Abstract
A protective role for antibodies has not previously been described for host defense against the pathogenic fungus Histoplasma capsulatum (Hc). Mouse mAb’s were generated from mice immunized with Hc yeast that binds the cell surface of Hc. Administration of mAb’s before Hc infection reduced fungal burden, decreased pulmonary inflammation, and prolonged survival in a murine infection model. Protection mediated by mAb’s was associated with enhanced levels of IL-4, IL-6, and IFN-γ in the lungs of infected mice. The mAb’s increased phagocytosis of yeast by J774.16 cells through a CR3-dependent process. Ingestion of mAb-opsonized Hc by J774.16 macrophage-like cells was associated with yeast cell growth inhibition and killing. The mAb’s bound to a 17-kDa antigen expressed on the surface of Hc. The antigen was identified as a histone H2B–like protein. This study establishes that mAb’s to a cell surface protein of Hc alter the intracellular fate of the fungus and mediate protection in a murine model of lethal histoplasmosis, and it suggests a new candidate antigen for vaccine development.
Introduction
The dimorphic fungus Histoplasma capsulatum var. capsulatum (Hc) is the most prevalent cause of fungal respiratory disease, infecting approximately 500,000 individuals in the US each year (1–3). Infection usually results in a mild, often asymptomatic respiratory illness but may progress to life-threatening systemic disease, particularly in individuals with AIDS (4, 5). In contrast to immunocompetent individuals, in whom dissemination is uncommon, 95% of individuals with AIDS present with disseminated disease (5, 6). Patients with AIDS are more likely to develop symptomatic disease that requires hospitalization and have a high fatality rate (7). Despite administration of amphotericin B, the fatality rate in severe disease (e.g., shock, respiratory failure) remains extremely high (6, 8). Even with highly active antiretroviral therapy (HAART) and the availability of prophylactic triazoles, there have not been significant changes in the rates of morbidity and mortality in patients with HIV infected with Hc (7). Since currently available medications for systemic fungal infections often fail to work in the setting of impaired immunity, and many AIDS patients require lifelong prophylaxis to prevent recurrent disease, new therapies are urgently needed.
Ab administration is associated with improved outcome in experimental infection with certain intracellular pathogens (9), but there is no evidence that humoral immunity is effective against Hc. Protective Ab’s function through diverse mechanisms, including complement-mediated lysis, enhancement or inhibition of phagocytosis, and Fc-mediated cytokine release. Exposure to Hc is known to induce an Ab response, and the IgG fraction contains complement-fixing and precipitating Ab’s (10). Passive immunization with immune serum has not been shown to mediate protection (11), B cell–deficient mice are not particularly susceptible to infection (12), and high titers of serum-specific Ab to Hc do not correlate with immunity (10). Consequently, the consensus in the field is that humoral immunity has little or no role in host defense. In fact, high titers of complement-fixing Ab are associated with progressive disease. Nevertheless, we hypothesized that mAb’s could be identified that are active against histoplasmosis. Here we describe the generation of protective mAb’s to Hc that bind to a histone H2B–like protein on the surface of the fungus. The results suggest that the histone H2B–like protein is a potential candidate for vaccine development.
Methods
Fungal strains.
Hc ATCC G217B was obtained from the American Type Culture Collection (ATCC, Rockville, Maryland, USA). Hc CIB 1980 was a gift from A. Restrepo (Corporación para Investigaciónes Biologícas, Medellin, Colombia). Yeast cells were grown at 37°C in Ham’s F-12 medium as described previously (13). The cells were washed three times in PBS and counted by hemacytometer. Additionally, Cryptococcus neoformans strain 24067 (ATCC), Candida albicans strain SC5314 (a gift from M. Ghannoum, Case Western Reserve University, Cleveland, Ohio, USA), Sporothrix schenckii strain CIB (a gift from A. Restrepo, Corporación para Investigaciónes Biologícas), and Saccharomyces cerevisiae strain LM23-3az (a gift from L. Marsh, Albert Einstein College of Medicine, New York, USA) were grown in Sabouraud dextrose broth (Becton Dickinson and Company, Sparks, Maryland, USA) with shaking at 37°C. Heat-inactivated Paracoccidioides brasiliensis strain 60995 yeast was a gift from A. Restrepo. For immunization studies, Hc were killed by heat at 56°C for 1 hour.
Generation and identification of mAb’s to Hc.
Five 6- to 8-week-old female BALB/c mice (National Cancer Institute, Rockville, Maryland, USA) were immunized with intraperitoneal injections of 106 heat-inactivated Hc cells, which were suspended in a 1:1 (vol/vol) emulsion of CFA (Sigma-Aldrich, St. Louis, Missouri, USA) and PBS. Additional doses of 106 heat-inactivated Hc were administered at weeks 2, 4, and 6 after initial immunization in 1:1 (vol/vol) emulsions of incomplete Freund’s adjuvant (Sigma-Aldrich) and PBS. Before, and 2 weeks after, each immunization, sera were obtained and analyzed for Ab’s to Hc. The Ab response was assessed using an Hc yeast cell ELISA developed for this study. Hc yeast cells were found to adhere to 96-well polystyrene plates (Costar 9018; Corning Inc., New York, New York, USA). The optimum number of yeast cells necessary to coat each well was 5 × 105 yeast per well by microscopic evaluation of the plates after serial washing in an ELISA washer (SkanWasher 400; Skatron Instruments, Lier, Norway). Wells were blocked to prevent nonspecific binding using 2% BSA (ICN Biomedicals Inc., Aurora, Ohio, USA) and 0.05% Tween-20 in TBS for 1 hour at 37°C. The plates were washed three times with 0.1% Tween-20 in TBS (TBST) after each incubation. Sera were serially diluted 1:2 in the blocking solution, added to wells of the Hc-coated plates, and incubated for 1 hour at 37°C. After washing, a 1:1,000 dilution of alkaline phosphatase–conjugated goat anti-mouse (GAM) IgG and IgM (Southern Biotechnology Associates Inc., Birmingham, Alabama, USA) was added to the wells and incubated for 1 hour at 37°C. Ab binding was detected by addition of p-nitrophenyl phosphate (Sigma-Aldrich) in 1.0 mM MgCl2 and 50.0 mM Na2CO3, pH 9.8, and measurement of OD at 405 nm with a Ceres 900HDi EIA Workstation (Bio-Tek Instruments Inc., Winooski, Vermont, USA).
The mouse with the highest Ab titer to Hc yeast cells (1:12,800) was boosted again at week 8 and used to generate hybridomas as described previously (14). The hybridomas producing Ab’s that bound to Hc, but not to the blocking solutions, were cloned twice using soft agar cloning to recover homogenous hybridoma cell lines. The heavy- and light-chain isotype of each mAb was determined using alkaline phosphatase–conjugated Ab’s specific for the heavy-chain constant region and for κ and λ light chains.
Localization of binding to Hc yeast.
Hc strains G217B and CIB 1980 were washed in PBS, dried on poly-L-lysine–coated slides (Sigma-Aldrich), and blocked with SuperBlock (Pierce Chemical Co., Rockford, Illinois, USA). The slides were incubated with 10 μg/ml of mAb to Hc (mAb 3B9, 5B8, or 9C7) or an isotype-matched control (mAb 5C11) for 1 hour at 37°C. mAb 5C11 is specific for lipoarabinomannan of Mycobacterium tuberculosis (15). After a wash, the slides were incubated with FITC-conjugated GAM IgM (Southern Biotechnology Associates Inc.) for 1 hour at 37°C. The slides were washed, mounted using a 50% glycerol/50% PBS/0.1 M N-propyl gallate solution, and viewed with an Olympus AX70 microscope (Olympus Optical Co., Melville, New York, USA) equipped with a FITC filter. The reactivity of the Hc-binding mAb’s and P. brasiliensis, S. schenckii, S. cerevisiae, C. albicans, and C. neoformans yeast cells were also examined.
Infected tissues were examined to determine whether the mAb’s could bind Hc in situ. C57BL/6 mice (6–8 weeks old; National Cancer Center, Frederick, Maryland, USA) were anesthetized with ketamine and xylazine and then intranasally infected with 5 × 106 Hc yeast. Mice were euthanized 14 days after infection. Lung tissue was used for immunofluorescent microscopy as described above.
