Abstract
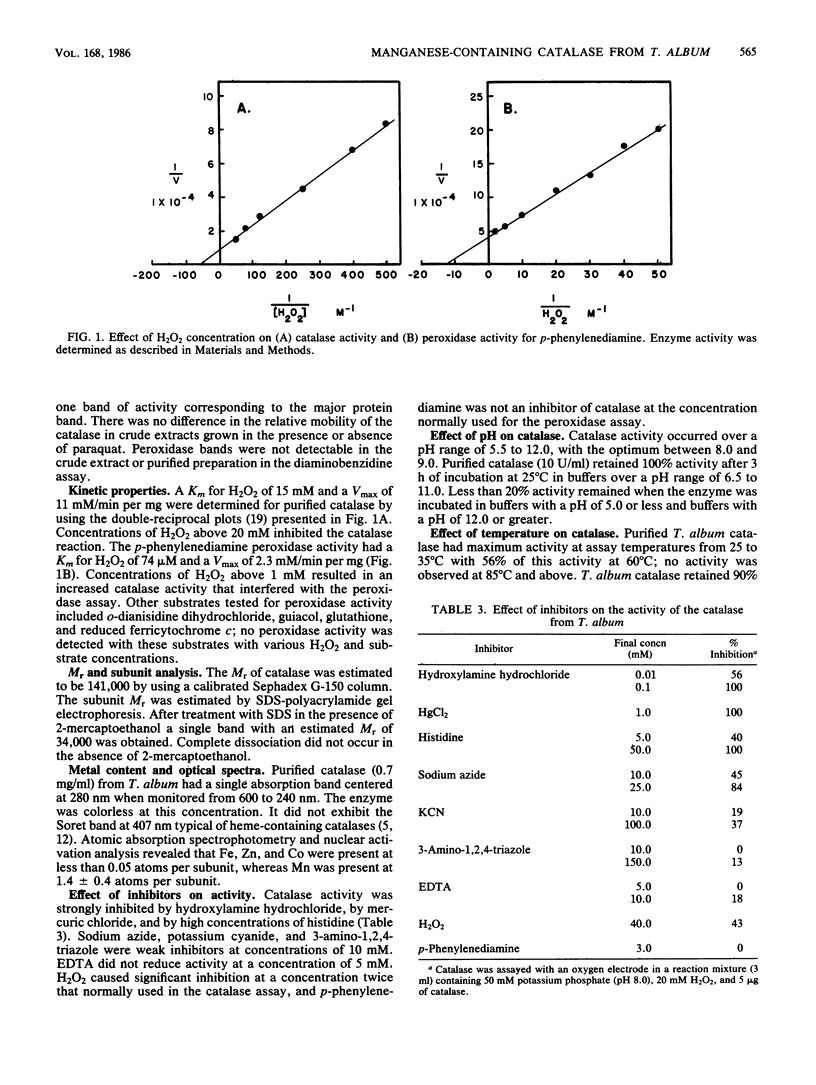
A manganese-containing catalase has been characterized from Thermoleophilum album NM, a gram-negative aerobic bacterium obligate for thermophily and n-alkane substrates. The level of catalase in cells was increased about ninefold by growth in the presence of paraquat (2.5 microM), a superoxide-generating toxicant. Superoxide dismutase levels were unaffected by this compound. The enzyme was purified from cultures grown in the presence of paraquat to greater than 95% homogeneity and had an Mr of 141,000. The enzyme was composed of four subunits, and each had an Mr of 34,000. There were 1.4 +/- 0.4 atoms of manganese present per subunit. The catalase had a Km for hydrogen peroxide of 15 mM and a Vmax of 11 mM/mg. Peroxidase activity, as measured with p-phenylenediamine, copurified with the catalase. Inhibitors of heme-catalase were weak inhibitors of the T. album enzyme. The optimum pH for catalase activity was 8 to 9. The enzyme was stable from pH 6.5 to 11 and retained activity at assay temperatures from 25 to 80 degrees C. The catalase was stable for 24 h of incubation at 60 degrees C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allgood G. S., Perry J. J. Oxygen defense systems in obligately thermophilic bacteria. Can J Microbiol. 1985 Nov;31(11):1006–1010. doi: 10.1139/m85-190. [DOI] [PubMed] [Google Scholar]
- Allgood G. S., Perry J. J. Paraquat toxicity and effect of hydrogen peroxide on thermophilic bacteria. J Free Radic Biol Med. 1985;1(3):233–237. doi: 10.1016/0748-5514(85)90123-0. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- CLAYTON R. K. Purified catalase from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1959 Nov;36:40–47. doi: 10.1016/0006-3002(59)90067-8. [DOI] [PubMed] [Google Scholar]
- Claiborne A., Fridovich I. Purification of the o-dianisidine peroxidase from Escherichia coli B. Physicochemical characterization and analysis of its dual catalatic and peroxidatic activities. J Biol Chem. 1979 May 25;254(10):4245–4252. [PubMed] [Google Scholar]
- Claiborne A., Malinowski D. P., Fridovich I. Purification and characterization of hydroperoxidase II of Escherichia coli B. J Biol Chem. 1979 Nov 25;254(22):11664–11668. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DELWICHE E. A. Catalase of Pedicoccus cerevisiae. J Bacteriol. 1961 Mar;81:416–418. doi: 10.1128/jb.81.3.416-418.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Visualization of catalase on acrylamide gels. Anal Biochem. 1974 Mar;58(1):57–62. doi: 10.1016/0003-2697(74)90440-0. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Herbert D., Pinsent J. Crystalline bacterial catalase. Biochem J. 1948;43(2):193–202. doi: 10.1042/bj0430193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. Catalase of the Lacto-bacillaceae. J Bacteriol. 1962 Apr;83:936–938. doi: 10.1128/jb.83.4.936-938.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. DISTRIBUTION AND CHARACTERISTICS OF THE CATALASES OF LACTOBACILLACEAE. J Bacteriol. 1965 Aug;90:347–351. doi: 10.1128/jb.90.2.347-351.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. ISOLATION AND CHARACTERIZATION OF THE CYANIDE-RESISTANT AND AZIDE-RESISTANT CATALASE OF LACTOBACILLUS PLANTARUM. J Bacteriol. 1965 Aug;90:352–356. doi: 10.1128/jb.90.2.352-356.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob G. S., Orme-Johnson W. H. Catalase of Neurospora crassa. 1. Induction, purification, and physical properties. Biochemistry. 1979 Jul 10;18(14):2967–2975. doi: 10.1021/bi00581a009. [DOI] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J Biol Chem. 1983 May 25;258(10):6015–6019. [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Stults F. H., Forstrom J. W., Chiu D. T., Tappel A. L. Rat liver glutathione peroxidase: purification and study of multiple forms. Arch Biochem Biophys. 1977 Oct;183(2):490–497. doi: 10.1016/0003-9861(77)90384-8. [DOI] [PubMed] [Google Scholar]
- WHITTENBURY R. HYDROGEN PEROXIDE FORMATION AND CATALASE ACTIVITY IN THE LACTIC ACID BACTERIA. J Gen Microbiol. 1964 Apr;35:13–26. doi: 10.1099/00221287-35-1-13. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woodbury W., Spencer A. K., Stahman M. A. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971 Nov;44(1):301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]
- Yamada T., Tanaka A., Fukui S. Properties of catalase purified from whole cells and peroxisomes of n-alkane-grown Candida tropicalis. Eur J Biochem. 1982 Jul;125(3):517–521. doi: 10.1111/j.1432-1033.1982.tb06712.x. [DOI] [PubMed] [Google Scholar]



