Abstract
Current antipsychotic treatments fail to fully address the range of symptoms of schizophrenia, particularly with respect to social and occupational dysfunctions. Recent work has highlighted the role of nicotinic in both cognitive and attentional deficits as well as deficient processing of repetitive sensory information. The predilection for schizophrenia patients to be extremely heavy cigarette smokers may be related to their attempt to compensate for a reduction in hippocampal α7 nicotinic cholinergic receptors by delivering exogenous ligand to the remaining receptors. Studies in rodent models of both learning and memory deficits and deficits in sensory inhibition have confirmed a role for α7 subtype of the nicotinic cholinergic receptors in these processes. Rodent studies also demonstrated the efficacy of a selective partial α7 nicotinic agonist, DMXBA, to improve these deficits. Subsequent human clinical trials demonstrated improved sensory inhibition in 12 schizophrenia patients and showed improvement in several subtests of the RBANS learning and memory assessment instrument. These data suggest that therapeutic agents selected for α7 nicotinic activity may have utility in treating certain symptoms of schizophrenia.
Keywords: Schizophrenia, nicotinic receptors, DMXBA, P50 auditory evoked potential, cognitive deficits, nicotine
Although the positive symptoms such as hallucinations and delusions of schizophrenia are partially treated with the available antipsychotic medications, social and occupational dysfunction remains a major issue in treating this disorder. Cognitive deficits have been identified as more closely related to ability to adequately function [1]. In the search for new and more effective therapeutic agents for schizophrenia, recent attention has turned the nicotinic cholinergic receptors system.
α7 and P50 Auditory Gating
People with schizophrenia, in addition to the cardinal symptoms of hallucinations and delusions, suffer from the inability to focus attention. This may stem from being overwhelmed by extraneous sensory stimuli [2] which impairs the person’s ability to think coherently. This “flooding” has been modeled in the laboratory physiologically by measuring the amplitude of the evoked responses to identical paired auditory stimuli separated by 500 ms. The P50 auditory-evoked response occurs 40-75 ms after the presentation of a brief click. This is called the “conditioning” response. On the presentation of the second “test” stimulus, inhibitory mechanisms are normally activated so the brain can tune out repetitive non-essential noise. This results in a diminished amplitude of the P50 component of the evoked response to the second stimulus relative to the first (Figure 1). Persons with schizophrenia generally show less ability to inhibit or filter out these extraneous second stimuli, as demonstrated by a a larger response to the second “test” stimulus, and a larger test wave when compared to the conditioning wave [,4,5,6,7] This deficit is correlated with impairment in sustained attention as measured by diminished performance on the Digit Vigilance Test[8].
Figure 1.

P50 auditory evoked potentials from a control and schizophrenia patient. The arrows indicate the onset of the stimuli. The tick marks delineate the peak and trough of the P50 evoked response wave. In the normal subject, the test amplitude is 12% of the conditioning amplitude demonstrating the suppression of response to the second of paired identical tones. The schizophrenic patient shows a test amplitude of 84% of the responses of the conditioning amplitude. (Adapted from Adler et al 1990.)
People with schizophrenia frequently smoke cigarettes and often smoke heavier than the normal population[9,10,11,12,13,14,15]. Additionally, they extract more nicotine from each cigarette they smoke presumably by deeper inhalation.[16]. The high level of smoking has been proposed as a form of self-medication to alleviate symptoms of their illness including depression, anxiety, anhedonia or amotivation[17,18,19,20]. Others have proposed that smoking alleviates symptoms of nicotine withdrawal or neuroleptic-induced side effects[21,22,23,24]. Finally, smoking may be an attempt to improve cognition and sensory gating[25,26,27].
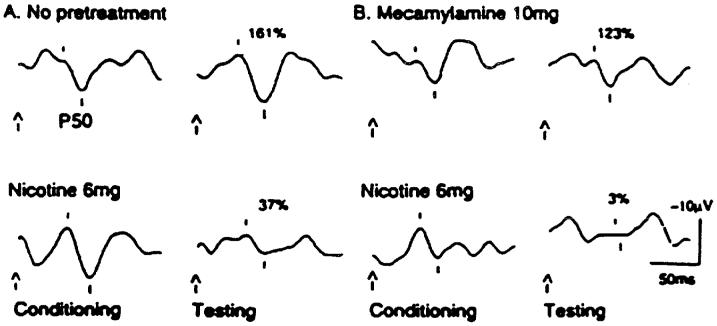
Nicotine appears to have a positive effect on P50 inhibition in schizophrenia[27]. When people with schizophrenia who have been withdrawn from nicotine smoke cigarettes, they are able to temporarily filter stimuli. However within approximately 30 minutes, their inhibitory deficit returns. Longer lasting effects are not seen with the transdermal patch, demonstrating that prolonged effects cannot be obtained with this method of administration because of tachyphylaxis[28]. Relatives of people with schizophrenia also have poor P50 suppression without the confounds of the additional pathological effects of schizophrenia, the effects of medications or chronic smoking[29,30,31,32]. People with schizophrenia treated with clozapine exhibit normalization of their P50 ratio coincident with improvement in their clinical symptoms[33]. Clozapine, which releases acetylcholine in the hippocampus[34], may thereby indirectly act on the nicotinic cholinergic receptors to normalize the P50 ratio, as people with schizophrenia also decrease the amount of cigarettes they smoke while taking this medication[35,36]. Clozapine also has the property of 5HT3 antagonism, which activates nicotinic cholinergic receptors. Nicotine gum and physostigmine also improve P50 suppression in relatives of people with schizophrenia[37]. Administration of high dose nicotine with 10 mg of mecamylamine, a high affinity α4ß2 nicotinic cholinergic receptors antagonist still produces improvement in P50 suppression in schizophrenia[38](Figure 2). Thus, since nicotine is a non-selective agonist and mecamylamine is blocking the high affinity receptors, the improvement in suppression appears to be mediated through the low affinity α7 nicotinic cholinergic receptors.
Figure 2.
A: The P50 auditory gating deficit in the relative of a schizophrenic patient (left upper panel) with a lack of inhibition. The arrows indicate the onset of the stimuli. The tick marks delineate the peak and trough of the P50 evoked response wave. The test amplitude is initially 161% of the conditioning amplitude. After nicotine gum, the P50 evoked response is normalized as indicated by a test amplitude of 37% of the conditioning amplitude (left lower panel); B: With pretreatment with mecamylamine (right upper panel) blockade of the α2ß4 high affinity receptor does not block the effect of nicotine(right lower panel) thus the test amplitude is 3% of the conditioning amplitude. The enhancement of the effect may be caused by mecamylamine’s blockade of the stimulatory effect of nicotine on catecholamine release, which is mediated through high affinity nicotinic receptors(Adapted from Freedman et al. 1994).
Additional independent evidence for involvement of the α7 cholinergic receptor in the P50 auditory evoked potential deficit is provided through genetics. Nine multiplex families with schizophrenia were studied in a genome-wide linkage analysis. Maximal linkage to the P50 deficit was found at chromosome 15q14 at a polymorphic marker < 120 kb from the α7 gene with a lod score of 5.3, Θ = 0.0[39]. Linkage of this region to schizophrenia was further replicated in families from the NIMH Schizophrenia Genetics Initiative[40] and in other studies[41,42,43,44], but there have also been some negative studies in this region[45,46]. Multiple single nucleotide polymorphisms (SNP)have been identified in the 15q14 gene promoter region that are more frequently present in people with schizophrenia and their family members than normal controls[47,48]. Furthermore, the presence of a SNP in the 15q14 gene CHRNA7 5′ core promoter is significantly associated with P50 suppression deficits[47]. Association of CHRNA7 polymorphisms with P50 gating has been replicated, but the specific allelic associations differ, which suggests that responsible mutations have not yet been unambiguously identified[47,48]. In addition to the deficits in P50 evoked potentials and the functional promoter polymorphisms in the CHRNA7 region, people with schizophrenia also have abnormalities in expression and regulation of central nicotinic cholinergic receptors. Decreased α7 nicotinic cholinergic receptor binding has been noted in the reticular nucleus of the thalamus, the hippocampus, the cingulate cortex and the frontal lobe regions[49,50,51,52].