Immunogold transmission electron microscopy was used to determine where the mAb’s bound Hc. Sections of Hc strain G217B were prepared for microscopy as described previously (16). Primary mAb was either mAb to Hc (9C7, 5B8, or 3B9) or polyclonal murine IgM (ICN Biomedicals Inc.) as a negative control. Secondary mAb was GAM IgM conjugated to 5 nm gold (Goldmark Biologicals, Phillipsburg, New Jersey, USA). The grids were viewed with a JEOL (Tokyo, Japan) 100 CX transmission electron microscope.
mAb specificity studies.
To assess mAb specificity, each IgM was purified, biotinylated, and used in competition experiments with the Hc yeast cell ELISA. The mAb’s were purified using agarose beads conjugated to GAM IgM (Sigma-Aldrich) according to the instructions of the manufacturer. Biotinylation was accomplished by the incubation of the mAb’s with an EZ-Link Sulfo-NHS-Biotin kit (Pierce Chemical Co.) according to the instructions of the manufacturer. A constant concentration of one of the biotinylated mAb’s was incubated with varying amounts of a different nonbiotinylated mAb for 1 hour at 37°C. After washing, avidin conjugated with alkaline phosphatase (Sigma-Aldrich) was added for 1 hour at 37°C. Binding of the biotinylated mAb was detected as described above.
Assays of direct mAb effects on Hc.
The growth rate of Hc opsonized with either 10 μg/ml or 100 μg/ml of specific mAb was compared with that of yeast grown in the presence of an isotype-matched control mAb. The Hc yeast cells were grown in F-12 medium as described above, aliquots were removed at various intervals, and the number of cells was determined by counting with a hemacytometer. To assess for agglutination, yeast cells were incubated with or without Hc-binding mAb (concentrations ranging from 1 to 1,000 μg/ml) for 2 hours at 37°C and viewed by a microscope. The cells were also plated onto brain-heart infusion (BHI) agar (Becton Dickinson) for CFU determination.
To determine whether opsonization of Hc with the mAb’s resulted in the permeabilization of the fungus, protein release was assessed in the presence and absence of specific mAb (17). Protein release was assayed for 5 × 106 organisms incubated in PBS with 10 μg/ml or 100 μg/ml of Hc-binding mAb (3B9, 5B8, or 9C7) or control mAb (5C11), or PBS for 15 minutes at 37°C, and then collected by centrifugation. Yeast cells were also incubated in PBS alone. The supernatant was incubated with 10 μM of the thiol-specific fluorophore ThioGlo 1 (Calbiochem, EMD Biosciences Inc., San Diego, California, USA) in the presence and absence of the protein-denaturing agent SDS. Thiol-containing proteins were detected using a fluorescent plate reader with excitation and emission wavelengths of 405 nm and 535 nm, respectively. The proteins were quantified by subtraction of the fluorescence due to released glutathione (i.e., from the signal obtained in the absence of SDS) from total fluorescence. The permeabilizing antimicrobial peptide mellitin (Sigma-Aldrich) was used as a positive control.
CFU and survival studies.
Two hours before infection, 6- to 8-week-old BALB/c or C57BL/6 mice (National Cancer Institute) were injected intraperitoneally with 100 μg of mAb to Hc (9C7, 5B8, or 3B9), isotype-matched control (mAb 5c11), or PBS. Prior to use in the animal and macrophage studies, the mAb’s and PBS were screened with the Limulus amebocyte assay (BioWhittaker Inc., Walkersville, Maryland, USA) to assure that the reagents were endotoxin free. To produce a sublethal infection, mice were anesthetized and infected intranasally with 5 × 106 Hc yeast. Mice were euthanized 14 days after infection. The left upper lobe of the lung was removed and placed into formalin for histology. For CFU determination, the remaining lung tissue, spleen, and liver were homogenized separately, diluted, plated on BHI agar with glucose (10 g/l), cysteine (0.1 g/l), penicillin-streptomycin, and sheep red blood cells (50 ml/l; Colorado Serum Co., Denver, Colorado, USA), and incubated at 37°C.
Lethal infections were also induced by intranasal infection of C57BL/6 mice with 1.25 × 107 Hc yeast. Mice were given 100 μg mAb to Hc (9C7, 5B8, or 3B9), control mAb (5C11), or PBS prior to infection and observed twice daily for survival. Survival experiments were also performed in which mice that received mAb 9C7, control mAb, or PBS prior to infection were subsequently given subinhibitory doses of amphotericin B. Administration of subinhibitory concentrations of amphotericin B in combination with GM-CSF has been shown to protect lethally infected mice (18). Amphotericin B sodium desoxycholate (Sigma-Aldrich) was administered intraperitoneally three times weekly beginning the day after infection at a dose of 0.125 μg/kg. A third survival study examined the effect of preincubation of mAb and Hc prior to infection. Since IgM mAb may not efficiently penetrate lung tissue, mice were infected intranasally with lethal concentrations of Hc yeast that were preincubated for 1 hour at 37°C with 1 mg of mAb 9C7, control mAb, or PBS.
Cytokine and chemokine determinations.
C57BL/6 mice were infected as described above with 5 × 106 Hc strain G217B. Experimental groups were given purified mAb 9C7 or PBS 2 hours before infection with yeast. Sham-infected groups were given mAb 9C7 or PBS 2 hours before intranasal administration of PBS. Mice were sacrificed at days 2 and 7 postinfection, and the lungs were homogenized in 2 ml PBS in the presence of protease inhibitors (Complete Mini; Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, Connecticut, USA). The homogenates were centrifuged at 6,000 g for 10 minutes to remove cell debris, and the supernatant was frozen at –80°C until tested. The supernatants were assayed for IL-2, IL-4, IL-6, IL-10, IL-12p70, monocyte chemoattractant protein-1 (MCP-1), TNF-α, and IFN-γ using ELISA (Becton Dickinson Biosciences Pharmingen, San Diego, California, USA; and R&D Systems Inc., Minneapolis, Minnesota, USA). The detection limits of cytokine assays are 3.1 pg/ml for IL-2, 7.8 pg/ml for IL-4, 15.6 pg/ml for IL-6 and TNF-α, 31.3 pg/ml for IL-10 and IFN-γ, and 62.5 pg/ml for IL-12p40, as stated by the manufacturer. The detection limit of the chemokine assay is 15.6 pg/ml for MCP-1, as determined by the manufacturer.
Phagocytosis assays.
The macrophage-like cell J774.16 is derived from a reticulum cell sarcoma and has been extensively used to study phagocytosis of intracellular microbes (19). J774.16 cells were cultured in DMEM with 10% heat-inactivated FCS, 10% NCTC-109 medium, and 1% nonessential amino acids. CHO cell lines stably transfected with LFA1 (CD18/CD11a), CR1 (CD35), CR3 (CD18/CD11b), and CR4 (CD18/CD11c) were obtained from D.T. Golenbock (University of Massachusetts Medical School, Boston, Massachusetts, USA). CHO cell lines were cultured in α minimum essential medium (Life Technologies Inc., Carlsbad, California, USA) with 10% heat-inactivated FCS. All macrophages were plated at 105 cells per well in an eight-chamber polystyrene tissue-culture glass slide (Becton Dickinson) and grown overnight before use in the phagocytosis assays.
Phagocytosis assays with Hc and macrophages using heat-killed yeast cells labeled with a fluorochrome were done according to standard protocols (20). Hc cells were collected after 3 days of growth, washed three times in PBS, and heat-killed. Yeast cells were incubated with 0.1 mg/ml Oregon Green 488 isothiocyanate (Molecular Probes Inc., Eugene, Oregon, USA) for 20 minutes at room temperature and then washed in PBS. The labeled cells were incubated with mAb 9C7, nonspecific mAb 5C11, or PBS for 1 hour at 37°C. Labeling with Oregon Green did not inhibit mAb specific to Hc from binding the yeast (data not shown). After washing, the samples were added to the macrophage monolayer at an effector-to-target ratio of 2:1, and the suspension was incubated at 37°C for 2 hours in media without complement. Trypan blue (1 mg/ml in PBS) was added for 15 minutes at room temperature to quench the fluorescence of uningested organisms. The phagocytic index was determined by microscopic examination at a magnification of ×600. For each experiment, five fields in each well were counted, and at least 200 macrophages were analyzed in each well. All conditions were tested in triplicate. The phagocytic index was the ratio of intracellular yeast to the number of macrophages counted.
