Abstract
The striated muscle Z-line, a multiprotein complex at the boundary between sarcomeres, plays an integral role in maintaining striated muscle structure and function. Multiple Z-line-associated proteins have been identified and shown to play an increasingly important role in the pathogenesis of human muscle disease. Cypher/ZASP, a PDZ-LIM protein in the Z-line, binds to α-actinin (via its PDZ domain) and has been suggested to function as a linker-strut to maintain cytoskeletal structural integrity during contraction. Cypher may also participate in signaling pathways by binding to protein kinase C (PKC) via its LIM domains. Analysis of Cypher deficient mice has revealed that Cypher plays an integral role in Z-line maintenance/integrity of striated muscles and the pathogenesis of congenital myopathies, including cardiomyopathy. These studies have led to the subsequent discovery of Cypher mutations in human patients with dilated cardiomyopathy, hypertrophic cardiomyopathy as well as skeletal muscle myopathies, which have been recently termed Zaspopathies. The recent discovery of various alternatively spliced isoforms of Cypher with potentially distinct structural and signaling roles brings a different level of complexity to the mechanisms underlying Cypher-based human myopathies. This review will focus on recent developments on the role of Cypher and its isoforms in striated muscle structure, signaling, and disease to provide insights into the mechanisms involved in the pathogenesis of Z-line-associated human myopathies.
Introduction
The striated muscle Z-line, alternatively termed the Z-band or Z-disc, is a multiprotein complex at the boundary between sarcomeres, the basic contractile unit of myofibrils. Within the Z-line, actin and titin filaments from adjacent sarcomeres are overlapping and cross-linked by α-actinin, allowing the transmission of tension between sarcomeres during contraction. Furthermore, the Z-line forms lateral connections between myofibrils and connects the contractile apparatus with the cytoskeleton and extracellular matrix to form the costamere (Ervasti 2003). Thus, the Z-line plays a pivotal role in striated muscle formation, maintenance, structure and function (reviewed in Clark et al. 2002, Frank et al. 2006) and is thought to be a focal point for signaling in striated muscle (Chen and Chien 1999, Frey et al. 2004, Furukawa et al. 2001, Hoshijima 2006, Knoll et a. 2002). Recently, a large number of Z-line-associated proteins have been identified and mutations in many of these have been linked to cardiac and skeletal myopathies in humans and mice (Clark et al. 2002, Frank et al. 2006, and Figure 1). Further characterization of structural and signaling roles of Z-line proteins in disease is essential for providing insights into treatment strategies for Z-line-associated human muscle myopathies.
Figure 1.
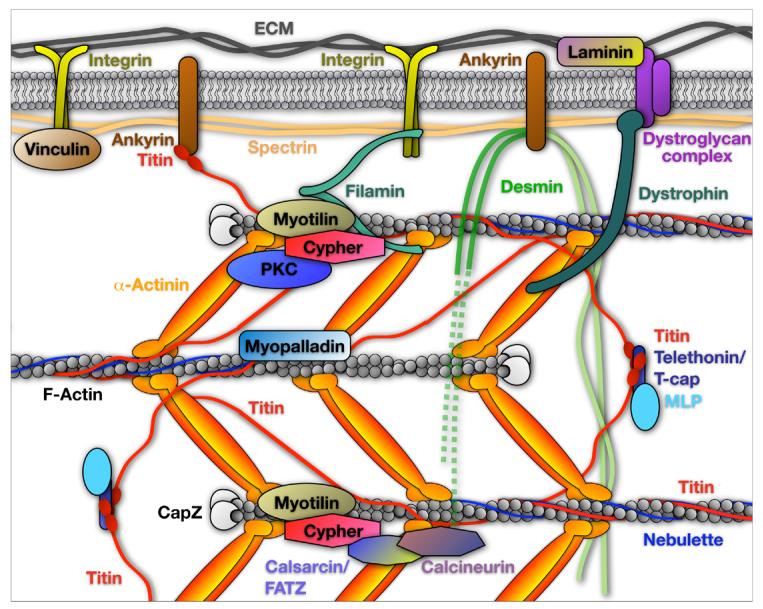
Model of the sarcomeric Z-line and costamere highlighting Cypher in the context of other Z-line associated proteins.