A similar deficit in auditory gating has been found in inbred mice. The DBA/2 strain exhibits a failure to suppress its response to the second stimulus in a paradigm identical to that used with humans, while the C3H shows a pattern comparable to normal humans[53] (Figure 3). Studies have shown that the auditory gating improves in the DBA/2 mouse with nicotine administration[54], just as it does in schizophrenia patients[27]. Additionally, similar effects were found for clozapine in both the DBA/2 mouse[55] and schizophrenia patients[33]. The mechanism of auditory gating has been clarified through the use of these animal models. The activation of the α7 cholinergic receptors release GABA from GABAergic interneurons[56,57] which then act on GABAB receptors which activates that decrease the release of glutamate thus preventing hippocampal neurons from responding to the second stimulus in the P50 paradigm [58]. Nitric oxide acts as a second messenger to prolong the effect of the α7 nicotinic cholinergic receptor stimulation. Additionally, a promoter region polymorphism exists between the CH3 and the DBA/2 mouse strains[59] which model the polymorphisms observed in the human promoter region[39]. Thus, the DBA/2 mouse represents a useful model of the auditory gating deficit in schizophrenia from both the phenotypic and genotypic standpoint. The DBA/2 mouse also shows a reduction in the number of hippocampal α7 nicotinic cholinergic receptors [60] similar to what is observed in humans with schizophrenia[50].
Figure 3.

Auditory evoked potentials in response to identical paired stimuli in 2 inbred strains of mouse. The arrows indicate the onset of the stimuli. The tick marks delineate the peak and trough of the N40 evoked response wave. The C3H strain models the pattern seen with human control subjects with a test amplitude that is 17% of the conditioning amplitude, demonstrating inhibition of the evoked potential. The DBA/2 mouse shows a pattern similar to that observed in schizophrenia patients, with a test amplitude of 114% of the conditioning amplitude, and a lack of inhibition of the evoked response. . (From Stevens et al 1996).
α7 in Learning and Memory
Although nicotine seems to improve cognition, the involvement of high or low affinity nicotinic cholinergic receptors is unclear. Withdrawal from nicotine in normal smokers has been shown to cause attention impairments[61]. Nicotine administration may just be relieving withdrawal and correcting those deficits. However, if low-dose nicotine is administered to normal non-smokers, thereby avoiding the confound of withdrawal, there is enhanced performance on the continuous performance test with decreased errors of omission without an increase in errors of commission[62] In Alzheimer’s disease, where nicotinic cholinergic receptors are known to be decreased in the cortex and hippocampus[63,64], nicotine injections or nicotine skin patches significantly improve attention, learning and memory[65,66,67,68,69,70]. Similarly, in adults with attention deficit disorder, nicotine skin patch reduces clinical symptoms and increases reaction time, but does not improve errors of omission in the Continuous Performance Test[71]. The effects of nicotine on neuropsychological measures in persons with schizophrenia, unlike the effects on the P50 auditory evoked potential less are conclusive. In smokers with schizophrenia, with abstinence from nicotine, working memory is decreased[72,73]. If nicotine is then reinitiated by smoking, working memory deficits are normalized[73]. There have been several studies using the nicotine patch to administer nicotine. One study induced memory deficits with haloperidol. The nicotine patch was then applied and was able to restore functioning to baseline as measured before the administration of haloperidol[74]. In another study, the nicotine patch also improved auditory working memory, attention, complex reaction times but not simple reaction times [75,76,74]. Nicotine gum shows mixed effects depending on the psychological realm and whether the subjects are smokers or are nonsmokers. While nicotine gum improves attention in nonsmokers, it may diminish attention in smokers. In contrast, nicotine gum has no effect in either smokers or nonsmokers on working memory or visuospatial memory[77]. Finally, nicotine nasal spray enhances verbal memory[78], visuospatial delayed recognition[79] and complex reaction times but has no effect on simple reaction times attention or working memory[74, 80, 79]. Thus, chronic exposure to nicotine in smokers, the mode of experimental nicotine delivery, the nicotine dose given, the particular neuropsychological test, clinical diversity and potentially other factors in these studies may account for the variability of these findings.
DMXBA as a Prototype Drug
Nicotine has several limitations as a therapeutic agent for schizophrenia. Nicotine induces tachyphylaxis and thus does not maintain sustained benefit. Additionally, the long-term health risks of chronic nicotine use are unknown. Nicotine is also addictive and without sustained use, people can experience symptoms of withdrawal[81]. Thus, alternative nicotinic agonists that are less potentially toxic would be helpful in the treatment of schizophrenia.
One of the few agents that has reached clinical trials is GTS-21 or 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXBA). This is one of a series of compounds derived from anabaseine, an alkaloid found in marine worms[82,83]. DMXBA is a partial agonist at the α7 nicotinic cholinergic receptor [84,85] and a weak competitive antagonist at the α4ß2 nicotinic cholinergic receptors and 5HT3 receptors[86,87]. The first step in testing of this compound was to administer DMXBA in DBA/2 mice. This compound produced a dose-dependent improvement in auditory gating in this model (Figure 4A) which occurred through a selective reduction in the response to the second stimulus (Figure 4B) [88]. This pattern is in concert with what is now known regarding nicotinic modulation of the 2 evoked potentials, that is that modulation of the response to the second stimulus is mediated by α7 nicotinic cholinergic receptor [89] while the response to the first stimulus is mediated, in part, by α4β2 nicotinic cholinergic receptor [90]. Thus, increased stimulation of the limited numbers of hippocampal α7 nicotinic cholinergic receptor with DMXBA in this mouse strain increases firing of the interneurons, on which they reside[91], which in turn inhibits firing of a subpopulation of pyramidal cells in response to the second stimulus. Antagonism of the α4β2 receptors with mecamylamine blocks any stimulation by endogenous acetylcholine, thus inhibiting increases in the response to the first stimulus, as is commonly observed with non-selective agonists such as nicotine[54]. The improvement in auditory gating has been replicated in isolation-reared rats which show deficient auditory gating[92], in C3H chronically treated with cocaine, which also show deficient auditory gating[93] and after oral administration of DMXA in DBA/2 mice[94].
Figure 4.
A. The effect of DMXBA on the ratio of the amplitude of the second auditory evoked response divided by the amplitude of the response to the first stimulus. DMXBA dose dependently lowered the ratio showing improvement in auditory gating in the DBA/2 mouse, a model of schizophrenia-like deficits in auditory gating. DMXBA was administered as indicated by the arrows labeled injection. Asterisks indicate ratios that are significantly different from vehicle. Filled circles indicate mean N40 ratio B. DMXBA was administered as indicated by the arrows labeled injection.The improvement in auditory gating with this compound was produced by a selective decrease in the response to the second stimulus demonstrating increased inhibition in the circuitry. The records were obtained in the CA3 region of the hippocampus in response to paired identical auditory stimuli. The DMXBA was administered subcutaneous and recordings obtained at 5 minute intervals. At 40 minutes, when the responses were back to baseline levels, a second identical injection was administered and recordings were obtained for an additional 40 mintues. Filled circles indicate mean conditioning amplitude. Open circles indicate mean test amplitude. Asterisks indicate amplitudes that are significantly different from vehicle (Adapted from Stevens et al 1998).
An alternate method for assessing deficiencies in sensory processing is the prepulse startle inhibition (PPI) paradigm in which a small auditory stimulus preceding a large startling auditory stimulus reduces the amplitude of the startle response induced by the large stimulus[95]. Schizophrenia patients do not show normal PPI[96], but smoking improves the deficit[97,98]. In rodent studies, nicotine improves PPI in a model of deficient schizophrenia-like PPI[99]. Administration of DMXBA also improves deficient PPI[100].