Ab’s to mouse CD14 (clone rmC5-3, rat [LOU] IgG1), CD35 (clone 8C12, rat [SD] IgG2a), CD18 (clone M18/2, rat IgG2a), CD11a (clone M17/4, rat [Wistar-Furth] IgG2a), CD11b (clone M1/70, rat [DA] IgG2b), and CD11c (clone HL3, Armenian hamster IgG, group 1) were obtained from Becton Dickinson Pharmingen. The cell-receptor mAb’s (20 μg/ml) were added to the macrophage monolayer 1 hour before the addition of the fungi that had been incubated with or without mAb specific for Hc. The phagocytosis assay continued as described above.
Transfected CHO cells (2 × 104 per well) were added to eight-chamber tissue-culture slides and grown overnight in α minimum essential medium. The cells were washed three times, and Hc incubated with or without yeast-specific mAb (4 × 104 per well; effector-to-target ratio 2:1) were added in α minimum essential medium without FCS. The remainder of the phagocytosis assay was performed as described with the macrophage monolayers described above.
Fate of Hc phagocytosed in the presence or absence of mAb specific to the fungus.
Intracellular replication of Hc preincubated with or without mAb specific to the yeast was quantified by RIA as described by Newman et al. (21). Macrophages were incubated overnight in 96-well tissue-culture plates and eight-well tissue-culture slides. Hc strain G217B cells preincubated with mAb 9C7 or 5C11 were added (effector-to-target ratio 5:1) with fresh medium for 1 hour to permit phagocytosis, and then 10 μM chloroquine was added as a postendocytic inhibitor. Fresh medium with or without [3H]leucine was added to the experimental wells. After 24 hours, unlabeled leucine and bleach were added. The contents of the wells were transferred to glass fiber filters by an automated harvester (Skatron Instruments, Sterling, Virginia, USA) and extensively washed to remove unincorporated tritium, and the incorporated tritium was quantified by scintillation counting (1414 liquid scintillation counter; PerkinElmer Life and Analytical Sciences Inc., Boston, Massachusetts, USA). The macrophages and yeast were simultaneously used to determine the phagocytic index as described above. The phagocytic index was used to normalize the counts according to the number of intracellular yeast at the time the [3H]leucine was added.
To determine whether the growth of intracellular Hc yeast was affected by prior opsonization with protective mAb, yeast cells preincubated with Hc-specific mAb 9C7, control mAb 5C11, or PBS were incubated with macrophage monolayers for 24 hours. Yeast cells were similarly incubated in wells without macrophages. The macrophages were lysed by the addition of sterile dH2O, and the contents of the wells were plated onto BHI agar to determine CFUs. The percentage growth was determined by comparison of the CFUs from Hc grown with macrophages to the CFUs from yeast grown in medium alone.
Identification of the mAb-binding antigen.
Cell wall/cell membrane preparations of Hc were generated according to published protocols (22, 23). Western blot analysis was performed by electrophoresis of the protein preparations in 10–20% SDS-PAGE gels (Bio-Rad Laboratories Inc., Hercules, California, USA), and the separated proteins were electroblotted onto nitrocellulose membranes. The membranes were blocked with 2% nonfat dried milk in TBS, incubated with mAb 3B9, 5B8, or 9C7, washed in TBST, and incubated with GAM IgM conjugated to HRP. The samples were developed with ECL substrate (SuperSignal; Pierce Chemical Co.) and exposed on X-Omat AR film (Eastman Kodak Co., Rochester, New York, USA). Samples were also treated with N-glycosidase, sodium metaperiodate, or proteinase K (Sigma-Aldrich) before Western blot analysis to determine whether the immunoreactive components of the preparations were carbohydrates or proteins.
The preparations were further purified by serial treatment with 1 mM DTT, 1% Triton, 10 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), and 100 mM sodium carbonate, pH 10 (Sigma-Aldrich). In situ digestion to isolate the desired protein was performed as described previously (24), and mass spectroscopy was performed with a PE Biosystems DE STR MALDI-TOF mass spectrometer (PerkinElmer Life and Analytical Sciences Inc.). The identified fragments were used for peptide mass matching by ProFound (25). The gene sequences of homologous proteins were used for BLAST searching against the Hc gene-sequence data at the Genome Sequencing Center at Washington University in St. Louis (26).
The putative gene encoding the identified protein was amplified from Hc to confirm its presence in the yeast and then cloned into Escherichia coli to determine whether the mAb to Hc could bind the recombinant protein. Hc RNA was prepared with TRIzol Reagent (Life Technologies Inc.) from yeast cells disrupted with a Mini-BeadBeater-8 (BioSpec Products Inc., Bartlesville, Oklahoma, USA). First-strand cDNA was generated by RT-PCR using 5 μg of Hc RNA and SuperScript II RNase H-Reverse Transcriptase (Invitrogen Corp., Carlsbad, California, USA) according the manufacturer’s directions with a Mastercycler gradient PCR (Eppendorf, Westbury, New York, USA). PCR was then performed using 1 μg of the cDNA in 50 μl volume containing 5 U of TaKaRa Taq polymerase (PanVera LLC, Madison, Wisconsin, USA), 10 mM of each dNTP, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 50 mM MgCl2, and 10 μM of each primer. The primers were prepared from the ends of the putative gene sequence with additional amino acids that facilitate cloning into the vector. The sense primer with an EcoRI site was 5′-TTAATGAATTCATATGCCACCAAAGGCC GCTGAG, and the antisense primer with a SalI site was 5′-TTAATGTCGACTTATTTGGCGGACGAGGAG. The reaction conditions were initial denaturation at 94°C for 2 minutes followed by 30 cycles at 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 1 minute. The gene product was sequenced with an Applied Biosystems model 377 DNA Sequencer (Foster City, California, USA).
The amplified cDNA of the 17-kDa protein gene was digested with EcoRI and SalI (Invitrogen Corp.) and cloned into the EcoRI-SalI site of pGEX-KG (27). The resulting plasmid was transformed into DH5α E. coli cells that were plated on Luria-Bertani (LB) agar with 100 μg/ml of ampicillin. To generate recombinant protein, a plasmid purified from a selected colony was transformed into E. coli, grown in LB medium with ampicillin, and then boiled for 5 minutes to lyse the bacteria. Western blot analysis was performed as described above. Western blot analysis was also done to assess whether an Ab response to the protein occurred during infection. After blocking, the recombinant protein electroblotted to nitrocellulose membranes was incubated with serum obtained before or 3 weeks after infection of C57BL/6 mice with 5 × 106 Hc. The blots were washed in TBST, incubated with GAM IgM/IgG conjugated to HRP, and then developed as described above.
Statistics.
Statistical significance was determined using the Student’s t test, Kruskal-Wallis nonparametric ANOVA test (Primer; McGraw-Hill, New York, New York, USA), or log-rank analysis (SPSS Inc., Chicago, Illinois, USA), depending on the data.
Results
Generation of mAb’s to Hc cell surface.
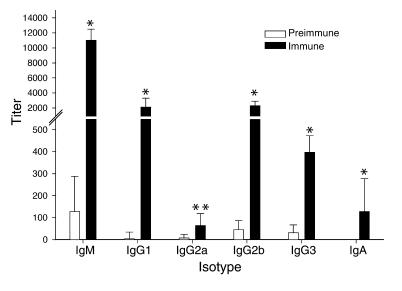
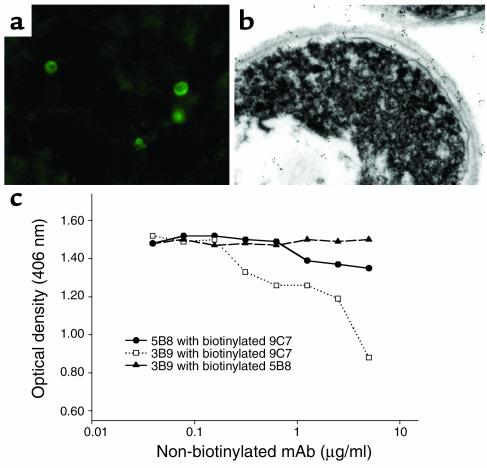
Immunization of mice with killed Hc elicited IgM, IgG, and IgA reactive to Hc yeast as measured by whole-cell ELISA (Figure 1). IgM was the most prevalent isotype. From the spleen of an immunized mouse, three IgM-κ hybridoma lines were generated (9C7, 5B8, and 3B9). No IgG isotype mAb’s were identified. Each of the three mAb’s bound to Hc strain G217B and strain CIB 1980 yeast in a circumferential pattern on the cell surface by immunofluorescent assay. The mAb’s to Hc did not bind P. brasiliensis, S. schenckii, S. cerevisiae, C. albicans, or C. neoformans yeast cells. Immunohistochemistry of infected BALB/c lung tissue showed that the mAb’s reacted with yeast cell walls (Figure 2a), establishing that the antigen is expressed during murine infection. Immunogold transmission electron microscopy confirmed that the mAb’s labeled antigen located in the cell wall (Figure 2b). Labeling also occurred on the interior of the cell, which suggests that the antigen is formed intracellularly and transported to the cell wall. Notably, more gold particles were identified using mAb 9C7 than using mAb 5B8 or 3B9. These results demonstrated that each of the three IgM mAb’s to Hc bound antigen located on the cell surface of the yeast.
