Abstract
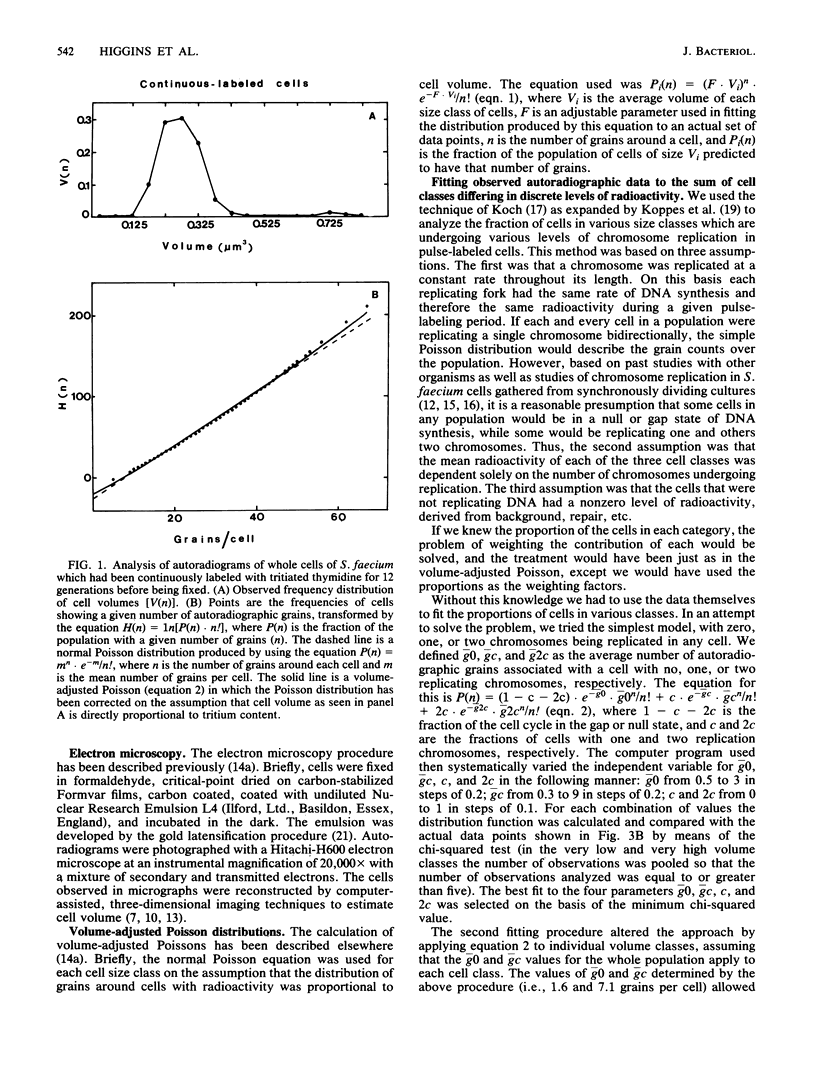
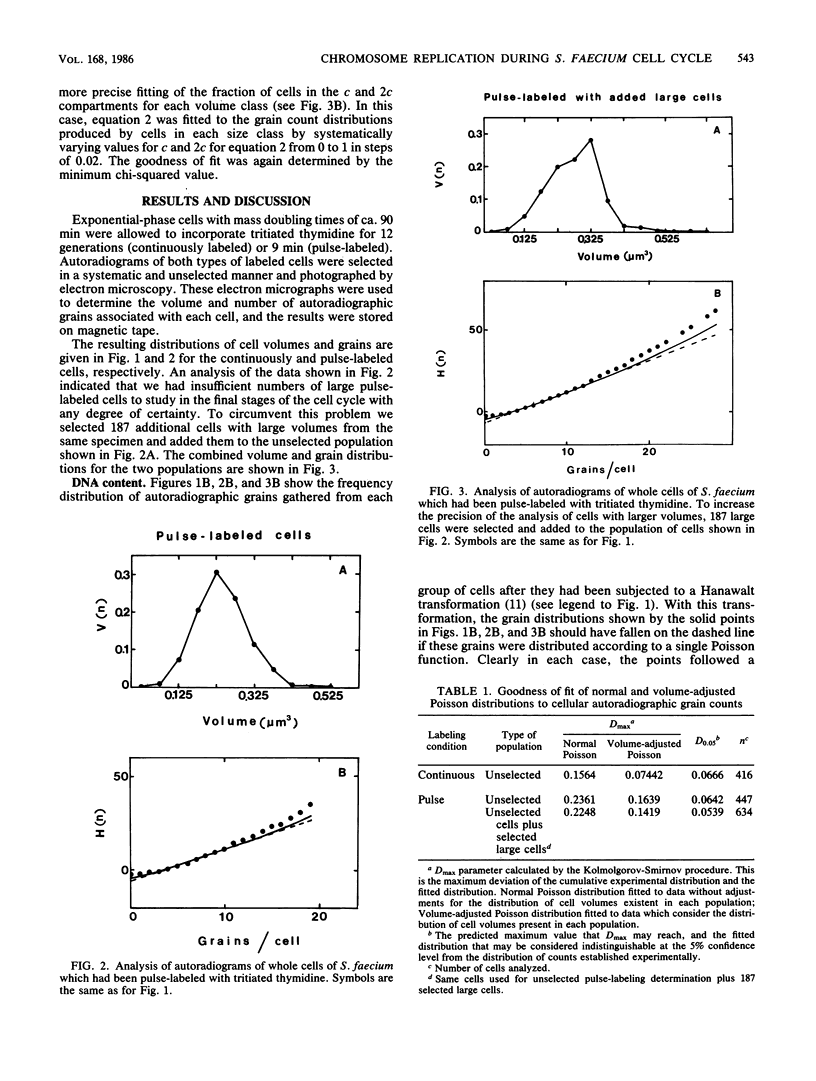
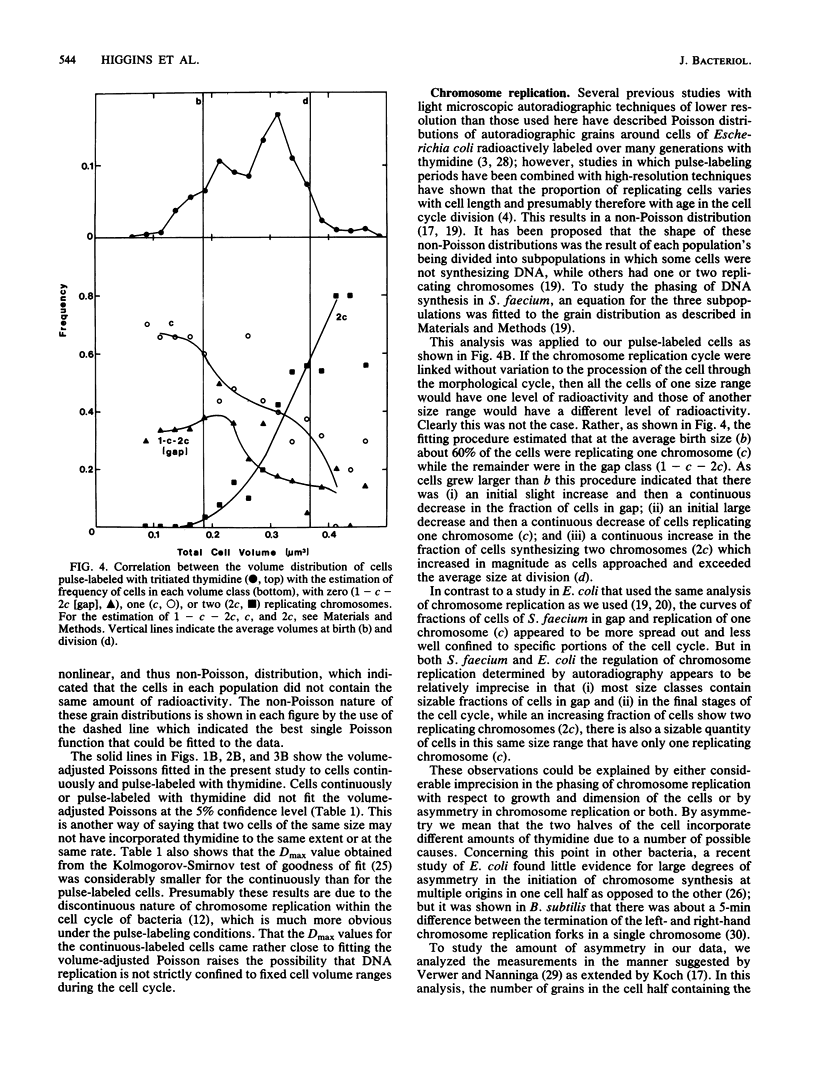
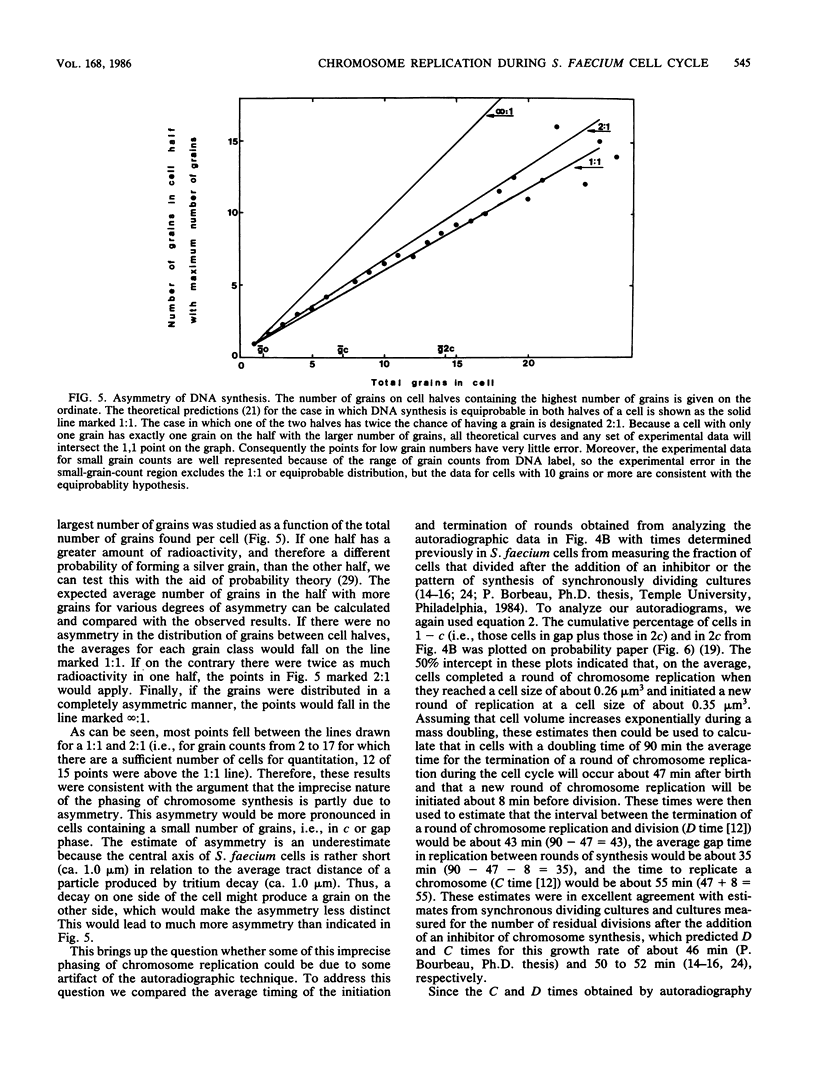
Analysis of the distribution of autoradiographic grains around cells of Streptococcus faecium which had been either continuously or pulse-labeled with tritiated thymidine (mass doubling time, 90 min) showed a non-Poisson distribution even when the distribution of cell sizes in the populations studied was taken into account. These non-Poisson distributions of grains were assumed to reflect the discontinuous nature of chromosome replication. To study this discontinuous process further, we fitted an equation to the grain distribution observed for the pulse-labeled cells that assumed that in any population of cells there were subpopulations in which there were zero, one, or two replicating chromosomes. This analysis predicted an average time for chromosome replication and for the period between completion of rounds of chromosome replication and division of 55 and 43 min, respectively, which were in excellent agreement with estimates made by other techniques. The present investigation extended past studies in indicating that the initiation and completion of rounds of chromosome replication are poorly phased with increases in cell volume and that the amount of chromosome replication may be different in different cell halves.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C., Handschumacher R. E. Inhibition of the synthesis of deoxyribonucleic acid in bacteria by 6-(p-hydroxyphenylazo)-2,4-dihydroxypyrimidine. I. Metabolic studies in Streptococcus fecalis. J Biol Chem. 1966 Jul 10;241(13):3083–3089. [PubMed] [Google Scholar]