Finally, cognitive and attentional deficits are a recognized symptom class in schizophrenia[101,102,103] which can be improved with nicotine administration[104,76,77,78]. Various paradigms have been developed to assess similar learning and memory, and attention functions in rodents and similar to what is seen in humans, nicotine has been shown to improve rodent deficits in these paradigms[105,106,107,108,109]. DMXBA improves monkey performance on a delayed matching to sample task, an effect that persists for 24 hours after drug administration[110]. The administration of DMXBA improves eyeblink classical conditioning acquisition in older rabbits who have lost cholinergic neurons[111] Mecamylamine, an α4ß2 antagonist has a deleterious effect on conditioned learning in young rabbits If young rabbits are given mecamylamine and DMXBA, their eyeblink classical conditioning acquisition is improved.[112]. DMXBA improves one-way active avoidance, Lashley III maze testing, and 17 arm radial maze test performance in aged rats[113,114]. Passive avoidance deficits are normalized in rats[115] and ischemia-induced hippocampal cell death and impaired passive avoidance performance in gerbils are prevented by treatment with DMXBA[116]. DMXBA also improves performance on the Morris water maze[117].
The first stage in human testing with DMXBA was to initially administer this compound to normal male subjects to assess for safety, tolerability, pharmacokinetics and possible effects on cognition prior to its study as a cognitive enhancer in Alzheimer’s disease[118]. Subjects were randomized to DMXBA (25, 75 and 150 mg) or placebo administered three times daily for 5 days with a 10 day washout period between drug-taking periods. All subjects were evaluated for performance on a computerized test battery to measure the effect of treatment on cognitive functioning including changes in attention (simple reaction time, choice reaction time, digit vigilance), numeric and spatial working memory, secondary episodic recognition memory (word and picture recognition, immediate and delayed word recall) and visual tracking. Peak plasma levels were achieved at 1-1.4 h after the first dose and 1-1.2h after 5 days of dosing. DMXBA was well tolerated at doses of up to 450 mg daily with no significant safety findings. DMXBA significantly improved performance on simple reaction time, choice reaction time, correct detection during digit vigilance, both word and picture recognition memory and both immediate and delayed word recall. Additionally, DMXBA improved subject performance speed on numeric and spatial working memory task. Improvement was generally seen with the 25 mg dose, with further improvement at the 75 mg dose and an equivalent effect at the 150 mg dose[118].
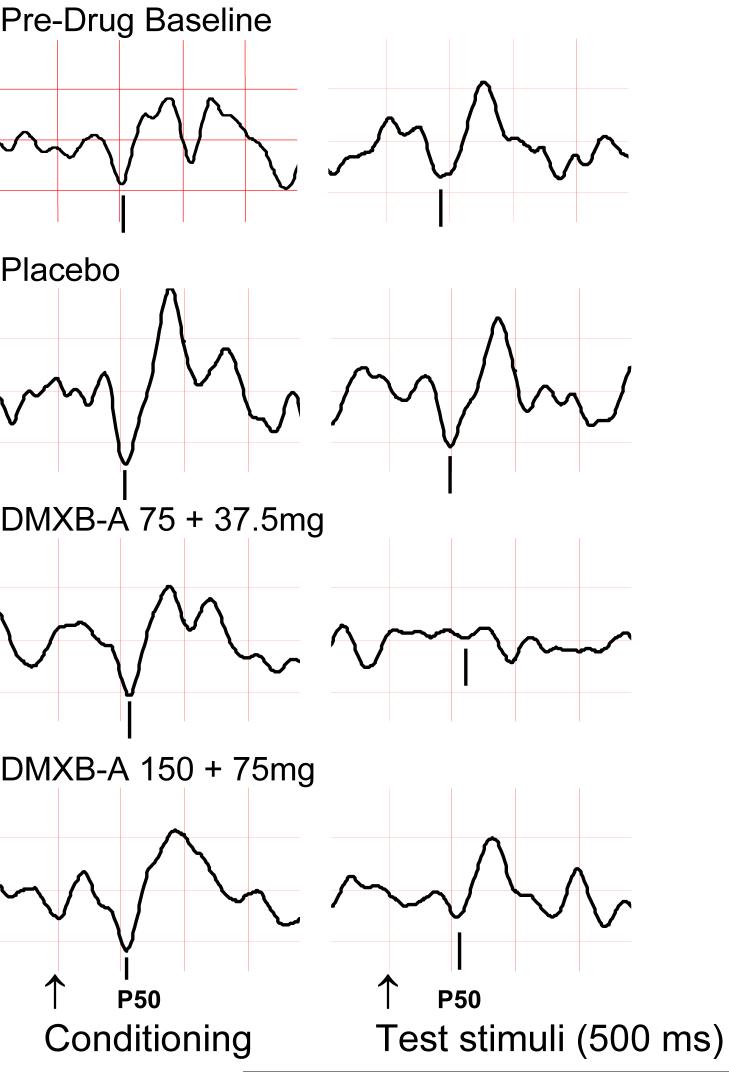
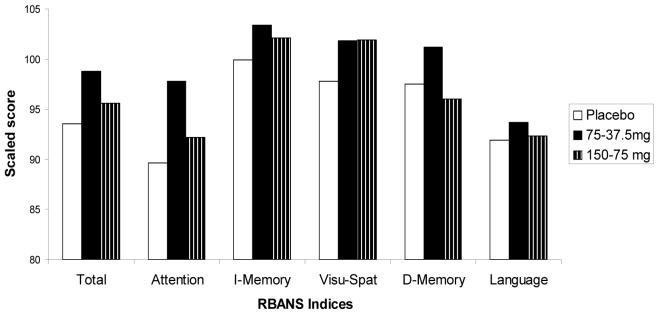
As this agent appeared safe and promising in enhancing cognition, DMXBA was studied in schizophrenia to prove that the α7 nicotinic cholinergic receptor activation is responsible for the normalization of the P50 auditory evoked potential deficit in schizophrenia. Additionally, the safety and effects of this agent on neurocognition in this population were also evaluated[119]. DMXBA was administered in a double-blind, placebo-controlled cross-over design to 12 male and female non-smokers with schizophrenia. All subjects had been stable on their normal antipsychotic medications for at least 3 months. DMXBA was administered orally (150 mg or 75 mg) followed two hours later by a half dose (75 mg or 37.5 mg). The half dose, administered at the predicted half-life of the first dose was chosen to extend the period of therapeutic drug levels during the behavioral measurements. Subjects received the high and low doses and identical appearing placebos, identical in appearance, randomly on 3 different days. Study days were at least 3 days apart. The Repeatable Battery for Assessment for Neuropsychological Status (RBANS), which assesses attention, immediate memory, visuo-spatial/construction, language and delayed memory[120], was administered immediately following the second dose on each experimental The P50 auditory evoked potential and Brief Psychiatric Rating Scales were recorded at baseline before drug administration, twice after the first and once after the second dose. DMXBA improved performance on both the RBANS Total Scale Score (effects size 1.8) as well as the Attention subscale (effect size 2.17; Figure 5; [119]. These effect sizes are much larger that those seen for nicotine on the RBANS (0.6 and 0.25 for the Total scale score and Attention subscale score, respectively[77]. DMXBA also normalized the P50 ratio (effect size of 2.36) as well as the test wave amplitude (effect size 1.45), a more specific measure of inhibition. These findings are an improvement over the study of nicotine on P50 auditory gating in relatives (effect size of 0.86; Figure 6; [119]. Increased inhibition of the P50 response during DMXBA is consistent with the hypothesized agonist effect on α7 receptors. DMXBA seems to be beneficial at low concentrations and less effective at higher concentrations. This suggests that there may be tachyphylaxis, but a larger number of subjects is required to establish this point. At this stage of the investigation, the difference between the two doses is not significant. In animal studies with DMXBA, there was no tachyphylaxis to repeated dosing, but a non-significant indication of decreased effect was observed at higher doses.
Figure 5.
Auditory evoked responses of a subject with schizophrenia. Stimuli were a conditioning auditory stimulus and an identical test stimulus, delivered 500 msec apart. Inhibition of the test P50 response is increased by DMXB-A administration, particularly during the lower dose (third row), compared to baseline and placebo responses above it. Arrows show the timing of the stimuli and vertical bars mark the location of the P50 wave in the tracings above. Positive polarity is downwards; vertical grid interval is 2 μV, and horizontal is 50 ms. (Adapted from Olincy et al. 2006).
Figure 6.
Effects of DMXB-A and placebo on the RBANS Total Scale Score and its specific Indices. I is immediate and D is delayed Memory Visu-Spat=visuospatial memory. (Adapted from Olincy et al. 2006).