Figure 1.
Whole-yeast-cell ELISA demonstrating the serological response to immunization with intact heat-killed Hc. Preimmune serum was obtained prior to immunization. The hyperimmune serum was taken 2 weeks after the fourth immunization. The sera were serially diluted in blocking solution. For comparison of preimmune and immune sera for each isotype, *P < 0.01 and **P = 0.018 by Student’s t test. The results were from the mouse with the strongest immune response, but results were similar in all mice.
Figure 2.
The mAb’s react with Hc yeast. (a) Immunofluorescence microscopy demonstrating labeling of Hc by mAb 9C7 in infected murine lung. Similar labeling was seen with mAb’s 5B8 and 3B9. No binding occurred with control Ab’s. (b) Immunogold transmission electron microscopy showing binding of mAb 9C7 to the cell wall of Hc. Binding also occurs intracellularly. Similar reactivity was seen with mAb’s 5B8 and 3B9. No binding occurred with control Ab’s. (c) Competition yeast cell ELISA using biotinylated and nonbiotinylated mAb’s to Hc. The concentration of biotinylated mAb was kept constant, whereas the amount of the second mAb was serially diluted. The experiment was repeated with similar results.
mAb’s bind to at least two different epitopes.
Competition ELISAs were performed to study mAb fine specificity. Binding of mAb 9C7 by mAb 3B9 was inhibited at concentrations greater than 0.5 μg/ml, in competition assays where the concentration of biotinylated mAb 9C7 was constant (Figure 2c). Competition was not observed when mAb 5B8 was incubated with either mAb 3B9 or 9C7. Hence, mAb’s 9C7 and 3B9 competed, whereas 5B8 did not compete. Competition does not necessarily indicate common epitope specificity, since IgMs can interfere with each other’s binding through steric interference. However, absence of competition is de facto evidence of binding to different epitopes. Hence, mAb 5B8 and mAb’s 9C7 and 3B9 bind to different epitopes, and mAb’s 9C7 and 3B9 may bind to the same or spatially related epitopes.
Assays of direct mAb effects.
Incubation of the Hc-binding mAb’s with Hc did not result in cell lysis or permeabilization. CFUs were similar for Hc incubated with or without the protective mAb’s (data not shown). Opsonization of Hc with the mAb’s did not result in agglutination of the yeast cells. No alterations in the protein concentrations were detected in the supernatants from cells incubated with or without the Hc-binding mAb’s using the ThioGlo 1 assay (limit of detection, 10 nM thiol-containing protein), indicating that there was no permeabilization of the fungus by the protective mAb’s (data not shown). After 36 hours of growth, the number of yeast cells in media was significantly decreased in cultures where 100 μg/ml mAb 9C7 was added, compared with cultures supplemented with control mAb (48 hours: 5.6 × 106 vs. 7.4 × 106, P < 0.001; 72 hours: 7 × 106 vs. 1.2 × 107, P < 0.001). No differences in growth were noted when 10 μg/ml mAb 9C7 or either concentration of mAb 3B9 or 5B8 was used (data not shown).
Passive immunization reduces fungal burden and inflammation and prolongs survival.
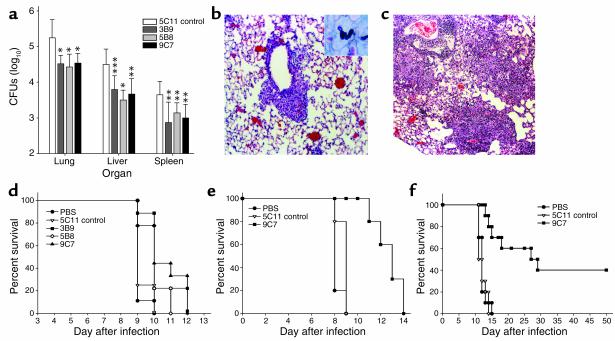
C57BL/6 mice given Hc-binding mAb prior to nonlethal intranasal infection with 5 × 106 yeast had significantly reduced average CFUs in the lungs, liver, and spleen compared with the control group (Figure 3a). BALB/c mice treated with mAb 9C7 or 5B8 also had significant reductions in CFUs, but administration of mAb 3B9 did not result in a significant reduction in fungal burden (data not shown).
Figure 3.
For the in vivo studies, ten mice were used for each condition studied in each experiment. Differences in fungal burden were analyzed by Kruskal-Wallis test, and differences in survival were analyzed by log-rank test. The experiments were repeated at least twice, and similar results were obtained. (a) CFU determinations of Hc 14 days after infection of C57BL/6 mice with 5 × 106 yeast. Bars are means of CFUs from four plates for each condition, and the error bars denote SD. The P values were generated by Kruskal-Wallis test comparing the median CFUs for mAb-treated mice with those for mice that received mAb 5C11 (control): *P < 0.001, **P < 0.01, ***P = 0.02. The experiment was repeated with similar results. (b and c) Histology of lungs from C57BL/6 mice pretreated with protective and nonprotective mAb and infected with 5 × 106 yeast. (b) Representative image of lung from a mouse treated with protective mAb 9C7, showing peribronchiolar inflammation and intact alveoli. (c) The lungs of mice given control mAb had dense inflammatory infiltrates filling much of the alveoli. The original magnification was ×250, and the samples were stained with H&E. The inset in b shows Hc by Gomori’s methenamine-silver staining. (d) mAb to Hc prolongs survival of lethally infected mice. Mice were pretreated with 100 μg of mAb to Hc, nonspecific mAb (5C11), or PBS and infected with 1.25 × 107 yeast. Compared with that of mice that received mAb 5C11 (control), survival was significantly prolonged with mAb to Hc (9C7, P = 0.002; 3B9, P = 0.004; 5B8, P = 0.007). (e) Preincubation of mAb 9C7 with Hc before lethal infection significantly prolonged survival compared with controls (9C7, P < 0.001). (f) Significant prolongation of the survival of lethally infected mice compared with controls (P < 0.001) was achieved by administration of mAb 9C7 before infection and treatment with subinhibitory concentrations of amphotericin B.
Histological examination revealed that mice given mAb prior to Hc infection had an altered inflammatory response to infection. Mice given Hc-binding mAb had less pulmonary inflammation (Figure 3b) than mice given non–Hc-binding mAb (Figure 3c). Even though mAb 3B9 did not affect pulmonary fungal burden in BALB/c mice, a greater decrease in inflammation was observed in the mAb 3B9–treated mice than in control mice, albeit the reduction in inflammation with 3B9 was less than with 5B8 or 9C7. The inflammation in mice treated with mAb to Hc was generally localized adjacent to bronchioles, with the alveolar airspaces appearing intact. In contrast, diffuse dense pneumonia was evident in control mice. These studies demonstrated that administration of Hc-binding mAb prior to infection with Hc reduced pulmonary CFUs and inflammation.
C57BL/6 mice given Hc-binding mAb prior to lethal infection (2.5 × 107 yeast) survived significantly longer than mice given nonspecific mAb or PBS (Figure 3d). Although statistically significant, the prolongation of survival was relatively brief, only 1–2 days. Since serum IgM can be expected to have poor penetration into the alveolar fluid, we modified the experiment by coincubating Hc with mAb prior to infection. The average survival of mice infected with Hc preincubated with mAb 9C7 was significantly longer than that of control mice (P < 0.001; Figure 3e). In a second set of experiments, subinhibitory concentrations of amphotericin B given in conjunction with mAb to Hc significantly extended the survival of mice (Figure 3f). Whereas mice given nonspecific Ab were dead by day 14, 40% of mice treated with mAb 9C7 and subinhibitory concentrations of amphotericin B were alive at day 50 (P < 0.001). When mAb 5B8 was combined with the antifungal drug, 30% of the infected animals were alive at day 50 (P = 0.004), and mAb 3B9 extended the survival of mice to day 29 (P = 0.04).
mAb treatment alters cytokine and chemokine expression.