- CARO L. G. Localization of macromolecules in Escherichia coli. I. DNA and proteins. J Biophys Biochem Cytol. 1961 Mar;9:539–553. doi: 10.1083/jcb.9.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai N. C., Lark K. G. Cytological studies of deoxyribonucleic acid replication in Escherichia coli 15T-: replication at slow growth rates and after a shift-up into rich medium. J Bacteriol. 1970 Oct;104(1):401–409. doi: 10.1128/jb.104.1.401-409.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Low R. L. Mutational alteration of Bacillus subtilis DNA polymerase 3 to hydroxyphenylazopyrimidine resistance: polymerase 3 is necessary for DNA replication. Biochem Biophys Res Commun. 1973 Mar 5;51(1):151–157. doi: 10.1016/0006-291x(73)90521-4. [DOI] [PubMed] [Google Scholar]
- Edelstein E. M., Rosenzweig M. S., Daneo-Moore L., Higgins M. L. Unit cell hypothesis for Streptococcus faecalis. J Bacteriol. 1980 Jul;143(1):499–505. doi: 10.1128/jb.143.1.499-505.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C. W., Daneo-Moore L., Higgins M. L. Analysis of initiation of sites of cell wall growth in Streptococcus faecium during a nutritional shift. J Bacteriol. 1984 Dec;160(3):935–942. doi: 10.1128/jb.160.3.935-942.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C. W., Daneo-Moore L., Higgins M. L. Cell wall assembly during inhibition of DNA synthesis in Streptococcus faecium. J Bacteriol. 1983 Jul;155(1):351–356. doi: 10.1128/jb.155.1.351-356.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C. W., Daneo-Moore L., Higgins M. L. Initiation of wall assembly sites in Streptococcus faecium. J Bacteriol. 1983 May;154(2):573–579. doi: 10.1128/jb.154.2.573-579.