Other Potential Nicotinic Targets
A series of other potential cholinergic receptor agonists have been developed as potential candidates for the treatment of schizophrenia and Alzheimer’s disease. Drugs currently in development include a 1,4 -diaza-bicyclo[3.2.2]nonane-4-carboxylic acid 4-pyridin-2yl-phenyl ester at Pfizer Inc., and a N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide (14 PHA-543,613) also at Pfizer, Inc.. The second compound demonstrates reversal of amphetamine-induced N40 gating deficit in anesthetized rats and improves the ability to discriminate between familiar and novel objects[121]. Targacept, Inc. has an (E)-N-methyl-5 (3-pyridinyl)-4-penten-2-amine and Pharmacia & Upjohn Company has a substituted-heteraryl-7-aza[2.2.1]bicycloheptane. SSR180711, a selective α7 nicotinic cholinergic receptor partial agonist from Sanofi-Adventis, enhances episodic memory in the object recognition task in rats preadministered methyllycaconitine, an α7 nicotinic cholinergic receptor antagonist and in mice. However, when administered to α7 knockout mice, there is not enhancement of long-term episodic memory[122]. AR-R 17779, an AstraZeneca product, is an acetylcholine analogue with full agonist properties at the α7 nicotinic cholinergic receptor. This compound does not enhance the inhibition of startle in DBA/2 mice[123,124,125] or improve accuracy and speed of response on a five-choice serial reaction time[126,127]. ABT-418, has some agonist properties at the α7 nicotinic cholinergic receptors, but is a less potent agonist than nicotine[128] and restores deficient auditory gating in DBA/2 mice as well as rats with fimbria-fornix lesions, but similar to nicotine, fails to produce continued improvement with a second dose[54].
Two compounds that have been assessed in humans have used alternatives strategies to the use of nicotinic agonists to increase the endogenous release of acetylcholine. Ondansetron, an antiemetic, increases acetylcholine levels via 5HT3 receptors antagonism. Ondansetron enhances P50 auditory suppression in persons with schizophrenia two hours after acute dosing[129]. Tropisetron, also a 5HT3 antagonist marketed outside the United States as an antinausea drug, also has efficacy as an α7 nicotinic cholinergic receptor agonist[130,131]. Low dose tropisetron increased the inhibition of the P50 auditory evoked potential differentially in non-smokers with schizophrenia[132], with no effect seen on smokers with schizophrenia. Consistent with a previous finding of the effect in smokers,[77] the authors proposed that the nicotinic cholinergic receptors were chronically densensitized and that additional nicotinic agonism was blocked in smokers.
Discussion
The α7 nicotinic cholinergic receptor role in schizophrenia has been established through multiple independent pathways. An initial clinical observation of increased frequency of smoking in schizophrenia lead to extraction of nicotine and heavy smoking[16, 11]. Examination of the symptom of being overwhelmed by extraneous sensory stimuli lead to finding a physiological deficit, the P50 auditory evoked potential which corrects with the administration of nicotine in both an animal model and in humans[54, 27]. This P50 auditory evoked potential deficit is linked to 15q14 at a polymorphic marker < 120 kb from the α7 gene [39]. Postmortem studies which demonstrate decreased α7 nicotinic cholinergic receptor binding have been noted in the reticular nucleus of the thalamus, the hippocampus, the cingulate cortex and the frontal lobe regions[49,50,51,52]. Nicotine has brief cognitive-enhancing effects in schizophrenia (74,75,76,77,78,79,80]. However, nicotine has several limitations as a therapeutic agent for schizophrenia. Nicotine induces tachyphylaxis and thus does not maintain sustained benefit. Additionally, the long-term health risks of chronic nicotine use are unknown and nicotine is also addictive. [81]. Thus, alternative nicotinic agonists that are less potentially toxic would be helpful in the treatment of schizophrenia. The first agent which has proved successful in early testing for enhancement of cognition is DMXBA, a partial agonist at the α7 nicotinic cholinergic receptor [84,85] and a weak competitive antagonist at the α4ß2 nicotinic cholinergic receptor and 5HT3 receptors [86,87,119]. Additionally, P50 auditory gating was also improved[119]. DMXBA needs to be tested further tested in longer trials to assess this drug’s potential to sustain its effects on cognition Additionally, as the testing was in a relatively uncommon population, people with schizophrenia that are non-smokers, to avoid interactions of nicotinic agonists with already desensitized nicotinic cholinergic receptors, a trial of these types of drugs in smokers is warranted. Furthermore, the half-life of DMXBA is relatively short with a peak effect at about 2 hours, requiring frequent administration and making it impractical for use in a cognitively impaired, non-adherent population. Thus, other delivery systems or other nicotinic agonists with longer half-lives are currently in development.
Abbreviations
- DMXBA
3-[2,4-Dimethoxybenzylidene]anabaseine
- PPI
prepulse startle inhibition
- RBANS
Repeatable Battery for Assessment for Neuropsychological Status
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ann Olincy, Department of Psychiatry, University of Colorado at Denver and Health Sciences Center, Denver, Colorado.
Karen E. Stevens, Department of Psychiatry, University of Colorado at Denver and Health Sciences Center, and Medical Research, Veterans Affairs Medical Center, Denver, Colorado
References
- [1].Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schiophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- [2].Venables PH. Hippocampal Function and Schizophrenia: Experimental Psychological Evidence. Annals New York Academy of Sciences. 1992;658:111–27. doi: 10.1111/j.1749-6632.1992.tb22841.x. [DOI] [PubMed] [Google Scholar]
- [3].Adler LE, Waldo MC, Freedman R. Neurophysiologic Studies of Sensory Gating in Schizophrenia: Comparison of Auditory and Visual Responses. Biological Psychiatry. 1985;20:1284–96. doi: 10.1016/0006-3223(85)90113-1. [DOI] [PubMed] [Google Scholar]
- [4].Boutros NN, Zouridakis G, Overall J. Replication and Extension of P50 Findings in Schizophrenia. Clinical Electroencephalography. 1991;22:40–5. doi: 10.1177/155005949102200109. [DOI] [PubMed] [Google Scholar]
- [5].Judd L, McAdams L, Budnick B, Braff DL. Sensory Gating Deficits in Schizophrenia: New Results. American Journal of Psychiatry. 1992;149:488–93. doi: 10.1176/ajp.149.4.488. [DOI] [PubMed] [Google Scholar]
- [6].Ward PB, Hoffer LD, Liebert B, Catts SV, O’Donnell M, Adler LE. Replication of a P50 auditory sensory gating deficit in Australian patients with schizophrenia. Psychiatry Research. 1996;64:121–35. doi: 10.1016/0165-1781(96)02876-4. [DOI] [PubMed] [Google Scholar]
- [7].Clementz BA, Geyer MA, Braff DL. P50 suppression among schizophrenia and normal comparison subjects: A methodological analysis. Biological Psychiatry. 1997;41:1035–44. doi: 10.1016/S0006-3223(96)00208-9. [DOI] [PubMed] [Google Scholar]
- [8].Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, et al. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophrenia Research. 1993;10:131–41. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- [9].Masterson E, O’Shea B. Smoking and Malignancy in Schizophrenia. British Journal of Psychiatry. 1984;145:429–32. doi: 10.1192/bjp.145.4.429. [DOI] [PubMed] [Google Scholar]