Cypher is a Member of the PDZ/LIM Domain Family
Cypher was first identified and cloned from mouse as a novel striated muscle-specific PDZ/LIM domain-containing protein localized in the Z-line (Zhou et al. 1999 and Figure 1). A human orthologue of Cypher, Z-band Alternatively Spliced PDZ-motif protein (ZASP), was independently identified (Faulkner et al. 1999). In addition, Cypher was also independently cloned from mouse as Oracle (Passier et al. 2000). Cypher is a member of the PDZ/LIM domain family, which contains a single central- or N-terminal-positioned PDZ domain as well as single or multiple LIM domains, positioned either N- or C-terminal from the PDZ domain (Te Velthuis et al. 2007). Both PDZ and LIM domains are protein-protein interaction domains with diverse functions (Te Velthuis et al. 2007). Based on phylogenetic studies, it is thought that the PDZ/LIM domain family evolved from a “one PDZ four LIM” ancestral gene (eat-1/tungus) (Te Velthuis et al. 2007). The one LIM domain containing actinin-associated LIM protein (ALP) subfamily includes ALP, Mystique, Elfin (CLP36), and RIL and is thought to arise through the loss of three (LIM 2-4) of the four LIM domains (Te Velthuis et al. 2007). Likewise, the three LIM domain containing Enigma subfamily, which includes Cypher/ZASP, Enigma (LMP-1), and Enigma homologue (ENH), is thought to arise through the loss of one LIM domain (LIM1) (Te Velthuis et al. 2007).
Identification of Cypher isoforms
Several alternatively spliced isoforms of cypher have been reported in various species. Mouse cypher contains 17 coding exons, which gives rise to six splice variants (Huang et al. 2003) that can be classified into cardiac and skeletal muscle forms based on the inclusion of exons, which are specific to cardiac (exon 4: Cypher c isoforms), or predominant in skeletal muscle (exon 5, 6, and 7: Cypher s isoforms) (Huang et al. 2003, Zhou et al. 1999). While cardiac isoforms of Cypher are not expressed in skeletal muscle, skeletal isoforms of Cypher are expressed in cardiac muscle (Vatta et al. 2003), which may explain why mutations in exon 6, an exon expressed predominantly in skeletal muscle, also lead to cardiomyopathy (Selcen and Engel 2005, Vatta et al. 2003). Within each muscle subtype, Cypher isoforms can be further subclassified as short (Cypher2c, 2s) or long [Cypher1c (Oracle 1), 1s, 3c (Oracle 2), 3s], based on the deletion or inclusion of exons encoding the C-terminal LIM domains, respectively (Huang et al. 2003). Both Cypher2 short isoforms contain exon 10 as the last exon, whereas exon 11 is differentially spliced to generate Cypher1 (included) or Cypher3 (excluded) isoforms (Huang et al. 2003). Thus, the N-terminal PDZ domain is present in both short and long isoforms, while the three LIM domains are unique to long isoforms (Huang et al. 2003). Human ZASP contains 16 exons (mouse exon 7 is absent), which, like in mouse, gives rise to six reported isoforms. These include ZASP-1, -2, -3, -4, -5, -6, which are analogous to mouse Cypher 2s, 1s, 3s, 2c, 1c, and 3c (Vatta et al. 2003). On the other hand, zebrafish cypher contains 18 exons and gives rise to at least 13 spice variants, only two of which have counterparts in mouse (Cypher 2s and Cypher 2c) (Van der Meer et al. 2006). Based on the large number of splice variants identified in zebrafish, it was suggested that other splice variants of cypher may be expressed in other species (Van der Meer et al. 2006).
A Structural Role for Cypher in Striated Muscle
A feature common to all PDZ/LIM domain family members is their ability to associate with the actin cytoskeleton (Te Velthuis et al. 2007). Cypher is localized in the Z-line, where it binds to α-actinin's C-terminal domain via its PDZ domain, which is common to all isoforms (Faulkner et al. 1999, Zhou et al. 1999, Zhou et al. 2001). In addition, recent studies have demonstrated that internal fragments of Cypher, containing a conserved sequence called the ZASP-like motif (ZM), are targeted to the Z-line and directly interact with α-actinin's rod domain. The ZM-motif, also present in ALP and CLP36, is localized within two alternatively spliced exons 4 and 6, which are found in the cardiac-specific and skeletal muscle predominant isoforms, respectively (Klaavuniemi and Ylänne 2006). Several other independent domains of Cypher are targeted to the Z-line, suggesting that Cypher may function as a linker-strut by interacting with several proteins in the Z-line (Zhou et al. 2001). Calsarcin, a calcium/calmodulin-dependent phosphatase, has also been identified as an interacting partner for Cypher at the Z-line (Frey et al. 2002); however, the domains required for binding and the biological relevance of this interaction as well as the identity of other potential interaction partners for Cypher in the Z-line remain to be determined.