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAWALT P. C., MAALOE O., CUMMINGS D. J., SCHAECHTER M. The normal DNA replication cycle. II. J Mol Biol. 1961 Apr;3:156–165. doi: 10.1016/s0022-2836(61)80042-9. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L., Boothby D., Shockman G. D. Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J Bacteriol. 1974 May;118(2):681–692. doi: 10.1128/jb.118.2.681-692.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Koch A. L., Dicker D. T., Daneo-Moore L. Autoradiographic studies of the synthesis of RNA and protein as a function of cell volume in Streptococcus faecium. J Bacteriol. 1986 Sep;167(3):960–967. doi: 10.1128/jb.167.3.960-967.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L. Three-dimensional reconstruction of whole cells of Streptococcus faecalis from thin sections of cells. J Bacteriol. 1976 Sep;127(3):1337–1345. doi: 10.1128/jb.127.3.1337-1345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks R. P., Daneo-Moore L., Shockman G. D. Approximation of the cell cycle in synchronized populations of Streptococcus faecium. J Bacteriol. 1978 Jun;134(3):1188–1191. doi: 10.1128/jb.134.3.1188-1191.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks R. P., Daneo-Moore L., Shockman G. D. Relationship between cellular autolytic activity, peptidoglycan synthesis, septation, and the cell cycle in synchronized populations of Streptococcus faecium. J Bacteriol. 1978 Jun;134(3):1074–1080. doi: 10.1128/jb.134.3.1074-1080.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Does the initiation of chromosome replication regulate cell division? Adv Microb Physiol. 1977;16:49–98. doi: 10.1016/s0065-2911(08)60047-8. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L. Control of wall band splitting in Streptococcus faecalis. J Gen Microbiol. 1984 Apr;130(4):735–745. doi: 10.1099/00221287-130-4-735. [DOI] [PubMed] [Google Scholar]