- [10].Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of Smoking Among Psychiatric Outpatients. American Journal of Psychiatry. 1986;143:993–97. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- [11].Goff DC, Henderson DC, Amico E. Cigarette Smoking in Schizophrenia: Relationship to Psychopathology and Medication Side Effects. American Journal of Psychiatry. 1992;149:1189–94. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- [12].Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW. Differential prevalence of cigarette smoking in patients with schizophrenia vs mood disorders. Schizophrenia Research. 1998;33:113–8. doi: 10.1016/s0920-9964(98)00045-0. [DOI] [PubMed] [Google Scholar]
- [13].Kelly C, McCreadie RG. Smoking Habits, Current Symptoms, and Premorbid Characteristics of Schizophrenic Patients in Nithsdale, Scotland. American Journal of Psychiatry. 1999;156:1751–57. doi: 10.1176/ajp.156.11.1751. [DOI] [PubMed] [Google Scholar]
- [14].Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and Mental Illness: A Population-Based Prevalence Study. JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- [15].De Leon J, Dadvand M, Canuso C, Odom White A, Stanilla JK, Simpson GM. Schizophrenia and Smoking: An Epidemiological Survey in a State Hospital. American Journal of Psychiatry. 1995;152:453–55. doi: 10.1176/ajp.152.3.453. [DOI] [PubMed] [Google Scholar]
- [16].Olincy A, Young DA, Freedman R. (1997) Increased Levels of the Nicotine Metabolite Cotinine in Schizophrenic Smokers Compared to Other Smokers Biological Psychiatry 1997421–5. [DOI] [PubMed] [Google Scholar]
- [17].Glassman AH. Cigarette Smoking: Implications for Psychiatric Illness. American Journal of Psychiatry. 1993;150:546–53. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- [18].Svensson TH, Grenhoff J, Engberg G. Effect of nicotine on dynamic function of brain catecholamine neurons. Ciba Foundation Symposium. 1990;152:169–80. doi: 10.1002/9780470513965.ch10. [DOI] [PubMed] [Google Scholar]
- [19].Tung CS, Grenhoff J, Svensson TH. Nicotine counteracts midbrain dopamine cell dysfunction induced by prefrontal cortex inactivation. Acta Physiologica Scandinavica. 1990;138:427–8. doi: 10.1111/j.1748-1716.1990.tb08868.x. [DOI] [PubMed] [Google Scholar]
- [20].Nisell M, Nomikos GG, Svensson TH. Nicotine Dependence, Midbrain Dopamine Systems and Psychiatric Disorders. Pharmacology and Toxicology. 1995;76:157–62. doi: 10.1111/j.1600-0773.1995.tb00123.x. [DOI] [PubMed] [Google Scholar]
- [21].Dalack GW, Meador-Woodruff JH. Smoking, smoking withdrawal and schizophrenia: case reports and a review of the literature. Schizophrenia Research. 1996;22:133–41. doi: 10.1016/s0920-9964(96)80441-5. [DOI] [PubMed] [Google Scholar]
- [22].Dalack GW, Becks L, Hill E, Pomerleau OF, Meador-Woodruff JH. Nicotine Withdrawal and Psychiatric Symptoms in Cigarette Smokers with Schizophrenia. Neuropsychopharmacology. 1999;21:195–202. doi: 10.1016/S0893-133X(98)00121-3. [DOI] [PubMed] [Google Scholar]
- [23].Decina P, Caracci G, Sandik R, Berman W, Mukherjee S, Scapicchio P. Cigarette Smoking and Neuroleptic-Induced Parkinsonism. Biological Psychiatry. 1990;28:502–8. doi: 10.1016/0006-3223(90)90483-i. [DOI] [PubMed] [Google Scholar]
- [24].Goff DC, Henderson DC, Amico E. Cigarette Smoking in Schizophrenia: Relationship to Psychopathology and Medication Side Effects. American Journal of Psychiatry. 1992;149:1189–94. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- [25].Taiminen TJ, Salokangas RKR, Saarijärvi S, Niemi H, Lehto H, Ahola V, et al. Smoking and Cognitive Deficits in Schizophrenia: A Pilot Study. Addictive Behaviors. 1998;23:263–66. doi: 10.1016/s0306-4603(97)00028-2. [DOI] [PubMed] [Google Scholar]
- [26].Nomikos GG, Schilström B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH. Role of Alpha-7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behavioural Brain Research. 2000;113:97–103. doi: 10.1016/s0166-4328(00)00204-7. [DOI] [PubMed] [Google Scholar]
- [27].Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of Auditory Physiology by Cigarette Smoking in Schizophrenic Patients. American Journal of Psychiatry. 1993;150:1856–61. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- [28].Griffith JM, O’Neill J, Petty F, Garver D, Young D, Freedman R. Nicotinic receptor desensitization and sensory gating deficits in schizophrenia. Biol. Psychiatry. 1998;44:98–106. doi: 10.1016/s0006-3223(97)00362-4. [DOI] [PubMed] [Google Scholar]
- [29].Siegal C, Waldo MC, Mizner G, Adler LE, Freedman R. Deficits in Sensory Gating in Schizophrenic Patients and their Relatives. Archives of General Psychiatry. 1984;41:607–12. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- [30].Waldo MC, Carey G, Myles-Worsley M, Cawthra E, Adler LE, Nagamoto HT, et al. Codistribution of a Sensory Gating Deficit and Schizophrenia in Multi-affected Families. Psychiatry Research. 1991;39:257–68. doi: 10.1016/0165-1781(91)90092-4. [DOI] [PubMed] [Google Scholar]
- [31].Clementz BA, Geyer MA, Braff DL. Poor P50 Suppression Among Schizophrenia Patients and Their First-Degree Biological Relatives. American Journal of Psychiatry. 1998;155:1691–94. doi: 10.1176/ajp.155.12.1691. [DOI] [PubMed] [Google Scholar]
- [32].Ross RG, Olincy A, Harris JG, Radant A, Hawkins M, Adler LE, et al. Evidence for Bilineal Inheritance of Physiological Indicators of Risk in Childhood-Onset Schizophrenia. American Journal of Medical Genetics. 1999;88:188–99. [PubMed] [Google Scholar]
- [33].Nagamoto HT, Adler LE, McRae KA, Huettl P, Cawthra E, Gerhardt G, et al. Auditory P50 in Schizophrenics on Clozapine: Improved Gating Parallels Clinical Improvement and Changes in Plasma 3-Methoxy-4-hydroxyphenylglycol. Neuropsychobiology. 1999;39:10–17. doi: 10.1159/000026553. [DOI] [PubMed] [Google Scholar]
- [34].Shirazi-Southall S, Rodriguez DE, Nomikos GG. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology. 2002;26:583–94. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- [35].McEvoy JP, Freudenreich O, Wilson W. Smoking and therapeutic response to clozapine in patients with schizophrenia. Biological Psychiatry. 1999;46:125–29. doi: 10.1016/s0006-3223(98)00377-1. [DOI] [PubMed] [Google Scholar]
- [36].Becker J, Gomes I, Ghisolfi ES, Schush A, Ramos FL, Ehlers JA, et al. Clozapine, but not typical antipsychotics, correct P50 suppression deficit in patients with schizophrenia. Clinical Neurophysiology. 2004;115:396–401. doi: 10.1016/j.clinph.2003.09.018. [DOI] [PubMed] [Google Scholar]
- [37].Adler LE, Hoffer LD, Griffith J, Waldo MC, Freedman R. Normalization by Nicotine of Deficient Auditory Sensory Gating in the Relatives of Schizophrenics. Biological Psychiatry. 1992;32:607–16. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- [38].Freedman R, Adler LE, Bickford PC, Byerley W, Coon H, Cullum CM, et al. Schizophrenia and Nicotinic Receptors. Harvard Review of Psychiatry. 1994:179–92. doi: 10.3109/10673229409017136. [DOI] [PubMed] [Google Scholar]
- [39].Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proceedings of the National Academy of Sciences, USA. 1997;94:587–92. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Leonard S, Gault J, Moore T, Hopkins J, Robinson M, Olincy A, et al. Further Investigation of a Chromosome 15 Locus in Schizophrenia: Analysis of Affected Sibpairs From the NIMH Genetics Initiative. American Journal of Medical Genetics. 1998;81:308–12. doi: 10.1002/(sici)1096-8628(19980710)81:4<308::aid-ajmg6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- [41].Riley BP, Makoff A, Mogudi-Carter M, Jenkins T, Williamson R, Collier D, et al. Haplotype Transmission Disequilibrium and Evidence for Linkage of the CHRNA7 Gene Region to Schizophrenia in Southern African Bantu Families. American Journal of Medical Genetics. 2000;96:196–201. doi: 10.1002/(sici)1096-8628(20000403)96:2<196::aid-ajmg15>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- [42].Tsuang DW, Skol AD, Faraone SV, Bingham S, Young KA, Prabhudesai S, et al. Examination of Genetic Linkage of Chromosome 15 to Schizophrenia in a Large Veterans Affairs Cooperative Study Sample. American Journal of Medical Genetics. 2001;105:662–68. [PubMed] [Google Scholar]
