Summary
The inhibitory receptor CTLA4 has a key role in peripheral tolerance of T cells for both normal and tumor-associated antigens. Murine experiments suggested that blockade of CTLA4 might have antitumor activity and a clinical experience with the blocking antibody ipilimumab in patients with metastatic melanoma did show durable tumor regressions in some patients. Therefore, a phase II study of ipilimumab was conducted in patients with metastatic renal cell cancer with a primary end point of response by Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Two sequential cohorts received either 3 mg/kg followed by 1 mg/kg or all doses at 3 mg/kg every 3 weeks (with no intention of comparing cohort response rates). Major toxicities were enteritis and endocrine deficiencies of presumed autoimmune origin. One of 21 patients receiving the lower dose had a partial response. Five of 40 patients at the higher dose had partial responses (95% confidence interval for cohort response rate 4% to 27%) and responses were seen in patients who had previously not responded to IL-2. Thirty-three percent of patients experienced a grade III or IV immune-mediated toxicity. There was a highly significant association between autoimmune events (AEs) and tumor regression (response rate = 30% with AE, 0% without AE). CTLA4 blockade with ipilimumab induces cancer regression in some patients with metastatic clear cell renal cancer, even if they have not responded to other immunotherapies. These regressions are highly associated with other immune-mediated events of presumed autoimmune origin by mechanisms as yet undefined.
Keywords: renal cancer, immunotherapy, CTLA-4, ipilimumab, costimulation
It has become clear that T-cell responses are under the control of both activating and inhibitory coreceptors.1,2 The interaction of the T-cell receptor with its cognate epitope, presented by the correct major histocompatibility complex molecule (‘‘signal one’’) is only one component of the complex signaling required to optimally activate a T cell to proliferate, differentiate, and exhibit effector functions.3 The critical role of inhibitory signaling in preventing, limiting, or terminating T-cell responses has recently become apparent.4 T-regulatory cells (T-regs) and inhibitory receptors such as CTLA4 and PD-1 are major regulators of the T-cell response and play significant roles in tolerance to ‘‘self.’’5 It is now known that the tumor-bearing host can mount T-cell responses to numerous tumor-associated antigens that can either be tissue differentiation antigens, overexpressed normal self proteins, or tumor-associated mutated proteins.6 Despite the presence of numerous immunogenic targets on both murine and human tumors, these tumors can still grow in immunocompetent hosts. The failure to reject such tumors can result from inadequate stimulation (‘‘ignorance’’) or immunologic tolerance, yet the relative contribution of these 2 mechanisms in human cancer remains controversial.
CTLA4 (CD152) is an inducible receptor expressed by T cells which ligates the B7-family of molecules (primarily CD80 and CD86) on antigen-presenting cells.7 When triggered, it inhibits T-cell proliferation and function. Mice genetically deficient in CTLA4 develop lymphoproliferative disease and autoimmunity.8 In pre-clinical models, CTLA4 blockade also augments anti-tumor immunity.9,10 These findings led to the development of antibodies that block CTLA4 for use in cancer immunotherapy. Initial studies in patients with melanoma showed that one such antibody, ipilimumab (previously MDX-010; Medarex Inc), could cause objective durable tumor regressions.11 Objective responses of measurable disease were only documented for patients with melanoma, although reductions of serum tumor markers was seen for some patients with ovarian or prostate cancer.12 Many of these patients had received concurrent or prior antitumor vaccines and based on murine models, it was hypothesized that these vaccines may have also contributed to the responses seen. Like melanoma, renal cell cancer (RCC) is responsive to immunotherapy with interleukin-2, yet few active vaccines exist which target RCC-associated antigens. Therefore, a phase II trial of single-agent ipilumimab in patients with metastatic renal cancer was undertaken to investigate whether other immunoresponsive tumors could respond to CTLA4 blockade and if this could occur in the absence of any vaccination.