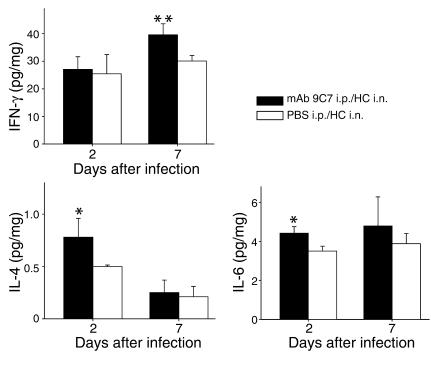
At day 2 postinfection, lung tissue of infected mice treated with mAb 9C7 contained significantly higher quantities of IL-4 and IL-6 than lung tissue of control mice (Figure 4). Administration of mAb 9C7 also produced a statistically significant increase in IFN-γ in the lungs of infected mice at day 7 (Figure 4). Ab administration did not affect the production of the other cytokines or chemokines. Additionally, no differences were noted between uninfected mice given mAb 9C7 or PBS.
Figure 4.
Cytokine expression in the lungs of mice in the presence or absence of mAb treatment. Mice were given mAb 9C7 or PBS intraperitoneally, followed by Hc or PBS intranasally. The cytokine levels were measured at days 2 and 7 postinfection. Values are normalized to total lung weight. Bars denote mean protein concentration, and error bars represent SD. n = 6 for mouse groups that received mAb 9C7 intraperitoneally plus Hc G217B intranasally (mAb 9C7 i.p./Hc i.n.) or PBS intraperitoneally plus Hc G217B intranasally (PBS i.p./Hc i.n.). *P = 0.01, **P = 0.04 by Student’s t test.
mAb to Hc promotes macrophage fungicidal activity in vitro.
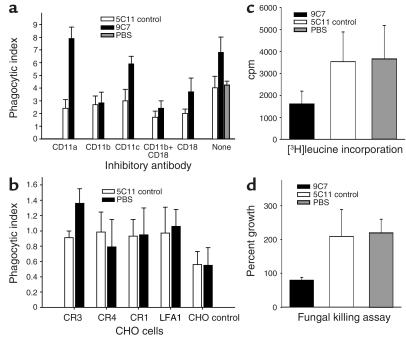
Hc yeast cells opsonized with mAb 9C7 were more avidly ingested by J774.16 cells than were cells incubated with isotype-matched control mAb or PBS (Figure 5a). The mAb-mediated increase in phagocytosis was significantly reduced when CD11b, CD18, or both were blocked with specific Ab’s (Figure 5a). Although Ab to CD11a and CD11c reduced phagocytosis in controls, no reduction occurred when the yeast were opsonized with protective mAb. There was no difference in phagocytosis of Hc with control mAb and PBS.
Figure 5.
Intracellular fate of Hc. Black bars represent 100 μg/ml mAb 9C7, white bars 5C11 isotype-matched control mAb, and gray bars PBS. Student’s t test was used to analyze the data in each panel. (a) Effect of IgM on Hc phagocytosis. Phagocytosis, by J774.16 macrophage-like cells, of Hc incubated with or without protective mAb in the presence or absence of mAb to CD11a, CD11b, CD11c, or CD18. Phagocytosis was performed without complement. Bars are the average of measurements from three wells (five fields each), and error bars denote SD. *P < 0.001 comparing phagocytic index of Hc with mAb 9C7 or control mAb for each condition examined. **P < 0.001 comparing phagocytic index of mAb 9C7 in the absence vs. the presence of complement receptor blockage. ***P < 0.001 comparing mAb 9C7 and PBS. (b) Phagocytosis of Hc by CHO cells. Phagocytosis of Hc incubated with protective or nonspecific mAb in the presence of CHO cells expressing CR1, CR3, CR4, LFA1, or no receptor. The experiment was done without complement. Bars are the average of measurements from three wells (five fields each), and error bars denote SD. A significant difference was seen between mAb’s in CHO cells expressing CR3 (*P < 0.001). (c) Effect of specific mAb on the growth of Hc. The incorporation of [3H]leucine was significantly lower for Hc phagocytosed by J774.16 cells in the presence of mAb 9C7 compared with controls (*P < 0.001). Bars are the average of measurements from five wells, and error bars denote SD. (d) Hc growth was inhibited and killing occurred when the yeast was phagocytosed by J77416 macrophages in the presence of protective mAb. Yeast cells replicated when phagocytosed in the absence of protective mAb. The difference in percentage growth between yeast incubated with mAb 9C7 and controls was significant (*P < 0.001). Bars are the average of measurements from five plates, and error bars denote SD. Each experiment was repeated at least once, and similar results were obtained.
The finding that the mAb-dependent increase in phagocytosis was mediated through CR3 (CD11b/CD18) was confirmed by studies with CHO cells expressing CR1, CR3, CR4, or LFA1 (Figure 5b). Phagocytosis of Hc opsonized with protective mAb was greatest with CHO cells transfected with CR3, and the phagocytic index was significantly greater than that from these cells incubated with yeast and control mAb (P < 0.001). The phagocytic indexes were lower with CHO cells, consistent with prior findings that the ingestion process in these cells is not as efficient as for murine macrophages (28). In summary, the protective mAb 9C7 promoted phagocytosis of Hc through CR3 in a complement-independent process.
The incorporation of [3H]leucine in mAb 9C7–opsonized Hc was reduced by 54% compared with that in yeast incubated with control mAb (P < 0.001; Figure 5c). There was no difference in the incorporation of radioactivity between yeast incubated with control mAb or PBS. Killing of intracellular Hc was measured by CFUs in the presence or absence of protective Ab grown with macrophage compared with culture in medium alone (Figure 5d). The growth of yeast incubated with mAb 9C7 in the presence of macrophage was reduced by approximately 30% compared with the growth of Hc in medium alone. In contrast, growth doubled for Hc incubated with nonspecific mAb or PBS in the presence of macrophage compared with yeast in medium alone. The results suggested that the intracellular growth of Hc was inhibited following phagocytosis of Hc opsonized with mAb.
Identification of the protective antigen on Hc.
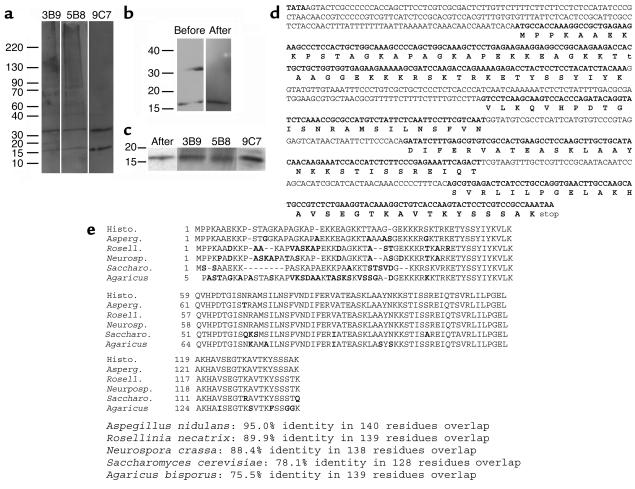
Immunoblots of cell wall proteins revealed that the three mAb’s each bound to proteins of 17 and 34 kDa (Figure 6a). This is noteworthy since the competition ELISAs detailed above (Figure 2c) demonstrated that the epitope recognized by mAb 5B8 was different from the epitope or epitopes recognized by mAb’s 9C7 and 3B9. Hence, the mAb’s appeared to bind different epitopes on the same or a similar antigen. No binding was detected when the cell wall/cell membrane preparations were treated with proteinase K prior to Western blot analysis (data not shown). Treatment of these preparations with N-glycosidase or sodium metaperiodate did not affect the reactivity of the mAb with the preparations (data not shown). Serial incubation of the cell wall/cell membrane preparations with DTT, Triton, CHAPS, and sodium carbonate significantly reduced the number of bands identified by Coomassie staining of Western gels, and the corresponding immunoblots reveal that the Hc-binding mAb’s bound only at 17 kDa (Figure 6b). Hence, the 34-kDa protein may be dimeric or have an associated anchor. Mass spectroscopy data on the 17-kDa protein recovered from the gel identified several fragments that were used for peptide mass matching by ProFound (25). Several high-probability sequences were identified, including histone H2B (protein ID no. 122046) of Aspergillus nidulans (probability 1, estimated Z value 1.66). BLAST searching was performed using the sequence of the A. nidulans H2B gene (gene no. 55547) against the currently available Hc gene sequence at the Genome Sequencing Center at Washington University in St. Louis (26). The deduced amino acid sequences in Hc were within F_HCG217B.contig_p9135 in the histoplasma database. The sequence of the cDNA generated from the PCR reactions and the protein encoded by the gene were identical to the predicted results (Figure 6d). The 17-kDa protein had a 95% identity with A. nidulans histone H2B, and significant homology with histone H2B sequences previously described in other fungi (Figure 6e). Western blot analysis demonstrated that the Hc-binding mAb’s reacted with the recombinant antigen, confirming that the histone-like antigen was indeed the molecule recognized (Figure 6c). Binding of immune serum Ab was detected at a 1:100 dilution, indicating the presence of a low level of Ab to the recombinant protein. No binding occurred with preimmune serum or dilutions of immune serum greater than 1:100.