- [43].Liu C-M, Hwu H-G, Lin M-W, Ou-Yang W-C, Lee SF-C, Fann CSJ, et al. Suggestive Evidence for Linkage of Schizophrenia to Markers at Chromosome 15q13-14 in Taiwanese Families. American Journal of Medical Genetics. 2001;105:658–61. doi: 10.1002/ajmg.1547. [DOI] [PubMed] [Google Scholar]
- [44].Xu J, Pato MT, Dalla Torre C, Medeiros H, Carvalho C, Basile VS, et al. Evidence of Linkage Disequilibrium Between the Alpha 7-Nicotinic Receptor Gene (CHRNA7) Locus and Schizophrenia in Azorean Families. American Journal of Medical Genetics. 2001;105:669–74. doi: 10.1002/ajmg.1549. [DOI] [PubMed] [Google Scholar]
- [45].Neves-Pereira M, Bassett AS, Honer WG, Lang D, King NA, Kennedy JL. No Evidence for Linkage of the CHRNA7 Gene Region in Canadian Schizophrenia Families. American Journal of Medical Genetics. 1998;81:361–63. doi: 10.1002/(sici)1096-8628(19980907)81:5<361::aid-ajmg3>3.0.co;2-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Curtis L, Blouin J-L, Radhakrishna U, Gehrig C, Lasseter VK, Wolyniec P, et al. No Evidence for Linkage Between Schizophrenia and Markers at Chromosome 15q13-14. American Journal of Medical Genetics. 1999;88:109–12. doi: 10.1002/(sici)1096-8628(19990416)88:2<109::aid-ajmg1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [47].Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, et al. Association of Promoter Variants in the alpha-7 Nicotinic Acetylcholine Receptor Subunit Gene With an Inhibitory Deficit Found in Schizophrenia. Archives of General Psychiatry. 2002;59:1085–96. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- [48].Houy E, Raux G, Thibaut F, Belmont A, Demily C, Allio G, et al. The promoter - 194 C polymorphism of the nicotinic alpha 7 receptor gene has a protective effect against the P50 sensory gating deficit. Molecular Psychiatry. 2004;9:320–22. doi: 10.1038/sj.mp.4001443. [DOI] [PubMed] [Google Scholar]
- [49].Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, et al. Neuronal Nicotinic Receptors in Dementia with Lewy Bodies and Schizophrenia: alpha-Bungarotoxin and Nicotine Binding in Thalamus. Journal of Neurochemistry. 1999;73:1590–97. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- [50].Freedman R, Hall M, Adler LE, Leonard S. Evidence in Postmortem Brain Tissue for Decreased Numbers of Hippocampal Nicotinic Receptors in Schizophrenia. Biological Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- [51].Marutle A, Zhang X, Court J, Piggot M, Johnson M, Perry R, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. Journal of Chemical Neuroanatomy. 2001;22:115–26. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- [52].Guan Z-Z, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha-7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–82. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- [53].Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, et al. Genetic Correlation of Inhibitory Gating of Hippocampal Auditory Evoked Response and alpha-Bungarotoxin-Binding Nicotinic Cholinergic Receptors in Inbred Mouse Strains. Neuropsychopharmacology. 1996;15:152–62. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- [54].Stevens KE, Wear KD. Normalizing Effects of Nicotine and a Novel Nicotinic Agonist on Hippocampal Auditory Gating in Two Animal Models. Pharmacology, Biochemistry and Behavior. 1997;57:869–74. doi: 10.1016/s0091-3057(96)00466-2. [DOI] [PubMed] [Google Scholar]
- [55].Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine Improves Deficient Inhibitory Auditory Processing in DBA/2 mice, via a Nicotinic Cholinergic Mechanism. Psychopharmacology. 2003;165:386–96. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- [56].Albuquerque EX, Pereira EFR, Braga MFM, Alkondon M. Contribution of nicotinic receptors to the function of synapses in the central nervous system: The action of choline as a selective agonist of alpha-7 receptors. Journal of Physiology (Paris) 1998;92:309–16. doi: 10.1016/s0928-4257(98)80039-9. [DOI] [PubMed] [Google Scholar]
- [57].Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddle TV. Acetylcholine Activates an alpha-Bungarotoxin-Sensitive Nicotinic Current in Rat Hippocampal Interneurons, But Not Pyramidal Cells. The Journal of Neuroscience. 1998;18:1187–95. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hershman KM, Freedman R, Bickford PC. GABA-B antagonists diminish the inhibitory gating of auditory response in the rat hippocampus. Neuroscience Letters. 1995;190:133–36. doi: 10.1016/0304-3940(95)11523-y. [DOI] [PubMed] [Google Scholar]
- [59].Mexal S, Jenkins PM, Lautner MA, Iacob E, Crouch E, Stitzel J. Alpha 7 nicotinic receptor gene promoter polymorphisms in inbred mice affect expression in a cell type-specific fashion. 2007 doi: 10.1074/jbc.M610694200. (in press) [DOI] [PubMed] [Google Scholar]
- [60].Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, et al. Genetic Correlation of Inhibitory Gating of Hippocampal Auditory Evoked Response and alpha-Bungarotoxin-Binding Nicotinic Cholinergic Receptors in Inbred Mouse Strains. Neuropsychopharmacology. 1996;15:152–62. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- [61].Hatsukami D, Fletcher L, Morgan S, Keenan R, Ambie P. The effects of varying cigarette deprivation duration on cognitive and performance tasks. J Sub Abuse. 1989;1:407–16. [PubMed] [Google Scholar]
- [62].Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–30. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- [63].Rinne JO, Myllykylä T, Lönnberg P, Marjamäki P. A post-mortem study of brain nicotinic receptors in Parkinson’s and Alzheimer’s disease. Brain Res. 1991:167–170. doi: 10.1016/0006-8993(91)90588-m. [DOI] [PubMed] [Google Scholar]
- [64].Schroder H, Giacobini E, Wevers A, Birtsch A, Schultz U. Nicotinic receptors in Altzheimer’s disease. In: Domino EF, editor. Brain Imaging of Nicotine and Tobacco Smoking. Npp Books, PO Box 1491; Ann Arbor, MI 48106: 1995. pp. 73–93. [Google Scholar]
- [65].Sahakian BJ, Jones GMM. The effects of nicotine on attention, information processing, and working memory in patients with dementia f the Altzheimer type. In: Adlkofer F, Thruau K, editors. Effects of Nocotine on Biological Systems. Birkhauser Berlag; Basel: 1991. pp. 230–623. [Google Scholar]
- [66].Jones GMM, Sahakian BJ, Levy R, Warburton DM, Gray JA. Effects of acute subcutaneous nicotine on attention, information processing and short-term memory in Altzheimer’s disease. Psychopharmacology. 1992;108:485–94. doi: 10.1007/BF02247426. [DOI] [PubMed] [Google Scholar]
- [67].White H, Levin ED. Chronic four week nicotine skin patch treatment effects on cognitive performance in Altzheimer’s disease. Psychopharmacology. 1999;143:158–65. doi: 10.1007/s002130050931. [DOI] [PubMed] [Google Scholar]
- [68].Newhouse PA, Sunderland T, Tariot PN, Blumhardt CL, Weingartner H, Mellow A, et al. Intravenous nicotine in Altzheimer’s disease: A pilot study. Psychopharmocology. 1988;95:171–75. doi: 10.1007/BF00174504. [DOI] [PubMed] [Google Scholar]
- [69].Parks R, Becker R, Rippey R, Gilbert D, Mathews J, Kabatatm E, et al. Increased regional cerebral glucose metabolism and semantic memory performance in Altzheimer’s disease: A pilot double blind transdermal nicotine positron emission tomography study. Neuropsychol. Rev. 1996;6:61–79. doi: 10.1007/BF01875368. [DOI] [PubMed] [Google Scholar]