MATERIALS AND METHODS
All treatment was administered under a Food and Drug Administration and Institutional Review Board-approved clinical protocol after obtaining the written, informed consent of the participants. Ipilimumab was provided by the Medarex Corp (Princeton, NJ). Patients over 16 years of age with measurable metastatic clear cell renal cancer and no systemic therapy within the past 3 weeks were considered eligible (patients recently discontinuing other therapies were required to show evidence of disease progression before entry). Patients were excluded if they had an Eastern Clinical Oncology Group performance status of ≥2, significant abnormalities in hematologic or major organ laboratory parameters, significant active viral or bacterial infections, prior CTLA4-directed therapies, a requirement of systemic steroids or renal cancer of nonclear cell type. Patients with preexisting autoimmune diseases were also excluded.
All patients were treated intravenously every 3 weeks with one of 2 dosing regimens in sequential cohorts: cohort A—patients received a loading dose of 3 mg/kg with all subsequent doses given at 1 mg/kg. Cohort B—patients received all doses at 3 mg/kg only. In cohort B, patients who had not had prior IL-2 could participate if they were ineligible for high-dose IL-2 or had limited or indolent disease documented. Evaluation for response was by Response Evaluation Criteria in Solid Tumors (RECIST) criteria and radiologic studies were performed before every other dose with designated treatment evaluation points after every 4 doses. Owing to study design and size, no intention to compare cohorts for rates of response was intended. Response durations are measured from initial treatment to date of relapse. Repeat treatment courses (for up to 1 y) were instituted unless limiting toxicity or objective tumor progression was documented.
RESULTS
Twenty-one patients were enrolled in cohort A. All had had previous IL-2 therapy. Their characteristics and the number of ipilimumab doses given are listed in Table 1. Three patients (14%) had grade III toxicity. All 3 had enteritis presenting with diarrhea, with biopsy evidence of duodenitis in one and colitis in the other 2 (the clinical course and treatment of this complication of ipilimumab is described in detail elsewhere13). One of these 3 patients also had extensive generalized rash and multiarticular arthritis. In 2 of these patients, high-dose systemic dexamethasone followed by a rapid taper was given to control diarrhea and the other patient responded to steroid enemas. The patient who had colitis, rash, and arthritis and who received indomethacin and steroid enemas, had a partial response in multiple lung and adrenal metastases that was maintained for 18 months before progressing with an isolated femoral bony metastasis.
TABLE 1.
Patient Characteristics
| Cohort B
|
|||
|---|---|---|---|
| Cohort A | Previous IL-2 | No Previous IL-2 | |
| Patients (n = ) | 21 | 26 | 14 |
| Median age (range) | 59 (31–70) | 52 (32–67) | 59 (34–69) |
| Sex (M/F) | 17/4 | 16/10 | 12/2 |
| ECOG PS | |||
| 0 | 15 | 16 | 6 |
| 1 | 6 | 10 | 8 |
| Sites of metastases | |||
| Lung only | 2 | 5 | 2 |
| Any liver and/or bone | 6 | 8 | 4 |
| Previous therapy | |||
| Nephrectomy | 21 | 26 | 13 |
| IL-2 | 21 | 26 | 0 |
| Chemotherapy | 6 | 2 | 0 |
ECOG PS indicates Eastern Clinical Oncology Group performance status.
Forty patients were enrolled in cohort B, 14 with no prior IL-2 and 26 after prior IL-2 therapy. Their characteristics and treatment features are listed in Table 1. Seventeen of these 40 patients (43%) incurred clinically significant immune-mediated toxicities (14 grade III, 2 grade IV, 1 grade V), thought to be of autoimmune etiology. Thirteen had enteritis alone, 1 had hypophysitis, 1 had both of these manifestations, 1 patient (with tumor in his sole remaining adrenal gland) showed primary adrenal insufficiency, and 1 patient had aseptic meningitis with cerebrospinal fluid lymphocytosis (Table 2). As reported separately,13 gastrointestinal toxicity from ipilimumab was ultimately treated with systemic dexamethasone in the majority of patients with resolution of symptoms within 2 weeks in all but 4 patients. Three patients in this study suffered colonic perforation and 1 underwent colectomy for bleeding. Of the 3 patients with perforation, 1 had no prodromal diarrhea, 1 patient treated early in cohort A had further ipilimumab after a brief bout of diarrhea, and 1 was noncompliant with steroid therapy. Noncompliance was also an issue in the patient with bleeding. Two patients died as a consequence of their perforations, with 1 declining intervention in the face of widely progressive renal cancer.