Figure 6.
Western blot analysis of mAb binding to Hc surface protein. (a) Immunoblots of cell wall proteins demonstrating binding of mAb’s 3B9, 5B8, and 9C7 to proteins at 17 and 34 kDa. (b) Binding of mAb 9C7 to Hc surface proteins before and after serial treatment with 1 mM DTT, 1% Triton, 10 mM CHAPS, and 100 mM sodium carbonate, pH 10. (c) Binding of mAb 9C7 to Hc proteins after serial treatment and reactivity of the Hc-binding mAb’s to recombinant protein (second through fourth lanes). (d) Gene sequence and deduced amino acid sequence of the protein recognized by the mAb to Hc. (e) The protein sequence is compared with that of histone H2B sequences reported in other fungi. These sequence data are available from GenBank/EMBL/DDBJ under accession no. AY316539.
Discussion
The data presented here demonstrate that mAb’s to Hc can modify the course of experimental murine histoplasmosis. The antigen recognized by the protective mAb’s is a histone H2B–like protein. Although histones are commonly associated with DNA, the presence of histones on cell surfaces has been described in both eukaryotic and prokaryotic cells. In particular, histone H2B is present on the cell surface of leukocytes (29), T cells (30–34), B cells (35), and a human lung carcinoma cell line (36). Extracellular histones can stimulate lymphoid cell proliferation and Ig synthesis (37). An interesting consequence of this polyclonal activation of B cells is that lupus Ab’s can bind cell surface histones with subsequent formation of immune complexes (38, 39). Mycobacterium leprae, an obligate intracellular pathogen, uses a histone-like protein in the binding of laminin on peripheral nerves (40–42), which facilitates invasion of the Schwann cells (42, 43). Similarly, Mycobacterium smegmatis produces a histone-like protein that is involved in binding to laminin on human pneumocytes and macrophages (44). Our observation that Hc expresses a histone-like protein on the surface is consistent with findings from other organisms. Furthermore, the association of histone-like proteins with mycobacterial attachment and virulence provides a precedent for interpreting our results.
Passive immunization with mAb to a surface histone-like protein of Hc reduced fungal burden, diminished inflammation, and prolonged survival. Although the administration of Hc-binding mAb prior to infection significantly increases survival of lethally infected mice, the efficiency of IgM-mediated phagocytosis must be interpreted in the context of the lung’s limitations with respect to IgM function (28, 45). Lung macrophages express low levels of CR3, which our work shows is the opsonic receptor for Hc IgM-mediated phagocytosis. Although an IgM Fc receptor has been reported in peritoneal macrophages (46–49), there is no evidence for an Fc receptor on pulmonary macrophages, and it is unclear whether the putative Fc receptor on peritoneal macrophages functions in a manner similar to that of IgG Fc receptors. IgM is primarily limited to the serum compartment, and penetration into alveolar spaces can be expected to occur at only a fraction of serum levels. Hence, the observation that direct opsonization of Hc by preincubation with specific mAb provided greater prolongation of survival can be interpreted in the context of higher efficacy as a consequence of the maximization of Ab interactions with the pulmonary phagocytic cells. Furthermore, we found that the administration of the mAb significantly improved the efficacy of antifungal therapy with subinhibitory concentrations of amphotericin B. In addition to its antifungal effects, amphotericin B can act as an immunomodulator (50). In C. neoformans, subinhibitory concentrations of amphotericin B can affect fungal cell charge and morphology, resulting in increased phagocytosis of yeast by macrophages (51). In histoplasmosis, subinhibitory concentrations of amphotericin B can act in an additive fashion with GM-CSF to protect mice lethally infected with Hc by stimulating nonadherent leukocytes to inhibit yeast cell growth (18).
The current paradigm for host control of histoplasmosis is based on activation of cellular immunity (52), since, in the absence of intact cellular immunity, progressive disease with dissemination occurs (12). Our observations do not challenge the existing paradigm that cellular immunity is central for controlling histoplasmosis; rather, they indicate that some Ab’s can also mediate protection when used as immunoprophylaxis. The finding that immune serum binds to the Hc histone-like protein indicates that the immune system recognizes the protein during infection, but the low level of Ab present in serum suggests that it is not an immunodominant antigen. Hc immunity is characterized by the development of a brisk cytokine response with rapid increases in IL-12 levels in the first days after infection followed by increased levels of IFN-γ (52). In our studies, mice given protective mAb had significantly higher levels of IFN-γ compared with controls, though IL-12 levels were similar. Protective mAb also increased production of IL-4, which is consistent with the reduction in inflammatory cells seen in infected tissues. Administration of IL-4 can protect mice against the deleterious effects of anti–IL-12 (13). Interestingly, administration of Ab to IL-4 has also been reported to decrease survival of infected mice (53). Although IL-4 production increased, IL-10 was not affected, which is consistent with previous findings that transcription of genes encoding IL-10 does not occur in murine histoplasmosis (52). The binding of nucleosomes by T cells can stimulate the release of IL-6 (54). The Hc-binding mAb’s may enhance the interactions of the histone-like protein and T cells. In histoplasmosis, profuse inflammatory responses can be detrimental, as illustrated by mediastinal fibrosis. The cytokine changes associated with mAb administration may reflect a more intense Th1-like response, as manifested by higher IFN-γ levels detected at day 7 and moderation of early inflammatory-mediated damage by higher IL-4 levels detected at day 2.
The role of phagocytic cells in histoplasmosis is complex. Macrophages are the primary effector cells in host resistance to Hc. However, they may initially serve as a protective environment for the fungus in the lung. In the absence of Ab or complement, Hc hsp60 binds to CR3 receptors on macrophages, is phagocytosed, and replicates intracellularly (20, 55). Ingested Hc survives in the phagolysosomes of macrophages by maintaining a pH of about 6.5 in the vacuole (56, 57) and, in some instances, inhibiting phagolysosomal fusion (57). Interestingly, studies with human peripheral blood have revealed that activated monocytes bind extracellular histones (58). Experiments with Ab’s to CD11a, CD11b, CD11c, and CD18 suggest that increased phagocytosis with mAb to Hc depends primarily on CD11b and CD18. The studies with CHO cells expressing CR receptors similarly indicate that the increase in phagocytosis seen with opsonized yeast occurs via the CR3 receptor (CD11b/CD18). Ingested yeast opsonized with Hc-binding mAb had reduced growth and could be killed. The mAb’s did not cause agglutination or direct toxicity to the fungus. The delay in growth of Hc in the presence of cell wall–binding mAb may be due to direct interference with the biosynthesis and organization of the cell wall, which is a mechanism proposed for mAb’s to cell wall constituents in C. neoformans (59, 60). The finding that IgM promoted phagocytosis through CR3 independently of complement is reminiscent of a recent report that specific IgM to C. neoformans promoted phagocytosis of that fungus by facilitating complement-independent polysaccharide-CR3 interactions (28).