- [70].Wilson AL, Langley LK, Monley J, Bauer T, Rottunda S, Mc-falls E, et al. Nicotine patches in Alzheimer’s disease: pilot study on learning, memory, and safety. Pharmacol Biochem Behav. 1995;51:509–14. doi: 10.1016/0091-3057(95)00043-v. [DOI] [PubMed] [Google Scholar]
- [71].Levin ED, Rezvani AH. Development of nicotinic drug therapy for cognitive disorders. European Journal of Pharmacology. 2000;393:141–46. doi: 10.1016/s0014-2999(99)00885-7. [DOI] [PubMed] [Google Scholar]
- [72].George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, et al. Effects of Smoking Abstinence on Visuospatial Working memory Function in Schizophrenia. Neuropsychopharmacology. 2001;26:75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- [73].Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, et al. Effects of Cigarette Smoking on Spatial Working Memory and Attentional Deficits in Schizophrenia. Archives of General Psychiatry. 2005;62:649–59. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- [74].Levin E, Wilson WH, Rose JE, McEvoy JP. Nicotine-Haloperidol Interaction and Cognitive Performance in Schizophrenics. Neuropsychopharmacology. 1996;15:429–36. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- [75].Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal J. Nicotine Effects on Brain Function and Functional Connectivity in Schizophrenia. Biological Psychiatry. 2004;55:850–58. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- [76].Dépatie L, O’Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NNY, Lal S. Nicotine and Behavioral Markers of Risk for Schizophrenia: A Double-Blind, Placebo-Controlled, Cross-Over Study. Neuropsychopharmacology. 2002;27:1056–70. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- [77].Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, et al. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–85. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- [78].Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of Cigarette Smoking and Nicotine Nasal Spray on Psychiatric Symptoms and Cognition in Schizophrenia. Neuropsychopharmacology. 2002;27:479–97. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- [79].Myers CS, Robles O, Kakoyannis AN, Sherr JD, Avila MT, Blaxton TA, et al. Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacology. 2004;174:334–40. doi: 10.1007/s00213-003-1764-8. [DOI] [PubMed] [Google Scholar]
- [80].Sherr JD, Myers C, Avila MT, Elliot A, Blaxton TA, Thaker GK. The Effects of Nicotine on Specific Eye Tracking Measures in Schizophrenia. Biological Psychiatry. 2002;52:721–28. doi: 10.1016/s0006-3223(02)01342-2. [DOI] [PubMed] [Google Scholar]
- [81].Benowitz NL. Summary: Risks and Benefits of Nicotine. In: Benowitz NL, editor. Nicotine Safety and Toxicity. Oxford University Press; New York: 1998. pp. 185–88. [Google Scholar]
- [82].Kem WR, Abbott BC, Coates RM. Isolation and Structure of a Hoplonemertine Toxin. Toxicon. 1971;9:15–22. doi: 10.1016/0041-0101(71)90039-0. [DOI] [PubMed] [Google Scholar]
- [83].Kem WR, Mahnir VM, Papke RL, Lingle CJ. Anabaseine Is a Potent Agonist on Muscle and Neuronal Alpha-Bungarotoxin-Sensitive Nicotinic Receptors. The Journal of Pharmacology and Experimental Therapeutics. 1997;283:979–92. [PubMed] [Google Scholar]
- [84].Briggs CA, McKenna DG, Piattoni-Kaplan M. Human alpha-7 Nicotinic Acetylcholine Receptor Responses to Novel Ligands. Neuropharmacology. 1995;34:583–90. doi: 10.1016/0028-3908(95)00028-5. [DOI] [PubMed] [Google Scholar]
- [85].De Fiebre CM, Meyer EM, Henry JC, Muraskin SI, Kem WR, Papke RL. Characterization of a Series of Anabaseine-Derived Compounds Reveals That the 3-(4)-Dimethylaminocinnamylidine Derivative Is a Selective Agonist at Neuronal Nicotinic alpha-7/125 I-alpha-Bungarotoxin Receptor Subtypes. Molecular Pharmacology. 1995;47:164–71. [PubMed] [Google Scholar]
- [86].Kem WR, Mahnir VM, Lin B, Prokai-Tartrai K. Two primary GTS-21 metabolites are potent partial agonists at alpha7 nicotinic receptors expressed in the Xenopus oocyte. Society of Neurosciences Abstract. 1996;22:268. [Google Scholar]
- [87].Papke RL, Meyer E, Nutter T, Uteshev VV. Alpha-7 Receptor-selective agonists and modes of alpha-7 receptor activation. European Journal of Pharmacology. 2000;393:179–95. doi: 10.1016/s0014-2999(00)00009-1. [DOI] [PubMed] [Google Scholar]
- [88].Stevens KE, Kem WR, Mahnir VM. Freedman R Selective alpha-7 nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology. 1998;136:320–27. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- [89].Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Research. 1992;587:130–36. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- [90].Radek RJ, Miner HM, Bratcher NA, Decker MW, Gopalakrishnan M, Bitner RS. Alpha4beta2 nicotinic receptor stimulation contributes to the effects of nicotine in the DBA/2 mouse model of sensory gating. Psychopharmacol. 2006;187:47–55. doi: 10.1007/s00213-006-0394-3. [DOI] [PubMed] [Google Scholar]
- [91].Freedman R, Wetmore C, Stromberg I, Leonard S, Olson L. Alpha-bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J. Neurosci. 1993;13:1965–75. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].O’Neill HC, Reiger K, Kem WR, Stevens KE. DMXB, an alpha7 nicotinic agonist, normalizes auditory gating in isolation-reared rats. Psychopharmacology. 2003;163:332–39. doi: 10.1007/s00213-003-1482-2. [DOI] [PubMed] [Google Scholar]
- [93].Stevens KE, Kem WR, Freedman R. Selective alpha 7 nicotinic receptor stimulation normalizes chronic cocaine-induced loss of hippocampal sensory inhibition in C3H mice. Biol Psychiat. 1999;46:1443–50. doi: 10.1016/s0006-3223(99)00200-0. [DOI] [PubMed] [Google Scholar]
- [94].Simosky JK, Stevens KE, Kem WR, Freedman R. Intragastric DMXB-A, an alpha7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biol Psychiat. 2001;50:493–500. doi: 10.1016/s0006-3223(01)01093-9. [DOI] [PubMed] [Google Scholar]
- [95].Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiat. 1994;51:139–54. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- [96].Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacol. 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- [97].George TP, Termine A, Sacco KA, Allen TM, Reutenauer E, Vessiccho JC, et al. A preliminary study of the effects of cigarette smoking on prepulse inhibition in schizophrenia: Involvement of nicotinic receptor mechanisms. Schizophrenia Res. 2006;87:307–15. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- [98].Kumari V, Soni W, Sharma T. Influence of cigarette smoking on prepulse inhibition of the acoustic startle response in schizophrenia. Hum Psychopharmaco. 2001;l16:321–26. doi: 10.1002/hup.286. [DOI] [PubMed] [Google Scholar]
- [99].Suemaru K, Yasuda K, Umeda K, Araki H, Shibata K, Choshi T, et al. Nicotine blocks apomorphine-induced disruption of prepulse inhibition of the acoustic startle in rats: possible involvement of central nicotinic alpha7 receptors. Br J Pharmacol. 2004;142:843–50. doi: 10.1038/sj.bjp.0705855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Schreiber R, Dalmus M, De Vry J. Effects of alpha 4/beta 2- and alpha 7-nicotine acetylcholine receptor agonists on prepulse inhibition of the acoustic startle response in rats and mice. Psychopharmacol. 2002;159:248–57. doi: 10.1007/s00213-001-0927-8. [DOI] [PubMed] [Google Scholar]