A Signaling Role for Cypher in Striated Muscle
Like other members of the Enigma family, long isoforms of Cypher can directly bind to protein kinase C (PKC) via its LIM domains and can be phosphorylated by PKC (Kuroda et al. 1996, Zhou et al. 1999). PKCs are a group of serine/threonine kinases, which have been shown to play a critical role in the development of cardiac hypertrophy and ischemic preconditioning in vitro and in vivo (Dempsey et al. 2000, Dorn and Force 2005). In addition, in vivo models of pressure overload-induced hypertrophy and human heart failure are associated with increased activity of a number of PKC isoforms (Bowling et al. 1999, Simpson 1999). Upon activation, PKC is translocated from one intracellular compartment to another by anchoring to specific RICKs (receptors for inactivated C kinase) and RACKs (receptors for activated C kinase) (Robia et al. 2001). The binding of Cypher long isoforms to PKC suggests that Cypher may have RICK or RACK-like functions and play a signaling role. Interestingly, a D626N mutation identified in Japanese DCM patients is located in exon 15, which encodes the third LIM domain (Arimura et al. 2004 and also see below). It has been found that the mutant Cypher protein has an increased affinity to PKC (Arimura et al. 2004), further suggesting that Cypher plays a signaling role in the heart.
Ablation of Cypher in Mice Leads to neonatal Lethality
A critical role for Cypher in striated muscle was demonstrated by conventional ablation of Cypher in mouse, which resulted in neonatal lethality within five days after birth due to functional failure in multiple striated muscle types and the development of a severe form of congenital myopathy, including dilated cardiomyopathy and heart failure (Zhou et al. 2001). Cypher knockout mice exhibited severely disorganized and fragmented Z-lines in both cardiac and skeletal muscle, while no Z-line abnormalities could be detected in the non-contracting diaphragm muscle before birth. This suggests that Cypher is dispensable for sarcomerogenesis but acts as a linker-strut to maintain the structural integrity of the Z-line during the stress of contraction (Zhou et al. 2001). These observations were supported by morpholino knockdown of cypher in zebrafish, which also resulted in cardiac dilation and marked ventricular wall thinning, which are features associated with dilated cardiomyopathy (DCM) (Van der Meer et al. 2006). Unlike in mouse, a role for Cypher in sarcomerogenesis was suggested in zebrafish studies (Van der Meer et al. 2006); however, it remains to be determined if this was due to species differences or the potential redundancy of other Enigma family members at early stages of mouse somite development.
A Role for Cypher Isoforms in Striated Muscle
Assessment of Cypher isoform expression during mouse development revealed that the short isoform of Cypher (Cypher2) is expressed at barely detectable levels during embryogenesis but expressed postnatally at increasing levels in both cardiac and skeletal muscle, suggesting that it may play an important role in muscle maturation (Huang et al. 2003). Long isoforms of Cypher have different developmental expression patterns dependent on the muscle type assessed. In cardiac muscle, Cypher3c is developmentally upregulated, while Cypher1c is the predominant long isoform expressed throughout development and into adulthood (Huang et al. 2003). In skeletal muscle, Cypher1s is the predominant long isoform during embryogenesis and in neonatal muscle, but is gradually replaced by Cypher3s with progressive aging (Huang et al. 2003). Rescue experiments using Cypher2s and Cypher3s knockin mice revealed that either short or long isoforms can partially rescue the neonatal lethality observed in Cypher knockout mice (Huang et al. 2003). All surviving Cypher2s and 3s knockin mice displayed growth retardation and exhibited muscle pathology (greater severity in Cypher3s knockin muscle), suggesting that short and long Cypher isoforms may likely play functionally redundant roles with respect to overall muscle viability, but have distinct functional roles that are required for normal muscle structure and function (Huang et al. 2003). Further studies will be required to determine if the cardiac muscle phenotype observed in the knockin models reflects the ablation of the cardiac muscle-specific isoforms or the requirement for both long and short isoforms. These studies will be important for the determination of the individual functional roles of these various Cypher isoforms in vivo.