- Koppes L. H., Woldringh C. L., Nanninga N. Size variations and correlation of different cell cycle events in slow-growing Escherichia coli. J Bacteriol. 1978 May;134(2):423–433. doi: 10.1128/jb.134.2.423-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes L. J., Overbeeke N., Nanninga N. DNA replication pattern and cell wall growth in Escherichia coli PAT 84. J Bacteriol. 1978 Mar;133(3):1053–1061. doi: 10.1128/jb.133.3.1053-1061.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Skarstad K., Steen H. B., Boye E. Escherichia coli DNA distributions measured by flow cytometry and compared with theoretical computer simulations. J Bacteriol. 1985 Aug;163(2):661–668. doi: 10.1128/jb.163.2.661-668.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN TUBERGEN R. P., SETLOW R. B. Quantitative radioautographic studies on exponentially growing cultures of Escherichia coli. The distribution of parental DNA, RNA, protein, and cell wall among progeny cells. Biophys J. 1961 Sep;1:589–625. doi: 10.1016/s0006-3495(61)86911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer R. W., Nanninga N. Pattern of meso-dl-2,6-diaminopimelic acid incorporation during the division cycle of Escherichia coli. J Bacteriol. 1980 Oct;144(1):327–336. doi: 10.1128/jb.144.1.327-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. S., Wake R. G. Restriction map of DNA spanning the replication terminus of the Bacillus subtilis chromosome. J Mol Biol. 1983 Dec 5;171(2):119–137. doi: 10.1016/s0022-2836(83)80349-0. [DOI] [PubMed] [Google Scholar]


