr hypoxic killing incubation. * p < 0.01 versus no HP incubation (two-tailed t-test).
We next tested the onset and duration of HP protection. Significant protection from hypoxic injury was not seen until the recovery time between the end of the preconditioning incubation and the hypoxic killing incubation was 8 hours. The protection lasted for up to 28 hours (Figures 1D). The precision of determining the onset and duration of HP was limited by the long 20 hr hypoxic killing incubation. Thus, sodium azide, a hypoxia mimetic that kills with only a 1 hr incubation, was used instead. Protection by HP from azide-induced death was not observed until 16 hrs after preconditioning and remained for at least 36 hrs (Figures 1E). The protection by HP against hypoxic and azide death and the lack of protection against thermal insult indicates that the induced protective response is relatively specific for hypoxia or hypoxia mimetics such as sodium azide. Finally, we measured the duration of the hypoxic preconditioning incubation required for protection. Only longer incubations of 2 and 4 hrs resulted in significant protection (Figure 1F), indicating that a strong hypoxic stimulus is required for HP in C. elegans. The onset and duration are similar to that for late preconditioning in mammalian tissue. No evidence for early preconditioning was found, indicating that C. elegans lacks the mechanism present in vertebrate cells necessary for early HP or, at least, a mechanism sufficient to protect the whole animal from death. Our results show that C. elegans has a mechanism for hypoxic preconditioning and that mechanisms for HP extend beyond the vertebrate subphylum.
Hypoxic Preconditioning Prevents Necrotic, Myocyte, and Neuronal Cell Death
Preconditioning has been studied most intensively in mammalian cardiac myocytes and neurons, which are particularly vulnerable to hypoxic injury. Thus, we asked whether C. elegans myocytes and neurons were protected by HP. Previously, we have shown that hypoxia produces characteristic pathological changes in both myocytes and neurons [5]. C. elegans myocyte nuclei become fragmented and are reduced in number after hypoxia. Neuronal hypoxic injury in C. elegans manifests as severe axonal beading similar to what is observed after traumatic spinal cord injury. HP markedly reduced both myocyte and neuronal pathology after hypoxia (Figures 2A–2B–2D–2E). The results suggest that C. elegans myocytes and neurons are capable of mounting a preconditioning adaptive response after hypoxia although a non-cell autonomous mechanism has not been ruled out. We have also previously shown that hypoxia induces a necrotic cell death in C. elegans. HP significantly reduced necrotic cell death as well (Figures 2C–2F).
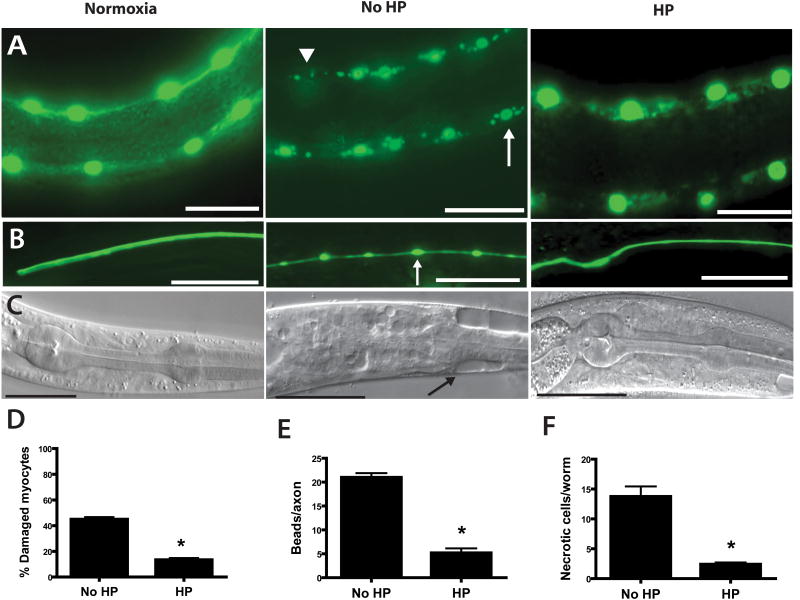
Figure 2. Hypoxia induced pathological cell defects and cell death blocked by HP.
L1 larvae underwent no hypoxic incubation (Normoxia) or a 20 hr hypoxic incubation that was preceded by 4 hr HP (HP) or normoxic preconditioning (No HP). After a 24 hr recovery, surviving animals were scored for cell pathological defects. Scale bars = 20μm.