A Role for Cypher in Human Muscle Disease
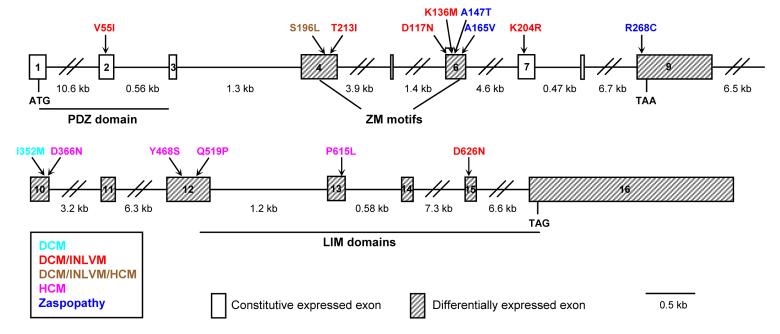
The lethal phenotype of Cypher knockout mice has led to an intensive search for Cypher/ZASP mutations associated with human muscle diseases. Cypher mutations were first identified in patients with human familial and sporadic dilated cardiomyopathy (Arimura et al. 2004, Vatta et al. 2003), a disease that has been linked to the disruption of the cytoskeletal architecture (Vatta et al. 2003). Mutations were identified in several domains within Cypher/ZASP, and found to be associated with a spectrum of different diseases. For example, mutations in the cardiac-specific domain (exon 4) of Cypher (T213I and S196L) were shown to be associated with isolated non-compaction of the left ventricular myocardium (INLVM) and dilated cardiomyopathy (DCM) (Vatta et al. 2003, Xing et al. 2006, Figure 2). Mutations in the skeletal muscle-predominant domain (exon 6) of Cypher (D117N and K136M) and exon 7 of Cypher (K204R) were associated with INLVM and DCM (Vatta et al. 2003, Figure 2), and a mutation in the long isoform specific exon 10 of Cypher (I352M) was associated with a “pure” form of DCM (Vatta et al. 2003, Figure 2). A mutation in exon 2 (V55I), which is common to all isoforms, was independently shown to be associated with INLVM in a Japanese population (Xing et al. 2006, Figure 2). Other independent studies identified Cypher/ZASP mutations (D626N) in a long isoform-specific exon (exon 15; within the third LIM domain) in Japanese patients exhibiting familial DCM and INLVM (Arimura et al. 2004, Xing et al. 2006, Figure 2). (Arimura et al. 2004). Cypher/ZASP mutations (D366N, Y468S, Q519P, and P615L), including one which was previously identified (S196L), have also been recently identified in a subset of human patients with hypertrophic cardiomyopathy (HCM), preferentially exhibiting a sigmoidal septal curvature (Theis et al. 2006, Xing et al. 2006, Figure 2). The D366N, Y468S, Q519P, and P615L mutations are localized in the long isoform-specific exons 10, 12, and 13, which further suggests that different splicing isoforms may have specific roles in cardiac function. Although the mechanisms underlying these disease-associated mutations remain to be determined, evidence suggests that several of these identified mutations may affect the binding affinity of Cypher to its interaction partners. For example, in vitro studies suggest that the D117N mutation found in DCM may exert its effects by disrupting the binding of Cypher/ZASP to proteins interacting with the actin cytoskeletal network (Vatta et al. 2003). Furthermore, the long isoform-specific D626N mutation has been shown to increase the affinity of the Cypher/ZASP LIM domain to PKC (Arimura et al. 2004), suggesting that Cypher plays a signaling role in the heart.
Figure 2.
The genetic organization of the human Cypher/ZASP gene with the identified mutations shown in different colors to illustrate diseases associated with each mutation. The PDZ domain, ZM motifs, and LIM domains are highlighted. Transcriptional start (ATG) and stop (TAG and TAA) codons are also delineated. INLVM: isolated non-compaction of the left ventricular myocardium; DCM: dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy. Exon 9 is the last exon of short Cypher isoforms, while exon 16 is the last exon of large Cypher isoforms.