TABLE 2.
Toxicities of Ipilimumab
| No. Patients | Doses of Ipilimumab Given | |
|---|---|---|
| Cohort A* | 21 | |
| Enteritis/colitis | 3 | 5, 5, 6 |
| Dermatitis | 1 | 5 |
| Arthralgia | 1 | 5 |
| Cohort B† | ||
| Previous IL2 | 26 | |
| Enteritis/colitis | 8 | 1, 1, 1, 2, 4, 4, 4, 6 |
| Hypopituitarism | 2 | 4, 5 |
| Aseptic meningitis | 1 | 4 |
| No previous IL2 | 14 | |
| Enteritis/colitis | 6 | 3, 4, 4, 4, 6, 6 |
| Adrenal insufficiency | 1 | 12 |
One patient with enteritis also had grade III dermatitis and arthalgia.
Seventeen patients experienced 18 grade III/IV/V toxicities.
The syndrome of hypophysitis and hypopituitarism with ipilimumab was similar to that described in patients with melanoma. The earliest manifestation can be pituitary swelling on magnetic resonance imaging scan, followed by depressed adrenocorticotropic hormone, thyroid-stimulating hormone, testosterone (in males), cortisol, and T4. Dopamine was typically normal or elevated. Patients received replacement doses of hydro-cortisone and thyroxine and testosterone (in males) was added if significant symptoms were noted. One patient had pituitary swelling, but a hormonal profile compatible with primary hypoadrenalism (elevated adrenocorticotropic hormone, normal T4, and thyroid-stimulating hormone). Both patients with hypopituitarism and the patient with adrenal insufficiency continue to require standard doses of corticosteroid replacement, but all thyroid or testosterone deficiencies and pituitary enlargement have resolved. The 1 patient with aseptic meningitis presented with severe headaches, photophobia, and mild cranial nerve deficits. Magnetic resonance imaging scan was unremarkable, but lumbar puncture was sterile and revealed 1375 leukocytes/mm3 with over 80% lymphocytes. All symptoms and the cerebrospinal fluid lymphocytosis rapidly resolved after beginning a short course of high-dose dexamethasone. One other patient developed a significant generalized rash, which resolved after a delay in ipilimumab dosing, but later developed hypophysitis with further treatment. There were no other significant or consistent treatment-related toxicities within these 40 patients.
Among the 40 patients in cohort B, there were 5 partial responses of 7, 8, 12, 17, and 21 months’ duration for an overall response rate of 12.5% (Fig. 1 and Table 3). The 2 shortest duration responses were in patients who had had previous IL-2 therapy (N = 26) and the 3 responses of longer duration among patients who had not had previous IL-2 (N = 14). One patient, who showed hypophysitis and was initially considered a responder, was later reassigned as a nonresponder due to the unrecognized appearance of his eventual site of progression. The incidence of autoimmune toxicities in cohort B was 7 of 14 (50%) in patients with no previous IL-2 and 10 of 26 (38%) in patients with previous IL-2 treatment (P = 0.52). There was a highly significant association between major autoimmune toxicity and objective tumor regression, with all 5 responses in cohort B occurring in the 17 patients showing autoimmunity and no responses occurring in the 23 patients not experiencing autoimmunity (P = 0.009). The number of doses given before the onset of autoimmunity or associated with tumor regression varied widely (Table 2). Indeed, 1 patient showed significant tumor growth after 2 doses of ipilimumab but experienced a mild bout of colitis after 2 additional doses and promptly demonstrated tumor regression that has been sustained for 21 months (Fig. 2). As with the responding patient in cohort A, the initial sites of relapse in 2 other responding patients in cohort B were solitary new sites, amenable to resection or radiation, without concurrent progression in other preexisting sites.
FIGURE 1.