(A,D) Muscle nuclear pathology reduced by HP. Muscle nuclei were visualized by nuclear-localized GFP driven by a muscle-specific promoter – Pmyo-3. Hypoxia produces damaged myocytes seen as nuclear fragmentation (arrow) and nuclear loss (arrowhead) [5] that is reduced by HP (* - p < 0.01 vs No HP, n=29 animals no HP, 44 HP).
(B,E) Axonal pathology reduced by HP. Touch sensory neurons were visualized by GFP driven by a touch neuron-specific promoter – Pmec-4. Hypoxia produces axonal beading pathology (arrow) [5] that is reduced by HP (* - p < 0.01 vs No HP, mean ± sem, n=124 axons no HP, 116 HP).
(C,F) Necrotic cell death reduced by HP. Hypoxia produces necrotic cell death (arrow) [5] that is reduced by HP (* - p < 0.01 vs No HP, n=29 animals no HP, 44 HP).
The Apaf-1 homolog CED-4 is Required for HP by a Novel Mechanism
A number of mechanisms have been proposed for delayed preconditioning [2, 8]. Among these mechanisms, the apoptosis pathway has repeatedly been implicated in HP. Evidence for two sorts of roles of apoptosis has been found. Numerous studies have found that HP can reduce apoptotic death [8, 9]. However, these studies do not generally address whether HP exclusively inhibits apoptotic death as opposed to other mechanisms of death such as necrotic cell death. The mechanism by which HP might reduce apoptotic death is not well-established but some evidence points to an increase in the activity of the apoptosis-inhibiting protein Bcl-2 [10-12]. A second potential role of the apoptosis pathway is as a trigger for hypoxic preconditioning. The evidence for such a role is scant and is essentially hypothetical [2, 13]. Apoptotic cell death in C. elegans is mediated through a well-studied core pathway [14]. The upstream most component in the pathway is the BH3-only domain protein EGL-1, which acts to inhibit the Bcl-2 homolog CED-9. CED-9 in turn sequesters the Apaf-1 homolog CED-4 on the mitochondrial surface. Upon disinhibition, by EGL-1, CED-4 is released from CED-9 where it multimerizes with the caspase CED-3, resulting in active caspase [14]. Developmental programmed somatic cell death and DNA-damage-induced germ cell death are both controlled by the entire core pathway whereas programmed germ cell death by only CED-9, 4, and 3.
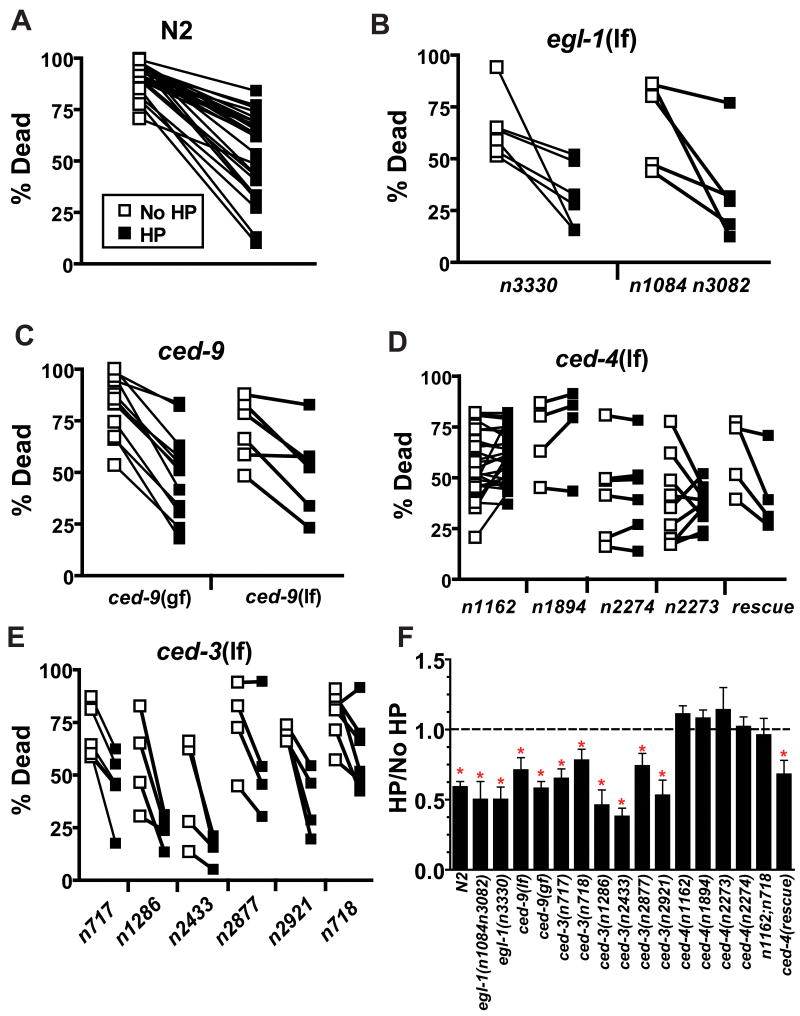
We utilized the extensive collection of core apoptosis pathway mutants to test the requirements of each gene product in HP. Two egl-1(null) mutants exhibited a normal HP response similar to that of the wild type strain N2 (Figures 3A–3B–3F, Table S1). Notably, both egl-1(null) strains were mildly but significantly hypoxia resistant. Indeed, all loss of function mutations in the apoptosis pathway were similarly hypoxia resistant (Figure 3, Table S1). This resistance could be derived from a general block of apoptotic cell death induced after hypoxia or could be due to the presence of additional cells, normally fated to die, that somehow confer a mild