- [101].Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr. Clin North Am. 2005;28:613–33. doi: 10.1016/j.psc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- [102].Joyce EM, Roiser JP. Cognitive heterogeneity in schizophrenia. Curr Opin Psychiat. 2007;20:268–72. doi: 10.1097/YCO.0b013e3280ba4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Tamminga CA. The neurobiology of cognition in schizophrenia. J Clin Psychiat. 2006;67:9–13. [PubMed] [Google Scholar]
- [104].Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacol. 2007 doi: 10.1038/sj.npp.1301423. (in press) [DOI] [PubMed] [Google Scholar]
- [105].Ciamei A, Aversano M, Cestari C, Castellano C. Effects of MK-801 and nicotine combinations on memory consolidation in CD1 mice. Psychopharmacol. 2001;154:126–30. doi: 10.1007/s002130000584. [DOI] [PubMed] [Google Scholar]
- [106].Moragrega I, Carrasco MC, Vicens Pl, Redolcat R. Spatial learning in male mice with different levels of aggressiveness: effects of housing conditions and nicotine administration. Behav Brain Res. 2003;147:1–8. doi: 10.1016/s0166-4328(03)00112-8. [DOI] [PubMed] [Google Scholar]
- [107].Puma C, Deschaux O, Molimard R, Bizot JC. Nicotine improves memory in an object recognition task in rats. Eur Neuropsychopharmacol. 1999;9:23–7. doi: 10.1016/s0924-977x(99)00002-4. [DOI] [PubMed] [Google Scholar]
- [108].Woodruff-Pak DS, Green JT, Coleman-Valencia C, Pak JT. A nicotinic cholinergic agonist (GST-21) and eyeblink classical conditioning: acquisition, retention and relearning in older rabbits. Exp Aging Res. 2000;26:323–26. doi: 10.1080/036107300750015723. [DOI] [PubMed] [Google Scholar]
- [109].Young J, Finalyson K, Spratt C, Marston H, Crawford N, Kelly J, Sharkey J. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacol. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- [110].Briggs CA, Anderson DJ, Brioni JD, Buccafusco JJ, Buckley MJ, Campbell JE, et al. Functional Characterization of the Novel Neuronal Nicotinic Acetylcholine Receptor Ligand GTS-21 in Vitro and In Vivo. Pharmacology, Biochemistry and Behavior. 1997;57:231–41. doi: 10.1016/s0091-3057(96)00354-1. [DOI] [PubMed] [Google Scholar]
- [111].Woodruff-Pak DS, Li Y-T, Kem WR. A nicotinic agonist (GTS-21), eye blink classical conditioning, and nicotinic receptor binding in rabbit brain. Brain Research. 1994;645:309–17. doi: 10.1016/0006-8993(94)91665-9. [DOI] [PubMed] [Google Scholar]
- [112].Woodruff-Pak DS. Mecamylamine reversal by nicotine and by a partial alpha-7 nicotinic acetylcholine receptor agonist (GTS-21) in rabbits tested with delay eye blink classical conditioning. Behavioral Brain Research. 2003;143:159–67. doi: 10.1016/s0166-4328(03)00039-1. [DOI] [PubMed] [Google Scholar]
- [113].Arendash GW, Sengstock GJ, Sanberg PR, Kem WR. Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res. 1995;674:252–59. doi: 10.1016/0006-8993(94)01449-r. [DOI] [PubMed] [Google Scholar]
- [114].Meyer E, Kuryatov A, Gerzanich V, Lindstrom J, Papke RL. Analysis of 3-(4-Hydroxy, 2-Methoxybenzylidene)Anabaseine Selectivity and Activity at Human and Rat Alpha-7 Nicotinic Receptors. The Journal of Pharmacology and Experimental Therapeutics. 1998;287:918–25. [PubMed] [Google Scholar]
- [115].Meyer EM, De Fiebre CM, Hunter BE, Simpkins CE, Frauworth N, De Fiebre NEC. Effects of Anabaseine-Related Analogs on Rat Brain Nicotinic Receptor Binding and on Avoidance Behaviors. Drug Development Research. 1994;31:127–34. [Google Scholar]
- [116].Nanri M, Yamamoto J, Miyake H, Watanabe H. Protective Effect of GTS-21, a Novel Nicotinic Receptor Agonist, on Delayed Neuronal Death Induced by Ischemia in Gerbils. Japanese Journal of Pharmacology. 1998;76:23–9. doi: 10.1254/jjp.76.23. [DOI] [PubMed] [Google Scholar]
- [117].Meyer EM, Tay EE, Papke RL, Meyers C, Huang G, deFiebre CM. 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rats α7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res. 1997;768:49–56. doi: 10.1016/s0006-8993(97)00536-2. [DOI] [PubMed] [Google Scholar]
- [118].Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE, et al. Safety, Pharmacokinetics, and Effects on Cognitive Function of Multiple Doses of GTS-21 in Healthy, Male Volunteers. Neuropsychopharmacology. 2003;28:542–51. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- [119].Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, et al. An α7-Nicotinic Cholinergic Agonist Enhances Cognitive Function in Schizophrenia. Arch Gen Psychiatr. 2006;63:630–8. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- [120].Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156:1944–1950. doi: 10.1176/ajp.156.12.1944. [DOI] [PubMed] [Google Scholar]
- [121].Wishka DG, Walker DP, Yates KM, Reitz SC, Shaojuan J, Meyers JK, et al. Discovery of N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl furo[2,3-c]pyridine-5-carboxamide, an agonist of the α7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: synthesis and structure-activity relationship. J Med Chem. 2006;49:4425–36. doi: 10.1021/jm0602413. [DOI] [PubMed] [Google Scholar]
- [122].Pichat P, Bergis OE, Terranova JP, Urani A, Duarte C, Santucci V, et al. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2007;32:17–34. doi: 10.1038/sj.npp.1301188. [DOI] [PubMed] [Google Scholar]
- [123].Mullen G, Napier J, Balestra M, DeCory T, Hale G, Macor J, et al. (-)-Spiro[1-azabicyclo[2.2.2]octane-3,5′-oxazolidin-2′-one], a Conformationally Restricted Analogue of Acetylcholine, Is a Highly Selective Full Agonist at the alpha-7 Nicotinic Acetylcholine Receptor. Journal of Medicinal Chemistry. 2000;43:4045–50. doi: 10.1021/jm000249r. [DOI] [PubMed] [Google Scholar]
- [124].Olivier B, Leahy C, Mullen T, Paylor R, Groppi VE, Sarnyai Z, et al. The DBA/2J strain and prepulse inhibition of startle: a model system to test antipsychotics? Psychopharmacology. 2001;156:284–90. doi: 10.1007/s002130100828. [DOI] [PubMed] [Google Scholar]
- [125].Schreiber R, Dalmus M, De Vry J. Effects of the alpha-4/beta-2 and alpha-7 nicotine acetylcholine receptor agonists on prepulse inhibition of the acoustic startle response in rats and mice. Psychopharmacology. 2002;159:248–57. doi: 10.1007/s00213-001-0927-8. [DOI] [PubMed] [Google Scholar]
- [126].Grottick A, Higgins G. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behavioral Brain Research. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- [127].Hahn B, Sharples CG, Wonnacott S, Shoaib M, Stolerman IP. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003;44:1054–67. doi: 10.1016/s0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- [128].Briggs CA, McKenna DG, Piattoni-Kaplan M. Human alpha-7 Nicotinic Acetylcholine Receptor Responses to Novel Ligands. Neuropharmacology. 1995;34:583–90. doi: 10.1016/0028-3908(95)00028-5. [DOI] [PubMed] [Google Scholar]
- [129].Adler LE, Cawthra EM, Donovan KA, Harris JG, Nagamoto HT, Olincy A, et al. Improved P50 Auditory Gating with Ondansetron in Medicated Schizophrenia Patients. American Journal of Psychiatry. 2005;162:386–88. doi: 10.1176/appi.ajp.162.2.386. [DOI] [PubMed] [Google Scholar]
- [130].Macor J, Gurley D, Lanthorn T, Loch J, III, Mack RA, Mullen G, et al. The 5-HT3 Antagonist Tropisetron (ICS 205-930) is a Potent Selective Alpha-7 Nicotinic Receptor Partial Agonist. Bioorganic and Medicinal Chemistry Letters. 2001;11:319–21. doi: 10.1016/s0960-894x(00)00670-3. [DOI] [PubMed] [Google Scholar]
- [131].Papke RL, Schiff HC, Jack BA, Horenstein NA. Molecular dissection of tropisetron, an alpha-7 nicotinic acetylcholine receptor-selective partial agonist. Neuroscience Letters. 2005;378:140–44. doi: 10.1016/j.neulet.2004.12.025. [DOI] [PubMed] [Google Scholar]
- [132].Koike K, Hashimoto K, Takai N, Shimizu E, Komatsu N, Watanabe H, et al. Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophrenia Research. 2005;76:67–72. doi: 10.1016/j.schres.2004.12.016. [DOI] [PubMed] [Google Scholar]