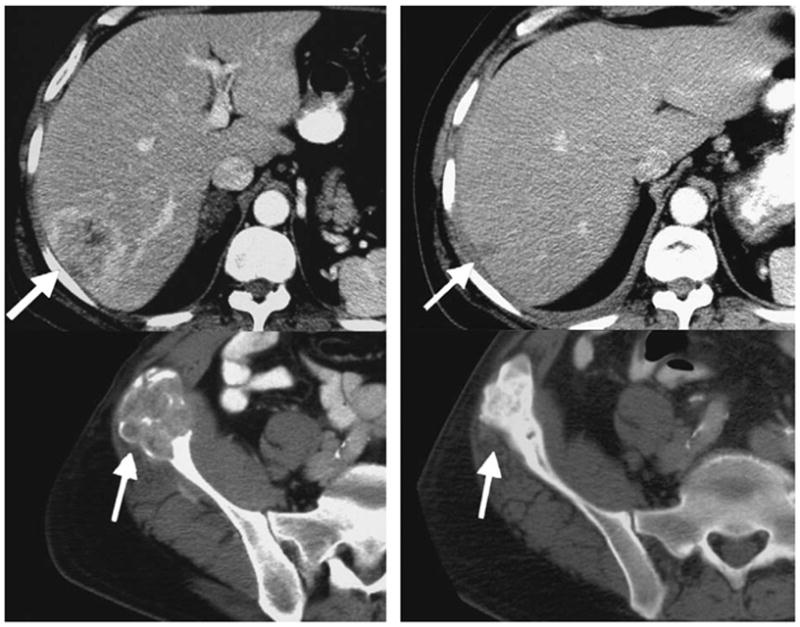
Patient with hepatic and multiple skeletal metastases from renal cell carcinoma who maintained a partial response for 17 months (baseline left, 2 y follow-up right). Tumor regressions illustrated continue 24 months after starting treatment, despite receiving high-dose dexamethasone for colitis at 4 months and showing progression in a solitary lymph node at 17 months.
TABLE 3.
Objective Tumor Regressions With Ipilimumab
| No. Patients | Doses of Ipilimumab | Response Duration | |
|---|---|---|---|
| Cohort A | 21 | ||
| PR | 1 | 5 | 18 mo |
| CR | 0 | — | — |
| Cohort B | |||
| Previous IL2 | 26 | ||
| PR | 2 | 4, 4 | 7, 8 mo |
| CR | 0 | — | — |
| No previous IL2 | 14 | ||
| PR | 3 | 3, 6, 4 | 12, 17, 21 mo |
| CR | 0 | — | — |
CR indicates complete response; PR partial response.
FIGURE 2.
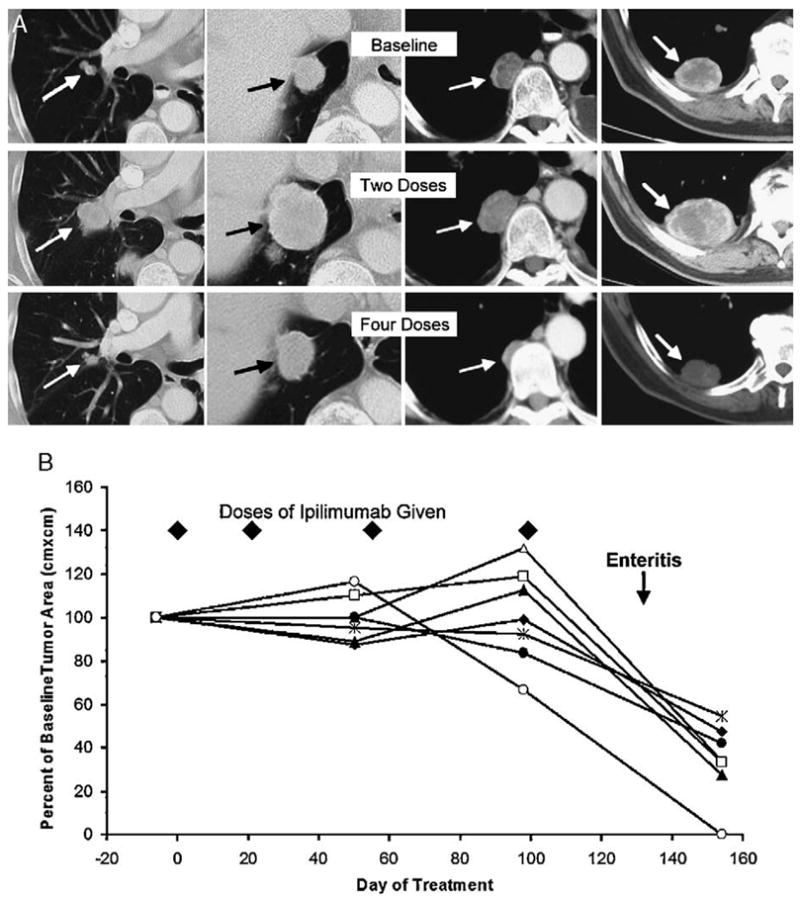
A, Patient with obvious tumor progression at multiple sites after 2 doses of ipilimumab, who then experienced mild colitis after 2 additional doses and had marked tumor regression that continues at 21+ months. B, Another patient’s measured tumor burden in 7 pulmonary metastases is shown as a percent of their baseline size. Tumor remained stable during 4 doses of ipilimumab administered without symptoms, but regressed promptly after patient experienced a bout of biopsy-documented enteritis.