protection from hypoxic injury. In any case, a block of developmental programmed cell death by egl-1(lf) does not alter HP. Likewise, neither ced-9(lf) nor ced-9(gf) block protection by HP (Figure 3C–3F, Table S1); thus, the C. elegans Bcl-2 homolog is not required for HP. Given these results, we were surprised to find that the canonical ced-4(lf) allele n1162 failed to show any protection by HP (Figures 3D–3F, Table S1). Testing of three additional ced-4(lf) alleles, along with rescue of HP protection by transformation of ced-4(n1162) with wild type ced-4, confirmed that ced-4 is required for HP (Figures 3D–3F, Table S1). Notably, the ced-4(n2273) mutant inconsistently failed to precondition. The n2273 mutation alters a splice acceptor sequence and decreases the function of two alternatively spliced CED-4 isoforms, CED-4S and CED-4L. CED-4S promotes programmed cell death; CED-4L prevents programmed cell death [15]. Because n2273 and all the other ced-4 mutants reduce the activity of both CED-4S and CED-4L, we cannot determine whether CED-4S, CED-4L, or both are required for HP. However, the partial reduction-of-function effect of n2273 may explain its weak HP blocking phenotype. CED-4 is known to activate the CED-3 caspase [16] but also has been shown to have CED-3 independent mechanisms for mediating cell death [17]. Multiple ced-3(lf) mutants exhibited normal protection by HP (Figures 3E–3F, Table S1). Additionally, the block in HP by ced-4(lf) does not require ced-3 as shown by the HP-defective phenotype of a ced-4(lf);ced-3(lf) double mutant (Figure 3F, Table S1). Thus, CED-4 regulates HP through a CED-3 independent mechanism. Indeed, CED-4 must act to control HP by some novel mechanism that does not require any of the other known core apoptosis genes.
Figure 3. The Apaf-1 homolog CED-4 is required for HP.
(A–E) open symbols are data for no HP treated animals, closed symbols are for HP-treated animals. Data are paired by trial with concurrent HP and No HP animals. (A) Paired values for the wild type strain N2; the 26 paired trials that were done as controls for all of the programmed cell death pathway experiments are shown. (B) egl-1 is not required for HP. egl-1(n3330) and egl-1(n1084 n3082), both null alleles, are significantly protected by HP. (CB) ced-9 is not required for HP. Neither ced-9(n1950 gf) nor ced-9(lf) [full genotype - unc-69(e587) ced-9(n1950gf n2161lf); ced-3(n2433)] block HP. (D) ced-4 is required for HP. n1162, n1894, and n2274 carry stop codon mutations in the ced-4 gene and likely represent null alleles [18]. The rescue strain has the genotype ced-1(e1735);ced-4(n1162);unc-31(e928);nEx7[unc-31(+);ced-4(+)]. (E) Multiple ced-3 mutations do not block HP. (F) Summary of the effect of programmed cell death pathway mutants on HP. The ratio of the % dead in HP to that for No HP in each trial, mean ± sem; dotted line indicates unity. * - HP versus No HP significantly different by paired t-test, p < 0.05.