Cypher/ZASP mutations have also been identified in skeletal muscle myopathies, which were recently named zaspopathies (Griggs et al. 2007, Selcen and Engel 2005). Cypher/ZASP skeletal muscle disease causing mutations have been identified in human patients exhibiting a novel autosomal dominant form of muscular dystrophy, termed myofibrillar myopathy (MFM) (Selcen and Engel 2005), which is characterized by disruption of the Z-line, followed by disintegration of myofibrils and subsequent abnormal protein accumulation. (Selcen and Engel 2005). Ten patients with MFM were identified, which carried mutations (A165V and A147T) in the skeletal muscle predominant exon (exon 6), also linked to cardiomyopathy, at or within the ZM motif, which is important for Cypher's interaction with α-actinin in the Z-line. In addition, a missense mutation (R286C) was identified in the short isoform-specific exon 9 in one patient with late-onset MFM. (Selcen and Engel 2005, Figure 2). The Cypher/ZASP A165V mutation was also recently shown to cause late-onset distal myopathy in a long-studied family (Griggs et al. 2007); however, further studies are required to determine the disease-causing mechanisms. Patients with myotonic dystrophy (DM), which is the most common adult form of muscle dystrophy, demonstrated missplicing of Cypher/ZASP isoforms in their skeletal muscle tissues (Machuca-Tzili et al. 2006). Interestingly, characterization of flies deficient in muscleblind, which is a Drosophila model of human DM and presents with Z-line abnormalities, also demonstrated abnormalities in CG30084 (Drosophila orthologue of Cypher) splicing, suggesting that muscleblind may be a direct regulator of Cypher/ZASP splicing and a potential cause for human DM (Machuca-Tzili et al. 2006). It should also be noted that Cypher is significantly downregulated in mice exhibiting skeletal muscle atrophy induced by fasting, tumor, uremia, or diabetes mellitus (Lecker et al. 2004).
Future Directions
Recent studies using Drosophila, zebrafish, mouse, and human genetics have revealed a pivotal role for Cypher in striated muscle structure, function, signaling, and disease. However, the precise molecular protein complexes associated with Cypher and molecular mechanisms underlying the functional role of these various Cypher isoforms as well as disease-causing mutations are still unresolved and will remain an active area of investigation. These studies will be necessary to not only determine the cause of the clinical heterogeneity of Cypher-specific human muscle myopathies but also the functional importance of these disease-associated mutations in the context of Z-line stability.
Acknowledgments
Work cited from the authors' laboratory was supported by NIH, AHA, CCF, and MDA grants (J.C.). F.S. is a recipient of the AHA National Scientist Development Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arimura T, Hayashi T, Terada H, et al. A Cypher/ZASP mutation associated with dilated cardiomyopathy alters the binding affinity to protein kinase C. J Biol Chem. 2004;279:6746–6752. doi: 10.1074/jbc.M311849200. [DOI] [PubMed] [Google Scholar]
- Bowling N, Walsh RA, Song G, et al. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- Chen J, Chien KR. Complexity in simplicity: monogenic disorders and complex cardiomyopathies. J Clin Invest. 1999;103:1483–1485. doi: 10.1172/JCI7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cyotarchitecture: An intricate web of form and function. Ann Rev Cell Dev Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- Dempsey EC, Newton AC, Mochly-Rosen D, et al. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–L438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Pallavicini A, Formentin E, et al. ZASP: a new Z-band alternatively spliced PDZ-motif protein. J Cell Biol. 1999;146:465–475. doi: 10.1083/jcb.146.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner G, Lanfranchi G, Valle G. Telethonin and other new proteins of the Z-disc of skeletal muscle. IUBMB Life. 2001;51:275–282. doi: 10.1080/152165401317190761. [DOI] [PubMed] [Google Scholar]
- Frank D, Kuhn C, Katus HA, Frey N. The sarcomeric Z-disc: a nodal point in signaling an disease. J Mol Med. 2006;84:446–468. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- Frey N, Olson EN. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J Biol Chem. 2002;277:13998–14004. doi: 10.1074/jbc.M200712200. [DOI] [PubMed] [Google Scholar]