Disseminated histoplasmosis occurs with increased frequency in individuals with impaired immunity, particularly in patients with AIDS. Currently available medications for systemic fungal infections often fail to work in the setting of impaired immunity, and many AIDS patients require lifelong prophylaxis to prevent recurrent disease. Vaccine development in Hc has focused on inducing cellular responses to the fungus (61–63). The histone-like protein could be combined with previously identified protective antigens to generate a vaccine that may produce a beneficial cellular and humoral response to Hc. Although vaccine development for Hc is an exciting area of research (64), vaccination may not be effective in immunocompromised individuals. Hence, it is conceivable that this population may benefit most from passive therapy with specific mAb to Hc. Combination of a mAb with antifungal therapy is currently being studied in a clinical trial for treatment of cryptococcosis in patients with AIDS (16), and combined therapy is considered by several mycology experts to be a valid approach to improving current antifungal regimens (65, 66). Since amphotericin B is usually used for initial treatment of histoplasmosis, the addition of mAb to therapy should be considered in future studies. Future investigations of protective mAb may reveal additional important insights into the pathogenesis of histoplasmosis and Ab function against Hc and, perhaps, other intracellular pathogens.
Acknowledgments
J.D. Nosanchuk is supported in part by NIH grant AI-52733 and an Albert Einstein College of Medicine Center for AIDS Research grant. J.N. Steenbergen is supported by NIH training grant T32GM 07491. G.S. Deepe, Jr., is supported by grants AI-34361 and AI-42747 from the NIH and by a Merit Review from the Department of Veterans Affairs. A. Casadevall is supported by NIH Awards AI-33774, AI-13342, AI-52733, and HL-59842. The authors gratefully acknowledge Stephanie Tucker, George Orr, and the Laboratory for Macromolecular Analysis and Proteomics at Albert Einstein College of Medicine for assistance in the identification of the 17-kDa protein. The Laboratory for Macromolecular Analysis and Proteomics is supported in part by the Albert Einstein Comprehensive Cancer Center (grant CA13330) and the Diabetes Research and Training Center (grant DK20541).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: Histoplasma capsulatum var. capsulatum (Hc); 0.1% Tween-20 in TBS (TBST); goat anti-mouse (GAM); brain-heart infusion (BHI); monocyte chemoattractant protein-1 (MCP-1); 3-[3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS).
References
- 1.Cano MV, Hajjeh RA. The epidemiology of histoplasmosis: a review. Semin. Respir. Infect. 2001;16:109–118. doi: 10.1053/srin.2001.24241. [DOI] [PubMed] [Google Scholar]
- 2.Retallack DM, Woods JP. Molecular epidemiology, pathogenesis, and genetics of the dimorphic fungus Histoplasma capsulatum. Microbes Infect. 1999;1:817–825. doi: 10.1016/s1286-4579(99)80084-7. [DOI] [PubMed] [Google Scholar]
- 3.Bradsher RW. Histoplasmosis and blastomycosis. Clin. Infect. Dis. 1996;22(Suppl. 2):S102–S111. doi: 10.1093/clinids/22.supplement_2.s102. [DOI] [PubMed] [Google Scholar]
- 4.Graybill JR. Histoplasmosis and AIDS. J. Infect. Dis. 1988;158:623–626. doi: 10.1093/infdis/158.3.623. [DOI] [PubMed] [Google Scholar]
- 5.Wheat LJ, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore). 1990;69:361–374. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Wheat J. Histoplasmosis in the acquired immunodeficiency syndrome. Curr. Top. Med. Mycol. 1996;7:7–18. [PubMed] [Google Scholar]
- 7.Hajjeh RA, et al. Multicenter case-control study of risk factors for histoplasmosis in human immunodeficiency virus-infected persons. Clin. Infect. Dis. 2001;32:1215–1220. doi: 10.1086/319756. [DOI] [PubMed] [Google Scholar]
- 8.Wheat LJ, et al. Factors associated with severe manifestations of histoplasmosis in AIDS. Clin. Infect. Dis. 2000;30:877–881. doi: 10.1086/313824. [DOI] [PubMed] [Google Scholar]
- 9.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–107. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 10.Chandler JW, Jr, Smith TK, Newberry WM, Jr, Chin TD, Kirkpatrick CH. Immology of the mycoses. II. Characterization of the immunoglobulin and antibody responses in histoplasmosis. J. Infect. Dis. 1969;119:247–254. doi: 10.1093/infdis/119.3.247. [DOI] [PubMed] [Google Scholar]
- 11.Tewari RP, Sharma D, Solotorovsky M, Lafemina R, Balint J. Adoptive transfer of immunity from mice immunized with ribosomes or live yeast cells of Histoplasma capsulatum. Infect. Immun. 1977;15:789–795. doi: 10.1128/iai.15.3.789-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allendorfer R, Brunner GD, Deepe GS., Jr Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J. Immunol. 1999;162:7389–7396. [PubMed] [Google Scholar]
- 13.Allendoerfer R, Biovin GP, Deepe GS., Jr Modulation of immune responses in murine pulmonary histoplasmosis. J. Infect. Dis. 1997;175:905–914. doi: 10.1086/513989. [DOI] [PubMed] [Google Scholar]
- 14.Rosas AL, et al. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect. Immun. 2000;68:2845–2853. doi: 10.1128/iai.68.5.2845-2853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glatman-Freedman A, Martin JM, Riska PF, Bloom BR, Casadevall A. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J. Clin. Microbiol. 1996;34:2795–2802. doi: 10.1128/jcm.34.11.2795-2802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casadevall A, et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormack FX, et al. Macrophage-independent fungicidal action of the pulmonary collectins. J. Biol. Chem. 2003;278:36250–36256. doi: 10.1074/jbc.M303086200. [DOI] [PubMed] [Google Scholar]
- 18.Deepe GS, Jr, Gibbons R. Recombinant murine granulocyte-macrophage colony-stimulating factor modulates the course of pulmonary histoplasmosis in immunocompetent and immunodeficient mice. Antimicrob. Agents Chemother. 2000;44:3328–3336. doi: 10.1128/aac.44.12.3328-3336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman SL, Bucher C, Rhodes J, Bullock WE. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J. Clin. Invest. 1990;85:223–230. doi: 10.1172/JCI114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman SL, Gootee L, Brunner G, Deepe GS., Jr Chloroquine induces human macrophage killing of Histoplasma capsulatum by limiting the availability of intracellular iron and is therapeutic in a murine model of histoplasmosis. J. Clin. Invest. 1994;93:1422–1429. doi: 10.1172/JCI117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez AM, Rhodes JC, Deepe GS., Jr Antigenicity and immunogenicity of an extract from the cell wall and cell membrane of Histoplasma capsulatum yeast cells. Infect. Immun. 1991;59:330–336. doi: 10.1128/iai.59.1.330-336.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Printen JA, Sprague GF., Jr Protein-protein interactions in the yeast pheromone response pathway: Ste5p interacts with all members of the MAP kinase cascade. Genetics. 1994;138:609–619. doi: 10.1093/genetics/138.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez J, Gharahdaghi F, Mische SM. Routine identification of proteins from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels or polyvinyl difluoride membranes using matrix assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS) Electrophoresis. 1998;19:1036–1045. doi: 10.1002/elps.1150190619. [DOI] [PubMed] [Google Scholar]
- 25.ProFound. http://prowl.rockefeller.edu/cgi-bin/ProFound.
- 26.Genome Sequencing Center. Washington University in St. Louis, School of Medicine, St. Louis, Missouri, USA. http://genome.wustl.edu/.