We examined two general mechanistic explanations for the requirement of CED-4 for HP. First, we considered the trivial possibility that ced-4 mutants might block HP by slowing oxygen depletion during the preconditioning incubation, perhaps by altering initial oxygen stores or gas exchange. If so, one would expect the kinetics of hypoxia-induced behavioral defects to differ between ced-4 mutant and wild type animals. However, the time course of hypoxia-induced paralysis was indistinguishable between the wild type strain and ced-4(n1162) (Figure S3). Second, we asked whether CED-4 transcript levels are induced or repressed by hypoxic preconditioning. Hypoxic induction of CED-4 transcripts would suggest the possibility that increases in CED-4 levels might somehow promote hypoxic preconditioning. Alternatively, repression of CED-4 transcripts would be consistent with HP suppressing a CED-4-mediated death. We observed a 4- to 5-fold induction in CED-4 transcript levels that peaked eight hours after a 4-hour hypoxic incubation and remained elevated up to 16 hrs later (Figure S4). However, a 4-hour normoxic preconditioning incubation produced a similar induction in CED-4 transcripts (Figure S4). Thus, induction of CED-4 transcription might be necessary for HP but it clearly is not sufficient.
CED-4 is Required during Adulthood Prior to, during, or after Preconditioning for HP
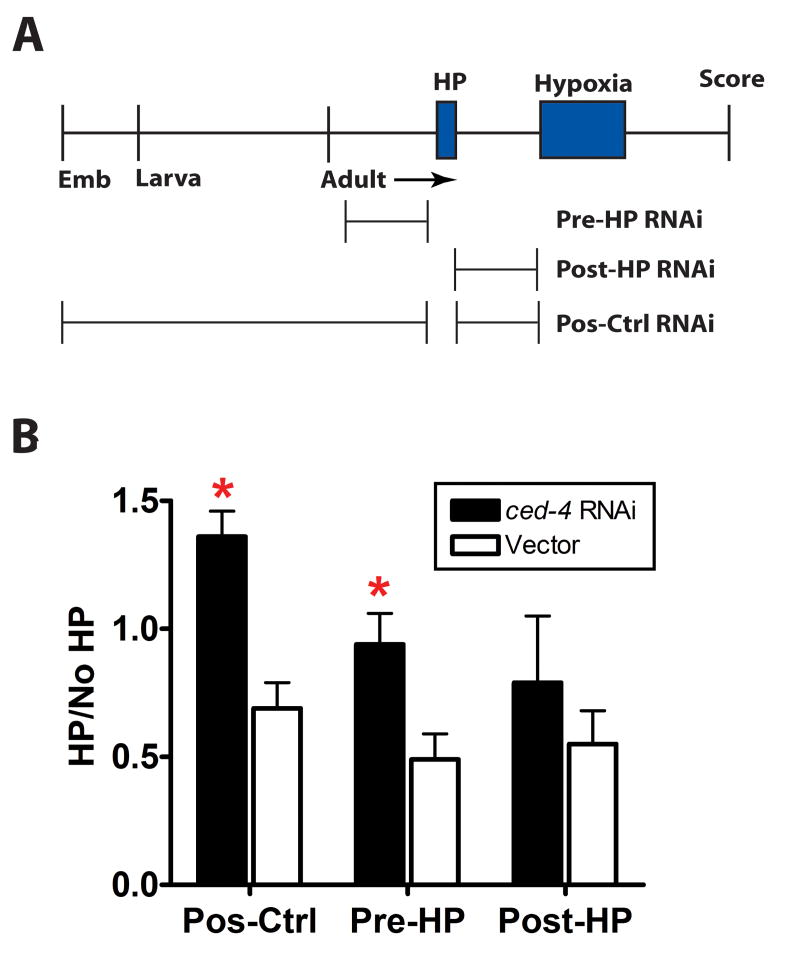
Finally, we asked when ced-4 is required for HP. In particular, we wanted to know whether as has been shown for programmed cell death, ced-4 acts primarily during early development to enable the HP mechanism or whether it might act in adults during or after preconditioning to permit activation of the HP mechanism. We fed animals bacteria carrying a ced-4(RNAi) construct or empty vector for three different time periods: Pos-Ctrl - from embryonic hatching until hypoxic injury, Pre-HP – 20 hrs immediately preceding the HP hypoxic incubation, Post-HP – 20 hrs immediately following the HP hypoxic incubation (Figure 4A). As expected animals fed ced-4(RNAi) throughout development and adulthood were HP defective and significantly different from empty vector animals (Figures 4B). Pre-HP animals were also HP defective; post-HP ced-4(RNAi) animals however were not significantly different from vector controls although there was a trend towards blocking HP (Figures 4B). Thus, CED-4 is required for HP during or after preconditioning, and the lack of normal developmental programmed cell death in ced-4(lf) is not responsible for blockade of HP.
Figure 4. CED-4 is required post-developmentally for HP.