- Frey N, Barrientos T, Shelton JM, et al. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy iin response to pathological biomechanical stress. Nat Med. 2004;10:1336–1343. doi: 10.1038/nm1132. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Ono Y, Tsuchiya H, et al. Specific interaction of the potassium channel beta-subunit mink with sarcomeric protein T-cap suggests a T-tubule-myofibril linking system. J Mol Biol. 2001;313:775–784. doi: 10.1006/jmbi.2001.5053. [DOI] [PubMed] [Google Scholar]
- Griggs R, Vihola A, Hackman P, et al. Zaspopathy in a large classic late-onset distal myopathy family. Brain. 2007;130:1477–1484. doi: 10.1093/brain/awm006. [DOI] [PubMed] [Google Scholar]
- Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–H1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Zhou Q, Liang P, Hollander MS, Sheikh F, Li X, Greaser M, Shelton GD, Evans S, Chen J. Characterization and in vivo functional analysis of splice variants of cypher. J Biol Chem. 2003;278:7360–7365. doi: 10.1074/jbc.M211875200. [DOI] [PubMed] [Google Scholar]
- Klaavuniemi T, Ylanne J. Zasp/Cypher internal ZM-motif containing fragments are sufficient to co-localize with alpha-actinin-analysis of patient mutations. Exp Cell Res. 2006;312:1299–1311. doi: 10.1016/j.yexcr.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Knoll R, Hoshijima M, Hoffman HM, et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–955. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Tokunaga C, Kiyohara Y, et al. Protein-protein interaction of zinc finger LIM domains with protein kinase C. J Biol Chem. 1996;271:31029–31032. doi: 10.1074/jbc.271.49.31029. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Jagoe RT, Gilbert A, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- Machuca-Tzili L, Thorpe H, Robinson TE, Sewry C, Brook JD. Flies deficient in Muscleblind protein model features of myotonic dystrophy with altered splice forms of Z-band associated transcripts. Hum Genet. 2006;120:487–499. doi: 10.1007/s00439-006-0228-8. [DOI] [PubMed] [Google Scholar]
- Passier R, Richardson JA, Olson EN. Oracle, a novel PDZ-LIM domain protein expressed in heart and skeletal muscle. Mech Dev. 2000;92:277–284. doi: 10.1016/s0925-4773(99)00330-5. [DOI] [PubMed] [Google Scholar]
- Robia SL, Ghanta J, Robu VG, Walker JW. Localization and kinetics of protein kinase C-epsilon anchoring in cardiac myocytes. Biophys J. 2001;80:2140–2151. doi: 10.1016/S0006-3495(01)76187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcen D, Engel AG. Mutations in ZASP define a novel form of muscular dystrophy in humans. Ann Neurol. 2005;57:269–276. doi: 10.1002/ana.20376. [DOI] [PubMed] [Google Scholar]
- Simpson PC. Beta-protein kinase C and hypertrophic signaling in human heart failure. Circulation. 1999;99:334–337. doi: 10.1161/01.cir.99.3.334. [DOI] [PubMed] [Google Scholar]
- Te Velthuis AJ, Isogai T, Gerrits L, Bagowski CP. Insights into the molecular evolution of the PDZ/LIM family and identification of a novel conserved protein motif. PLoS ONE. 2007;2:e189. doi: 10.1371/journal.pone.0000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis JL, Martijn Bos J, Barleson VB, et al. Echocardiographic-determined septal morphology in Z-disc hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2006;351:896–902. doi: 10.1016/j.bbrc.2006.10.119. [DOI] [PubMed] [Google Scholar]
- Van der Meer DL, Marques IJ, Leito JT, et al. Zebrafish cypher is important for somite formation and heart development. Dev Biol. 2006;299:356–372. doi: 10.1016/j.ydbio.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Vatta M, Mohapatra B, Jimenez S, et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol. 2003;42:2014–2027. doi: 10.1016/j.jacc.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Xing Y, Ichida F, Matsuoka T, et al. Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol Genet Metab. 2006;88:71–77. doi: 10.1016/j.ymgme.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Ruiz-Lozano P, Martone ME, Chen J. Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C. J Biol Chem. 1999;274:19807–19813. doi: 10.1074/jbc.274.28.19807. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Chu PH, Huang C, Cheng CF, Martone ME, Knoll G, Shelton GD, Evans S, Chen J. Ablation of Cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J Cell Biol. 2001;155:605–612. doi: 10.1083/jcb.200107092. [DOI] [PMC free article] [PubMed] [Google Scholar]