DISCUSSION
Immune-based therapies using high-dose interleukin-2 to treat advanced renal cancer have a 15% to 25% objective response rate, but are of singular value because of their durability and curative potential. The majority of patients who achieve a complete response will not relapse even after decades of follow-up.14 Therefore, the mechanisms which affect the immune response to human renal cancer are of great interest to enhance the frequency and durations of responses to IL-2 and to develop other immunotherapeutic approaches to this disease. Preclinical studies identified the inhibitory receptor CTLA4 as a major influence in damping or blunting T-cell responses, and as a significant mechanism for maintaining immune tolerance to normal tissues. The development of ipilimumab, a fully human IgG which binds and blocks CTLA4, led to early phase clinical testing and evidence for immune-mediated antitumor responses was seen in patients, particularly those with metastatic melanoma. Summarizing a large single-institution experience with ipilimumab in patients with melanoma, covering a wide dose range (all given intravenously every 3 wk), the response rate for patients with measurable metastatic melanoma was approximately 13%.15 Again, as seen with IL-2, many of these responses were of striking durability with some ongoing at their maximum follow-up of over 4 years. They were seen at a variety of visceral and peripheral sites, and previous IL-2 therapy did not seem to affect the ability to respond to ipilimumab. Durable regressions of melanoma and autoimmunity have also been reported in patients receiving another anti-CTLA4 antibody, temelimumab (CP-675206).16
A significant minority of patients given ipilimumab developed a variety of toxicities that were presumed to be of autoimmune origin. The most frequent major organ toxicities in patients with melanoma were enteritis (particularly colitis with diarrhea) and hypophysitis that occurred in 21% and 5% of patients, respectively.13,17 Dermatitis and sporadic cases of arthritis, hepatitis, nephritis, iritis, and vitiligo were also seen. Many of these toxicities required and responded to brief courses of high-dose corticosteroids. The significant exception to this outcome seemed to be adrenocortical insufficiency as a component of panhypopituitarism, which usually persisted despite recovery of other pituitary endocrine functions.
The most striking observation in these trials was a significant association between immune-mediated adverse events and tumor regression. For patients with melanoma who evidenced such adverse events, the antitumor response rate was 36%, but it was only 5% in those who showed no such toxicities.15 This experience using ipilimumab to treat patients with metastatic clear cell renal cancer closely parallels the results seen in patients with melanoma. Again immune-mediated toxicities were seen with enteritis and hypophysitis the most frequent. The overall response rate was 5% in cohort A (3 mg/kg loading dose followed by 1 mg/kg every 3 wk) and 12.5% in cohort B (3 mg/kg every 3 wk for all doses) although there are disparities in the characteristics of these 2 groups, preventing comparisons of efficacy. The association between toxicity and tumor regression was again striking. Among the 20 patients with significant auto-immune toxicity, the response rate was 30% yet among the 41 patients free of such toxicity it was 0% (P = 0.0007; both cohorts combined). The major difference from the experience in melanoma is that no complete or durable regressions of renal cancer were documented in this small study.