(A) A timeline of the experimental protocol is drawn to scale. Animals were exposed to ced-4(RNAi) or empty vector control at the periods indicated. Emb – embryo
(B) Comparison of HP protection for ced-4(RNAi) versus vector. * - p < 0.05 compared to empty vector (paired t-test); mean±sem of at least 3 independent trials, > 50 animals/trial.
In summary, hypoxic preconditioning has previously only been reported in vertebrate organisms. The results reported here show that an invertebrate has a mechanism for HP. This decidedly broadens the phylogenetic scope of HP and offers a forward-genetically-tractable model organism for the study of HP. The HP mechanism in C. elegans requires the Apaf-1 homolog CED-4. CED-4 acts to control HP by a novel mechanism distinct from its classic role in programmed cell death. In particular, release of CED-4 from mitochondrial tethering by CED-9 and subsequent activation of CED-3 caspase is not essential to the HP mechanism. Our findings beg the question, what is the pathway through which CED-4 acts. Identification of this pathway should provide novel insights into metazoan adaptations to hypoxic injury.
Supplementary Material
Acknowledgments
We thank Barbara Conradt and Robert Horvitz for sharing of unpublished strains and results. This work was supported by NINDS (R01 NS045905), an American Heart Association Established Investigator Award, and a McKnight Endowment Fund for Neuroscience, Neuroscience of Brain Disorders Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor of KATP channel. Annu Rev Physiol. 2000;62:79–109. doi: 10.1146/annurev.physiol.62.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 3.Jiang H, Guo R, Powell-Coffman JA. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci USA. 2001;98:7916–7921. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padilla PA, Nystul TG, Zager RA, Johnson AC, Roth MB. Dephosphorylation of Cell Cycle-regulated Proteins Correlates with Anoxia-induced Suspended Animation in Caenorhabditis elegans. Mol Biol Cell. 2002;13:1473–1483. doi: 10.1091/mbc.01-12-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott BA, Avidan MS, Crowder CM. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science. 2002;296:2388–2391. doi: 10.1126/science.1072302. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall AR, LaRue B, Padilla PA. Glyceraldehyde-3-phosphate dehydrogenase mediates anoxia response and survival in Caenorhabditis elegans. Genetics. 2006;174:1173–1187. doi: 10.1534/genetics.106.061390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron. 2003;37:765–773. doi: 10.1016/s0896-6273(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 8.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 9.Murphy E, Steenbergen C. Preconditioning: The Mitochondrial Connection. Annual Review of Physiology. 2007;69 doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu S, Nagayama T, Jin KL, Zhu L, Loeffert JE, Watkins SC, Graham SH, Simon RP. bcl-2 Antisense treatment prevents induction of tolerance to focal ischemia in the rat brain. J Cereb Blood Flow Metab. 2001;21:233–243. doi: 10.1097/00004647-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Maulik N, Engelman RM, Rousou JA, Flack JE, 3rd, Deaton D, Das DK. Ischemic preconditioning reduces apoptosis by upregulating anti-death gene Bcl-2. Circulation. 1999;100:369–375. doi: 10.1161/01.cir.100.suppl_2.ii-369. [DOI] [PubMed] [Google Scholar]
- 12.Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, Henshall DC, Simon RP. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:234–246. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin B, Hartnett KA, Erhardt JA, Legos JJ, White RF, Barone FC, Aizenman E. Caspase 3 activation is essential for neuroprotection in preconditioning. PNAS. 2003;100:715–720. doi: 10.1073/pnas.0232966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lettre G, Hengartner MO. Developmental apoptosis in C. elegans: a complex CEDnario. Nat Rev Mol Cell Biol. 2006;7:97–108. doi: 10.1038/nrm1836. [DOI] [PubMed] [Google Scholar]
- 15.Shaham S, Horvitz HR. An alternatively spliced C. elegans ced-4 RNA encodes a novel cell death inhibitor. Cell. 1996;86:201–208. doi: 10.1016/s0092-8674(00)80092-6. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka H, Yokota H, Jover T, Cappuccio I, Calderone A, Simionescu M, Bennett MV, Zukin RS. Ischemic preconditioning: neuronal survival in the face of caspase-3 activation. J Neurosci. 2004;24:2750–2759. doi: 10.1523/JNEUROSCI.5475-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloss TA, Witze ES, Rothman JH. Suppression of CED-3-independent apoptosis by mitochondrial betaNAC in Caenorhabditis elegans. Nature. 2003;424:1066–1071. doi: 10.1038/nature01920. [DOI] [PubMed] [Google Scholar]
- 18.Yuan J, Horvitz HR. The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development. 1992;116:309–320. doi: 10.1242/dev.116.2.309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.