- 27.Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 28.Taborda CP, Casadevall A. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 2001;166:2100–2107. doi: 10.4049/jimmunol.166.3.2100. [DOI] [PubMed] [Google Scholar]
- 29.Rekvig OP, Muller S, Briand JP, Skogen B, Van Regenmortel MH. Human antinuclear autoantibodies crossreacting with the plasma membrane and the N-terminal region of histone H2B. Immunol. Invest. 1987;16:535–547. doi: 10.3109/08820138709087100. [DOI] [PubMed] [Google Scholar]
- 30.Ojcius DM, Muller S, Hasselkus-Light CS, Young JD, Jiang S. Plasma membrane-associated proteins with the ability to partially inhibit perforin-mediated lysis. Immunol. Lett. 1991;28:101–108. doi: 10.1016/0165-2478(91)90106-k. [DOI] [PubMed] [Google Scholar]
- 31.Watson K, et al. Extra-nuclear location of histones in activated human peripheral blood lymphocytes and cultured T cells. Biochem. Pharmacol. 1995;50:299–309. doi: 10.1016/0006-2952(95)00142-m. [DOI] [PubMed] [Google Scholar]
- 32.Shaunak S, et al. Infection by HIV-1 blocked by binding of dextrin 2-sulphate to the cell surface of activated human peripheral blood mononuclear cells and cultured T-cells. Br. J. Pharmacol. 1994;113:151–158. doi: 10.1111/j.1476-5381.1994.tb16187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson K, Gooderham NJ, Davies DS, Edwards RJ. Nucleosomes bind to cell surface proteoglycans. J. Biol. Chem. 1999;274:21707–21713. doi: 10.1074/jbc.274.31.21707. [DOI] [PubMed] [Google Scholar]
- 34.Khan IU, Wallin R, Gupta RS, Kammer GM. Protein kinase A-catalyzed phosphorylation of heat shock protein 60 chaperone regulates its attachment to histone 2B in the T lymphocyte plasma membrane. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10425–10430. doi: 10.1073/pnas.95.18.10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mecheri S, Dannecker G, Dennig D, Poncet P, Hoffmann MK. Anti-histone autoantibodies react specifically with the B cell surface. Mol. Immunol. 1993;30:549–557. doi: 10.1016/0161-5890(93)90029-b. [DOI] [PubMed] [Google Scholar]
- 36.Bilozur ME, Biswas C. Identification and characterization of heparan sulfate-binding proteins from human lung carcinoma cells. J. Biol. Chem. 1990;265:19697–19703. [PubMed] [Google Scholar]
- 37.Bell DA, Morrison B, VandenBygaart P. Immunogenic DNA-related factors. Nucleosomes spontaneously released from normal murine lymphoid cells stimulate proliferation and immunoglobulin synthesis of normal mouse lymphocytes. J. Clin. Invest. 1990;85:1487–1496. doi: 10.1172/JCI114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubota T, Kanai Y, Miyasaka N. Interpretation of the cross-reactivity of anti-DNA antibodies with cell surface proteins: the role of cell surface histones. Immunol. Lett. 1990;23:187–193. doi: 10.1016/0165-2478(90)90190-2. [DOI] [PubMed] [Google Scholar]
- 39.Koutouzov S, et al. Binding of nucleosomes to a cell surface receptor: redistribution and endocytosis in the presence of lupus antibodies. Eur. J. Immunol. 1996;26:472–486. doi: 10.1002/eji.1830260230. [DOI] [PubMed] [Google Scholar]
- 40.Pessolani MC, Hunter SW, Brennan PJ. Relationship between host histones and armadillo-derived Mycobacterium leprae. Int. J. Lepr. Other Mycobact. Dis. 1993;61:381–388. [PubMed] [Google Scholar]
- 41.Marques MAM, et al. Further biochemical characterization of Mycobacterium leprae laminin-binding proteins. Braz. J. Med. Biol. Res. 2001;34:463–470. doi: 10.1590/s0100-879x2001000400004. [DOI] [PubMed] [Google Scholar]
- 42.Marques MAM, et al. Bacterial and host-derived cationic proteins bind a2-laminins and enhance Mycobacterium leprae attachment to human Schwann cells. Microbes Infect. 2000;2:1407–1417. doi: 10.1016/s1286-4579(00)01294-6. [DOI] [PubMed] [Google Scholar]
- 43.Shimoji Y, Ng V, Matsumura K, Fischetti VA, Rambukkana A. A 21-kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc. Natl. Acad. Sci. U. S. A. 1999;96:9857–9862. doi: 10.1073/pnas.96.17.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pethe K, et al. Mycobacterium smegmatis laminin-binding glycoprotein shares epitopes with Mycobacterium tuberculosis heparin-binding haemagglutinin. Mol. Microbiol. 2001;39:89–99. doi: 10.1046/j.1365-2958.2001.02206.x. [DOI] [PubMed] [Google Scholar]
- 45.Taborda CP, Casadevall A. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity. 2002;16:791–802. doi: 10.1016/s1074-7613(02)00328-x. [DOI] [PubMed] [Google Scholar]
- 46.Isaac L, Mariano M. The IgM receptor in mouse peritoneal macrophages. Clin. Exp. Immunol. 1988;72:516–520. [PMC free article] [PubMed] [Google Scholar]
- 47.Uher F, Dobronyi I, Gergel J. IgM-Fc receptor-mediated phagocytosis of rat macrophages. Immunology. 1981;42:419–425. [PMC free article] [PubMed] [Google Scholar]
- 48.Medgyesi GA, Foris G, Fust G, Bazin H. Regulation of Fc mu receptor-mediated functions of resident and provoked peritoneal macrophages. Immunobiology. 1984;167:293–300. doi: 10.1016/s0171-2985(84)80001-7. [DOI] [PubMed] [Google Scholar]
- 49.Shibuya A, et al. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat. Immunol. 2000;1:441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 50.Nosanchuk JD, Casadevall A. Amphotericin B: activity beyond fungal cell sterols. Recent Research Developments Antimicrobial Agents & Chemotherapy. 1999;3:7–15. [Google Scholar]
- 51.Nosanchuk JD, Cleare W, Franzot SP, Casadevall A. Amphotericin B and fluconazole affect cellular charge, macrophage phagocytosis, and cellular morphology of Cryptococcus neoformans at subinhibitory concentrations. Antimicrob. Agents Chemother. 1999;43:233–239. doi: 10.1128/aac.43.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cain JA, Deepe GS., Jr Evolution of the primary immune response to Histoplasma capsulatum in murine lung. Infect. Immun. 1998;66:1473–1481. doi: 10.1128/iai.66.4.1473-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou P, et al. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-gamma. J. Immunol. 1995;155:785–795. [PubMed] [Google Scholar]
- 54.Hefeneider SH, et al. Nucleosomes and DNA bind to specific cell-surface molecules on murine cells and induce cytokine production. Clin. Immunol. Immunopathol. 1992;63:245–251. doi: 10.1016/0090-1229(92)90229-h. [DOI] [PubMed] [Google Scholar]
- 55.Bullock WE, Wright SD. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J. Exp. Med. 1987;165:195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eissenberg LG, Goldman WE, Schlesinger PH. Histoplasma capsulatum modulates the acidification of phagolysosomes. J. Exp. Med. 1993;177:1605–1611. doi: 10.1084/jem.177.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strasser JE, et al. Regulation of the macrophage vacuolar ATPase and phagosome-lysosome fusion by Histoplasma capsulatum. J. Immunol. 1999;162:6148–6154. [PubMed] [Google Scholar]
- 58.Emlen W, Holers VM, Arend WP, Kotzin B. Regulation of nuclear antigen expression on the cell surface of human monocytes. J. Immunol. 1992;148:3042–3048. [PubMed] [Google Scholar]
- 59.Rosas AL, Nosanchuk JD, Casadevall A. Passive immunization with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infect. Immun. 2001;69:3410–3412. doi: 10.1128/IAI.69.5.3410-3412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodrigues ML, et al. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 2000;68:7049–7060. doi: 10.1128/iai.68.12.7049-7060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gomez FJ, Allendoerfer R, Deepe GS., Jr Vaccination with recombinant heat shock protein 60 from Histoplasma capsulatum protects mice against pulmonary histoplasmosis. Infect. Immun. 1995;63:2587–2595. doi: 10.1128/iai.63.7.2587-2595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allendoerfer R, Maresca B, Deepe GS., Jr Cellular immune responses to recombinant heat shock protein 70 from Histoplasma capsulatum. Infect. Immun. 1996;64:4123–4128. doi: 10.1128/iai.64.10.4123-4128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deepe GS, Jr, Gibbons R. Protective efficacy of H antigen from Histoplasma capsulatum in a murine model of pulmonary histoplasmosis. Infect. Immun. 2001;69:3128–3134. doi: 10.1128/IAI.69.5.3128-3134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dixon DM, et al. Development of vaccines and their use in the prevention of fungal infections. Med. Mycol. 1998;36:57–67. [PubMed] [Google Scholar]
- 65.Stevens DA, et al. Combined treatment: antifungal drugs with antibodies, cytokines or drugs. Med. Mycol. 2000;38:305–315. [PubMed] [Google Scholar]
- 66.Casadevall A, Pirofski L. Adjunctive immune therapy for fungal infections. Clin. Infect. Dis. 2001;33:1048–1056. doi: 10.1086/322710. [DOI] [PubMed] [Google Scholar]