The basis for the association of ipilimumab-induced autoimmunity and tumor regression is not known. Other examples of such an association have been described with IL-2 therapy where vitiligo and hypothyroidism are associated with improved tumor response rates.18,19 In allotransplantation for renal cancer and leukemia, patients showing graft-versus-host disease have improved therapeutic outcomes, presumably due to a graft-versus-tumor response.20 For ipilimumab therapy, a variety of possible mechanisms exist to explain the findings. An antigenic target shared by renal cancer, melanoma, gastrointestinal mucosa, pituitary, and other major organs is unlikely, particularly in view of the low frequency of multiple autoimmune sequelae occurring in the same patient. Immunosuppressive regulatory T cells (Treg) are known to constitutively express high levels of CTLA4 and are critical in controlling autoimmune colitis and gastritis in mice. They also seem to play a role in suppressing tumor rejection in some mouse models. Yet our investigation of the impact of ipilimumab on Treg did not support elimination or a reduction in these cells.21 Alternatively, activation of CD4 cells in response to ipilimumab-induced autoimmunity may nonspecifically augment an endogenous T-cell response against the intrinsically immunogenic human tumors, melanoma, and renal cancer. This may be mediated either by paracrine cytokine stimulation or CD4 activation of dendritic cells, which can then effectively present tumor-associated antigens. Finally, the association between autoimmunity and tumor regression may not be causal, but rather a reflection of genetically based differences in immune reactivity mediated by polymorphisms in genes that interact with CTLA4. Human population-based studies have shown that defined polymorphisms in the CTLA4 gene are associated with increases in the incidence of type 1 diabetes, Graves’ disease, and autoimmune hypothyroidism, all thought to have an autoimmune component.22 This is an active area of ongoing research in patients receiving ipilimumab.
Blockade of the T-cell–inhibitory receptor CTLA4 with a monoclonal antibody seems to both induce autoimmunity involving a diverse array of target tissues and cause the regression of metastatic human renal cancer. The occurrence of these 2 phenomena are significantly associated in patients. The immune-mediated toxicities can have serious consequences for a small minority of patients and it remains to be determined if the higher incidence of major complications from enteritis in patients with renal cancer compared to those with melanoma is genuine or a reflection of a limited experience. An algorithm for rapidly and vigorously addressing bowel toxicity is currently being instituted to determine if the frequency of such complications can be reduced.13 Again it is not yet clear if previous IL-2 therapy has any impact on the frequency or duration of responses in these patients and ongoing studies are investigating this issue. Surprisingly, the application of high-dose corticosteroids to control autoimmune toxicities did not seem to coincide with a subversion or truncation of an ongoing antitumor response. Therefore, there seem to be methods for addressing toxicities from this agent which can still permit tumor regressions in patients either not eligible for or not responding to standard immunotherapies. The new availability of other United States FDA-approved agents such as sunitinib and sorafenib for advanced renal cancer has broadened the choices available for patients with this disease. Yet complete regression with these agents is rarely achieved and most patients will show tumor progression within a year,23,24 so other treatments with different mechanisms of action are needed. The fact that other immunotherapies have been curative for a minority of patients with widespread renal cancer compels a continued interest in agents which act via the immune system. A larger experience with ipilimumab in RCC is needed to more accurately determine its relative risks and benefits and its role in the overall approach to renal cancer. Additional experience and correlative laboratory investigations may contribute to a better understanding of how ipilimumab induces the immunologic rejection of this and other cancers.
Footnotes
Financial Disclosure: Dr Israel Lowry is an employee of Medarex Corp and as such receives salary and holds stock options from Medarex. The remaining authors have declared there are no financial conflicts of interest related to this work.
References
- 1.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 4.Saito T, Yamasaki S. Negative feedback of T cell activation through inhibitory adapters and costimulatory receptors. Immunol Rev. 2003;92:143–160. doi: 10.1034/j.1600-065x.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 7.Chambers CA, Kuhns MS, Egen JG, et al. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 8.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 9.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 10.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by CTLA-4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, White DE, et al. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2. Ann Surg. 1998;228:319. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 17.Blansfield JA, Beck KE, Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19:81–84. [PubMed] [Google Scholar]
- 19.Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19:3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 20.Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 21.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 24.Rini BI, Sosman JA, Motzer RJ. Therapy targeted at vascular endothelial growth factor in metastatic renal cell carcinoma: biology, clinical results and future development. BJU Int. 2005;96:286–290. doi: 10.1111/j.1464-410X.2005.05616.x. [DOI] [PubMed] [Google Scholar]


